电化学Fe掺杂增强FTO薄膜的可见光光电化学性能
肖仕清,郝丽华,谢萌阳,马荣伟,牛振江*
(浙江师范大学物理化学研究所,浙江 金华 321004)
电化学Fe掺杂增强FTO薄膜的可见光光电化学性能
肖仕清,郝丽华,谢萌阳,马荣伟,牛振江*
(浙江师范大学物理化学研究所,浙江 金华 321004)
通过在FeSO4溶液中将FTO(F掺杂SnO2)导电玻璃电化学阴极极化,随后在500 °C空气中热氧化,制备出对可见光有响应的Fe掺杂FTO薄膜。用扫描电子显微镜(SEM)、X射线衍射(XRD)和X射线光电子能谱(XPS)表征了薄膜的形貌、结构和表面特性。在可见光下测试了薄膜在1.0 mol/L NaOH 溶液中零偏压下的光电流−时间曲线。结果显示,电化学修饰后的FTO薄膜表面呈纳米多孔形貌,薄膜中有1%(原子分数)左右的Fe元素掺杂且存在正交结构SnO2和四方结构SnO2两种物相。Fe掺杂FTO的光电流密度为0.5 μA/cm2,比无Fe掺杂的FTO薄膜(0.019 μA/cm2)显著增强。
氟掺杂二氧化锡;铁掺杂;阴极极化;可见光;光电流
从Fujishima等[1]的开创性工作开始,利用太阳能分解水或降解有机污染物,减少化石燃料的消耗和解决紧迫的环境问题,已成为世界范围的研究热点。开发各种高性能、低成本的光催化材料,是发展光催化技术的关键之一。
SnO2是n型半导体,在酸碱介质中性质稳定,但由于禁带宽度为3.6 eV,仅能吸收太阳光谱的紫外部分,且其本征的光催化活性很低。通过金属离子(如Mg[2]、V[3]、Zn[4-5]、W[4]、Cu[6]和Fe[7-10])的掺杂或与其他氧化物(如Fe2O3[11-13]、ZnO[14])的复合,可以有效地提高SnO2材料的光催化性能。F掺杂的SnO2(FTO)薄膜,已被广泛用作液晶显示屏和各种太阳能电池、电致变色材料的基底,也被用作许多光电催化材料的导电层[15-18]。而经过强阴极极化处理[19-20],能增强FTO薄膜的光电化学性能,使FTO薄膜能够直接用于光电催化反应。
本文通过在FeSO4溶液中对市售的FTO导电玻璃进行阴极极化,再经热氧化,制备出具有较好光电化学性能的改性FTO薄膜,并通过结构、形貌和表面特性的分析表征,讨论了电化学修饰增强FTO薄膜光电化学性能的机理。
1 实验
1. 1 样品制备
以市售的FTO导电玻璃(深圳华南湘城科技有限公司,表面方阻为13 Ω)为基底,依次用丙酮、无水乙醇超声清洗20 min,再经超纯水清洗后,放入0.1 mol/L FeSO4+ 0.1 mol/L (NH4)2SO4电解液中,以Pt片为阳极,恒电流密度6 mA/cm2阴极极化350 s,两电极之间的距离固定为2.0 cm。电化学处理后清洗,于空气气氛下500 °C恒温加热2 h,得到电化学修饰的FTO薄膜(F-FTO)。在0.1 mol/L Na2SO4溶液中以相同电流密度阴极极化和相同热处理制备的FTO薄膜(C-FTO)与直接进行热处理的薄膜(A-FTO)分别作为比较和空白样品。
1. 2 形貌、结构和光学特性表征
通过日本Hitachi S4800扫描电子显微镜(SEM)分析薄膜表面形貌。采用荷兰PANalytical X’pert PRO X射线衍射仪(XRD)分析薄膜的结构。用英国Renishaw inVia激光显微拉曼光谱仪进行拉曼光谱检测,激发波长为532 nm。用美国Thermo Scientific公司的ESCALAB 250Xi型X射线光电子能谱仪(XPS)分析样品元素的表面组成和化学状态,Al Kα射线(1 486.6 eV),以样品表面污染碳(C─H或C─C)C1s的结合能284.8 eV为内标校正电子结合能。通过日本岛津XRF-1800型X−荧光光谱仪(XRF)对样品进行元素定量分析。
1. 3 光电化学性能测试
以FTO薄膜(受光面积1.0 cm2)为工作电极,Pt片为对电极,采用两电极体系,在1.0 mol/L NaOH溶液中测试样品在零偏压下的光电流−时间( j−t)曲线。实验时每次光照50 s后闭光50 s为一次循环,同一样品经过5次循环以上的测试。所用光源为Xe灯(北京纽比特科技有限公司),用紫外截止滤光片ZJB380滤去紫外光部分,照射到样品表面的光强度为100 mW/cm2。
本文所有电化学实验均在室温下进行,所用仪器为CHI660C电化学工作站(上海辰华),所用化学试剂均为分析纯,溶液用超纯水配制。
2 结果与讨论
2. 1 薄膜的光电化学性能
图1为经过不同处理的FTO薄膜在零偏压下的j−t曲线。在可见光照射下,未经过电化学阴极极化处理的FTO薄膜(A-FTO)基本没有产生光电流。而经过在Na2SO4溶液中阴极极化处理的FTO薄膜(C-FTO)和在含FeSO4溶液中阴极极化后的FTO薄膜(F-FTO)都产生了较明显的阳极光电流,显示出n型半导体的特性。可见阴极极化显著增强了FTO的光电流,与文献的报道[19-20]一致。F-FTO的光电流密度为0.5 μA/cm2,比C-FTO的光电流密度(1.85 × 10−2μA/cm2)大了近26倍,表明在FeSO4溶液中阴极极化处理更能有效提高FTO薄膜的光电化学性能。
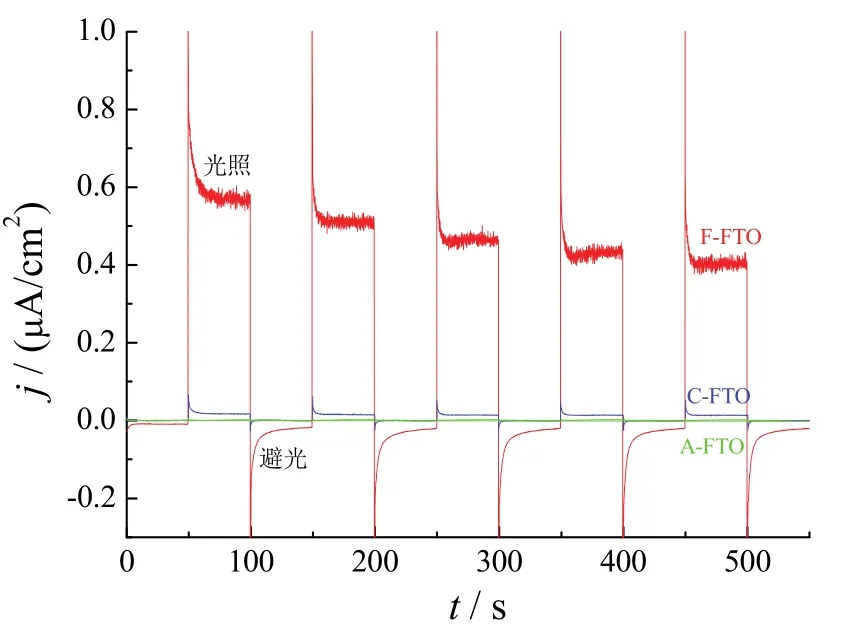
图1 FTO薄膜在可见光下的光电流−时间曲线(1.0 mol/L NaOH,零偏压)Figure 1 Photocurrent vs. time curves of FTO films in 1 mol/L NaOH electrolyte under visible light and zero bias
碱性溶液中零偏压下的光电流测试结果表明,电化学改性和修饰能够显著增强FTO薄膜在可见光下的光电化学性能。为了探讨电化学改性增强FTO薄膜光电化学性能的机理,进一步对不同处理的FTO样品进行了形貌、结构和表面特性的分析。
2. 2 FTO薄膜的形貌和结构
2. 2. 1 薄膜的形貌
图2为不同FTO薄膜的表面和截面形貌。从SEM分析结果可见,A-FTO为不规则的亚微米颗粒形成的致密薄膜,厚度约为500 nm。C-FTO薄膜表面是由大小不一的颗粒堆积成的多孔形貌,一些颗粒的尺寸明显增大,致密性降低,厚度增加到约600 nm。F-FTO的表面则呈较均匀的纳米颗粒堆积成的多孔形貌,截面出现分层,底部为厚度约200 nm的致密层,上部为厚约600 nm的更加疏松的纳米多孔结构。
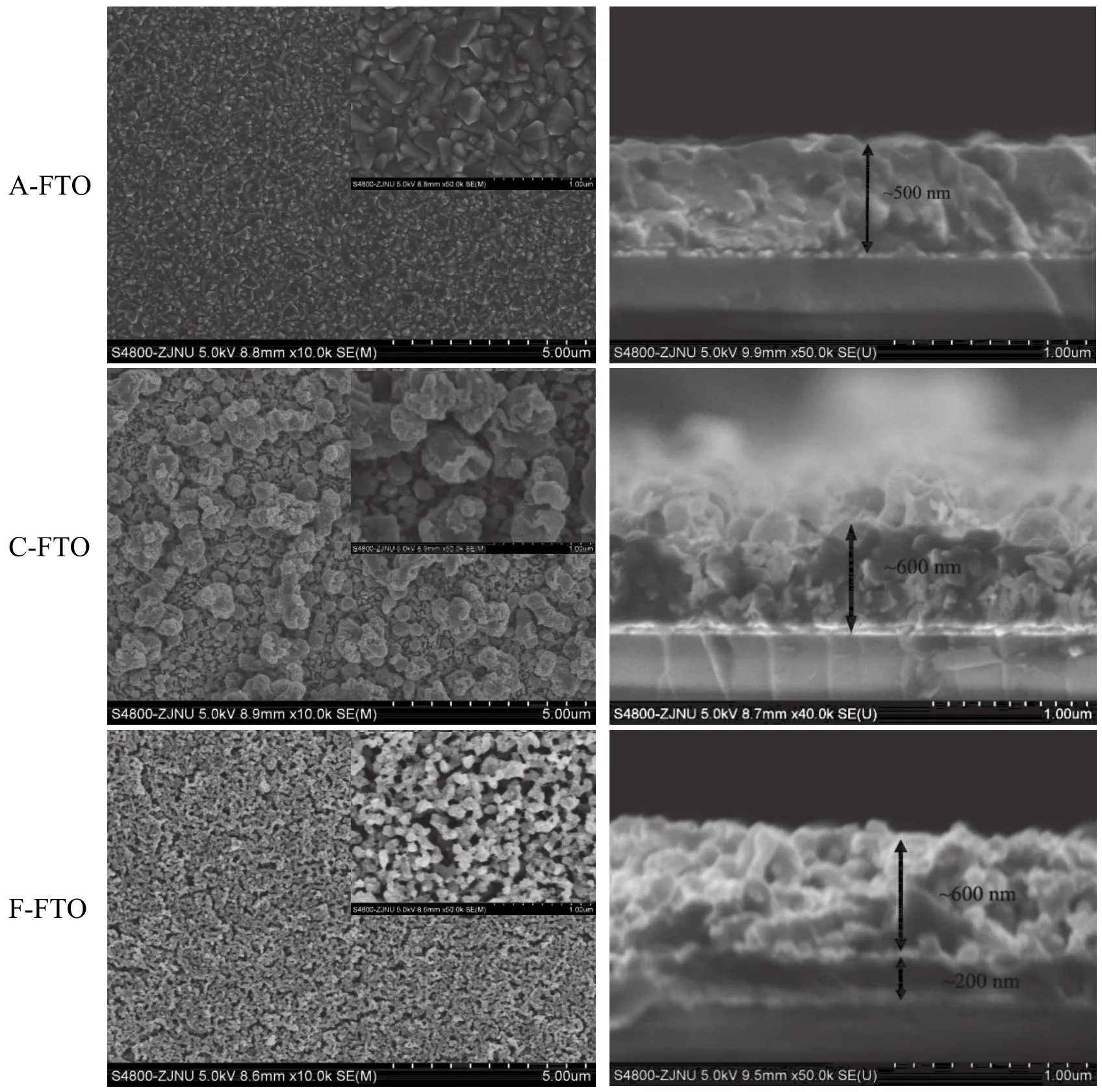
图2 FTO薄膜的表面和截面微观形貌Figure 2 Microscopic morphologies of surface and cross-section of FTO films
SEM分析表明,电化学阴极极化处理后,FTO薄膜的表面由致密转变为多孔,这是薄膜光电化学性能增强的一个原因。F-FTO的纳米多孔形貌明显提高了薄膜的光电流密度。实验中也发现,A-FTO是完全无色透明的,C-FTO呈浅乳白色,F-FTO则呈浅橘黄色。薄膜颜色的变化既改变了薄膜吸收可见光的性质,更利于提高可见光光电化学性能,也揭示了薄膜的颗粒尺寸、结构和组成发生了相应的变化。实验结果还表明,在相同的阴极极化条件下,FTO薄膜的形貌变化与溶液中的金属离子种类(Na+或Fe2+)有关。
2. 2. 2 薄膜的结构
图3为三种样品的XRD谱图。从中可见,A-FTO样品在2θ为26.45°、33.6°、37.7°、51.6°、54.5°、61.4°和65.5°处有衍射峰,可归属于四方结构SnO2的(110)、(101)、(200)、(211)、(220)、(310)和(112)晶面的衍射(JCPDS No.88-0287)。而C-FTO和F-FTO样品中,原来属于四方结构的几个衍射峰的中心位置向高衍射角偏移,而且有所减弱、宽化。峰中心向高衍射角偏移表明衍射晶面距减小,而衍射峰变宽则可能由晶粒细化和晶体内应力增大而引起。
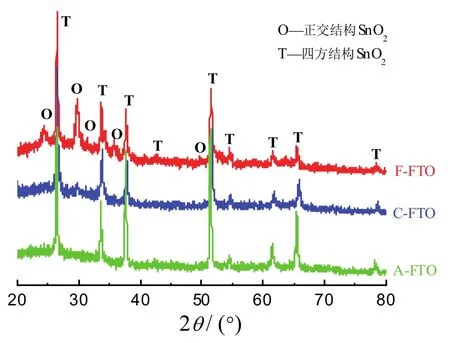
图3 FTO薄膜的X射线衍射特征Figure 3 XRD patterns of FTO films
更值得注意的是,C-FTO和F-FTO样品的XRD图谱中,在2θ为24.3°、29.7°、31.4°、34.0°、35.7°、50.0°、53.1°、58.0°处出现新的弱衍射峰,并且在F-FTO的谱图中更加明显。但这些衍射峰不能归属于Fe2O3或其他铁氧化物的衍射。虽然不能完全排除存在正交结构SnO的可能性[21],但根据本文实验条件和文献的研究结果,可以合理地将这些新的衍射峰归属于正交结构SnO2(110)/(111)、(113)、(020)/(021)、(022)/(114)、(023)、(220)、(132)/(223)和(119)晶面的衍射(JCPDS No.78-1063)。一般认为,正交结构的SnO2只能在高压下存在,但近几年来已发现可以在常压下通过各种不同的途径制备出正交结构的SnO2[22-26]。温和条件下形成正交SnO2的原因与氧化物形成过程中产生的内应力有关,尤其是当有掺杂元素(如Mn[27])存在时,掺杂元素引起的结构变形更有利于形成正交结构的SnO2。
笔者课题组[18]曾通过在含Cu2+溶液中阴极沉积后热氧化的方法在FTO基底上制备Cu2O薄膜,整个过程中都没有发现FTO基底本体有明显的结构或形貌变化。而本文的结果表明,在含Na+或Fe2+离子的溶液中阴极极化,FTO薄膜的形貌和结构都发生了变化。这可能是因为,在酸性或中性溶液中,Cu2+、Fe2+、Na+的标准还原电位分别为0.34、−0.45和−2.71 V,而SnO2无论还原成Sn还是SnO,其标准还原电位均约为−0.11 V[28],所以在含Cu2+溶液中阴极极化,Cu2+的还原将是主要的反应,对FTO基底的影响不大。但在含Fe2+或Na+的溶液中,SnO2的还原将成为主要的反应。文献中也报道了在酸性水溶液中可以将SnO2阴极还原为金属Sn[29]。溶液中的Fe3+离子也有助于FTO的阴极还原[30]。在Na2SO3溶液中,−2.1 V(相对于SCE)阴极电势下能将FTO还原[19]。本文实验中观察到,FTO薄膜经过6.0 mA/cm2阴极极化后薄膜的颜色都变深,表明FTO薄膜发生了类似的还原反应。Plȧ Cid等[31]在pH = 7.0的Na2SO4溶液中以0.26 mA/cm2的阴极电流对FTO极化处理2 750 s后,没有发现FTO的结构和形貌发生变化,可能是由于他们所用的阴极极化电流太低。
经阴极还原的FTO样品在热氧化过程中必然经历SnO中间态[32],而SnO的存在有利于进一步氧化时形成正交结构的SnO2[33]。如果薄膜中存在Fe元素,Fe和Sn离子半径的差异将引起SnO2晶格的畸变,晶距收缩且内应力增大[34],从而驱动正交结构SnO2的形成。因此F-FTO薄膜中形成了更多正交结构的SnO2,在XRD谱图中呈现出正交SnO2的衍射峰。
研究显示,正交结构SnO2与四方结构SnO2共存能提高SnO2材料的传感性能[35-37]。因此,经过阴极极化改性之后,FTO薄膜的表面由致密平整变为多孔的形貌,而且存在正交和四方两种结构的SnO2,加上可能有Fe元素的掺杂效应,使得薄膜的光电化学性能得到极大的提高。
2. 3 薄膜的XPS分析
为证实在F-FTO薄膜中是否存在Fe元素,对样品进行了XPS分析,结果见图4和图5。在图4的XPS宽谱中,三种样品没有明显的差别,主要的电子结合能峰都来自SnO2[38-41],27 eV和122 eV处的电子结合能分别归属于Sn4d和Sn4s的芯能级电子,487 eV和493 eV归属于Sn3d的芯能级电子,717 eV和758 eV归属于Sn3p的芯能级电子,1 060 eV左右处则是SnMNN俄歇电子峰;530 eV处的结合能峰归属于O1s芯能级电子,966 eV和976 eV两处的结合能归属于OKLL俄歇电子峰。

图4 FTO薄膜的XPS全谱Figure 4 XPS survey of FTO films

图5 FTO薄膜的Sn、Fe、O、F元素XPS精细谱图Figure 5 High-resolution XPS spectra of Sn, Fe, O and F elements for FTO films
由图5的各元素精细谱可见,三种样品中的O1s和Sn3d电子结合能峰的峰形和峰位相同。O1s的结合能分别为529.8 eV和531.5 eV,前者归属于常见的晶格O−2状态[41],后者则归属于吸附•OH或者水分子等物种[42]。Sn3d的结合能为485.8 eV和494.2 eV,分别归属于Sn(IV)价态的Sn3d5/2和Sn3d3/2[43-44]。F1s电子的结合能峰位于985 eV,与文献报道[45]一致,但F-FTO薄膜中F1s电子的结合能峰显著减小,可能是在FeSO4溶液里阴极极化及热处理过程中,样品内的F元素有所流失。O、Sn和F的精细XPS谱表明不同FTO样品的主要成分都是SnO2。
从Fe2p精细谱可见,三种样品都在717 eV处出现归属于Sn3p电子的结合能峰[46]。但F-FTO薄膜中,在Sn3p电子结合能峰的两侧存在肩峰,表明样品中存在Fe元素。通过高斯−洛仑兹分峰拟合后,可确定F-FTO中Fe2p的电子结合能为710.6 eV和724.2 eV,半峰宽超过5 eV。从电子结合能数值可推断在F-FTO薄膜中Fe以+3价态存在[47]。由于Fe2p的电子能谱峰形较差,因此难以根据元素的灵敏度因子定量计算Fe的含量。XRF成分分析表明,F-FTO中Fe的含量为1%(原子分数),证明F-FTO为Fe掺杂的FTO。
已有许多研究显示,在TiO2[48]、WO3[49]、SnO2[7-10]等氧化物半导体材料中掺杂Fe元素,能改变材料的光学带隙和缺陷密度,有效提高其光催化/光电化学性能。本文采用在FeSO4溶液中阴极极化后热处理的方式,在普通的FTO导电玻璃上制备出纳米多孔形貌的Fe掺杂FTO薄膜,显著提高了FTO薄膜在可见光下的光电流。本文的研究为宽禁带氧化物的掺杂、改性提供了新的途径。通过优化电化学阴极极化和热处理的条件,可望得到性能更为优异的掺杂FTO光催化材料,并阐明电化学掺杂改性增强光催化性能的机理。相关研究有待后续系统地展开。
3 结论
(1) 普通FTO导电玻璃通过在FeSO4溶液中阴极极化后热处理,能够制备出纳米多孔形貌的Fe掺杂FTO薄膜,它具有良好的可见光光电化学活性。
(2) Fe掺杂FTO薄膜中铁的原子分数为1%,存在正交结构SnO2和四方结构SnO2两种物相。
致谢
感谢厦门大学林敬东和金鹏老师帮助进行样品的XRD测试。
[1] FUJISHIMA A, HONDA K. Electrochemical photolysis of water at a semiconductor electrode [J]. Nature, 1972, 238 (5358): 37-38.
[2] VADIVEL S, RAJARAJAN G. Effect of Mg doping on structural, optical and photocatalytic activity of SnO2nanostructure thin films [J]. Journal of Materials Science: Materials in Electronics, 2015, 26 (5): 3155-3162.
[3] MAZLOOM J, GHODSI F E, GOLMOJDEH H. Synthesis and characterization of vanadium doped SnO2diluted magnetic semiconductor nanoparticles with enhanced photocatalytic activities [J]. Journal of Alloys and Compounds, 2015, 639: 393-399.
[4] ENESCA A, ANDRONIC L, DUTA A. Optimization of opto-electrical and photocatalytic properties of SnO2thin films using Zn2+and W6+dopant ions [J]. Catalysis Letters, 2012, 142 (2): 224-230.
[5] JIA X H, LIU Y Y, WU X Y, et al. A low temperature situ precipitation route to designing Zn-doped SnO2photocatalyst with enhanced photocatalytic performance [J]. Applied Surface Science, 2014, 311: 609-613.
[6] VADIVEL S, RAJARAJAN G. Influence of Cu doping on structural, optical and photocatalytic activity of SnO2nanostructure thin films [J]. Journal of Materials Science: Materials in Electronics, 2015, 26 (8): 5863-5870.
[7] DAVIS M, HUNG-LOW F, HIKAL W M, et al. Enhanced photocatalytic performance of Fe-doped SnO2nanoarchitectures under UV irradiation: synthesis and activity [J]. Journal of Materials Science, 2013, 48 (18): 6404-6409.
[8] ZHANG J H, YE J F, CHEN H, et al. One-pot synthesis of echinus-like Fe-doped SnO2with enhanced photocatalytic activity under simulated sunlight [J]. Journal of Alloys and Compounds, 2017, 695: 3318-3323.
[9] AGARWAL S, TYAGI I, GUPTA V K, et al. Iron doped SnO2/Co3O4nanocomposites synthesized by sol–gel and precipitation method for metronidazole antibiotic degradation [J]. Materials Science and Engineering: C, 2017, 70 (Part 1): 178-183.
[10] OTHMEN W B H, SIEBER B, CORDIER C, et al. Iron addition induced tunable band gap and tetravalent Fe ion in hydrothermally prepared SnO2nanocrystals: application in photocatalysis [J]. Materials Research Bulletin, 2016, 83:481-490.
[11] KANG J, KUANG Q, XIE Z-X, et al. Fabrication of the SnO2/α-Fe2O3hierarchical heterostructure and its enhanced photocatalytic property [J]. The Journal of Physical Chemistry C, 2011, 115 (16): 7874-7879.
[12] PRADHAN G K, REDDY K H, PARIDA K M. Facile fabrication of mesoporous α-Fe2O3/SnO2nanoheterostructure for photocatalytic degradation of malachite green [J]. Catalysis Today, 2014, 224: 171-179.
[13] ZHANG S W, LI J X, NIU H H, et al. Visible-light photocatalytic degradation of methylene blue using SnO2/α-Fe2O3hierarchical nanoheterostructures [J]. ChemPlusChem, 2013, 78 (2): 192-199.
[14] LIN C-C, CHIANG Y-J. Preparation of coupled ZnO/SnO2photocatalysts using a rotating packed bed [J]. Chemical Engineering Journal, 2012, 181/182: 196-205.
[15] TANG M, SUN B, HUANG J, et al. High performance white-light-controlled resistance switching memory of an Ag/α-Fe2O3/FTO thin film [J]. RSC Advances, 2016, 6 (30): 25028-25033.
[16] SHINDE P S, ANNAMALAI A, KIM J H, et al. Exploiting the dynamic Sn diffusion from deformation of FTO to boost the photocurrent performance of hematite photoanodes [J]. Solar Energy Materials and Solar Cells, 2015, 141: 71-79.
[17] LU X-H, WANG D, LI G-R, et al. Controllable electrochemical synthesis of hierarchical ZnO nanostructures on FTO glass [J]. The Journal of Physical Chemistry C, 2009, 113 (31): 13574-13582.
[18] 罗晶晶, 范旭良, 马荣伟, 等. 超薄SnO2修饰Cu2O多孔薄膜的可见光光电化学性能[J]. 电镀与涂饰, 2015, 34 (12): 650-655.
[19] XIE Y, LU X Q, HUANG W, et al. Blacking FTO by strongly cathodic polarization with enhanced photocurrent [J]. Applied Surface Science, 2015, 347: 321-324.
[20] MU S L, SHI Q F. Photoelectrochemical properties of bare fluorine doped tin oxide and its electrocatalysis and photoelectrocatalysis toward cysteine oxidation [J]. Electrochimica Acta, 2016, 195: 59-67.
[21] SONG P X, WEN D S. Experimental investigation of the oxidation of tin nanoparticles [J]. The Journal of Physical Chemistry C, 2009, 113 (31): 13470-13476.
[22] RAJ D V, PONPANDIAN N, MANGALARAJ D, et al. Electrodeposition of macroporous SnO2thin films and its electrochemical applications [J]. Materials Focus, 2015, 4 (3): 245-251.
[23] RAJ D V, PONPANDIAN N, MANGALARAJ D, et al. Electrochemical behavior of nanostructured SnO2thin films in aqueous electrolyte solutions [J]. Materials Science in Semiconductor Processing, 2014, 26: 55-61.
[24] CHEN Z W, LAI J K L, SHEK C-H. Facile strategy and mechanism for orthorhombic SnO2thin films [J]. Applied Physics Letters, 2006, 89 (23): 231902.
[25] KIM S T, KIM D-H, HONG S-H. Epitaxial growth of orthorhombic SnO2films on various YSZ substrates by plasma enhanced atomic layer deposition [J]. Journal of Crystal Growth, 2012, 348 (1): 15-19.
[26] LAMELAS F J, REID S A. Thin-film synthesis of the orthorhombic phase of SnO2[J]. Physical Review B, 1999, 60 (13): 9347-9352.
[27] ZHANG Q, LIU P, MIAO C J, et al. Formation of orthorhombic SnO2originated from lattice distortion by Mn-doped tetragonal SnO2[J]. RSC Advances, 2015, 5 (49): 39285-39290.
[28] 吴维昌, 冯洪清, 吴开治. 标准电极电位数据手册[M]. 北京: 科学出版社, 1991: 81, 93, 151, 217.
[29] LAITINEN H A, VINCENT C A, BEDNARSKI T M. Behavior of tin oxide semiconducting electrodes under conditions of linear potential scan [J]. Journal of the Electrochemical Society, 1968, 115 (10): 1024-1028.
[30] DAVIS D G, MURRAY R W. Surface electrochemistry of iron porphyrins and iron on tin oxide electrodes [J]. Analytical Chemistry, 1977, 49 (2): 194-198.
[31] PLÁ CID C C, SPADA E R, SARTORELLI M L. Effect of the cathodic polarization on structural and morphological proprieties of FTO and ITO thin films [J]. Applied Surface Science, 2013, 273: 603-606.
[32] SANGALETTI L, DEPERO L E, ALLIERI B, et al. Oxidation of Sn thin films to SnO2. Micro-Raman mapping and X-ray diffraction studies [J]. Journal of Materials Research, 2011, 13 (9): 2457-2460.
[33] LAMELAS F J. Formation of orthorhombic tin dioxide from mechanically milled monoxide powders [J]. Journal of Applied Physics, 2004, 96 (11): 6195-6200.
[34] ARAGÓN F H, COAQUIRA J A H, GONZALEZ I, et al. Fe doping effect on the structural, magnetic and surface properties of SnO2nanoparticles prepared by a polymer precursor method [J]. Journal of Physics D: Applied Physics, 2016, 49 (15): 155002.
[35] KUMAR M, KUMAR A, ABHYANKAR A C. Occurrence of non-equilibrium orthorhombic SnO2phase and its effect in preferentially grown SnO2nanowires for CO detection [J]. RSC Advances, 2015, 5 (45): 35704-35708.
[36] GU C D, ZHENG H, WANG X L, et al. Superior ethanol-sensing behavior based on SnO2mesocrystals incorporating orthorhombic and tetragonal phases [J]. RSC Advances, 2015, 5 (12): 9143-9153.
[37] HU D, HAN B Q, DENG S J, et al. Novel mixed phase SnO2nanorods assembled with SnO2nanocrystals for enhancing gas-sensing performance toward isopropanol gas [J]. The Journal of Physical Chemistry C, 2014, 118 (18): 9832-9840.
[38] SHINDE P S, ANNAMALAI A, KIM J H, et al. Photoelectrochemical, impedance and optical data for self Sn-diffusion doped Fe2O3photoanodes fabricated at high temperature by one and two-step annealing methods [J]. Data in Brief, 2015, 5: 796-804.
[39] CHOI S-J, JANG B-H, LEE S-J, et al. Selective detection of acetone and hydrogen sulfide for the diagnosis of diabetes and halitosis using SnO2nanofibers functionalized with reduced graphene oxide nanosheets [J]. ACS Applied Materials & Interfaces, 2014, 6 (4): 2588-2597.
[40] CHEN X-T, WANG K-X, ZHAI Y-B, et al. A facile one-pot reduction method for the preparation of a SnO/SnO2/GNS composite for high performance lithium ion batteries [J]. Dalton Transactions, 2014, 43 (8): 3137-3143.
[41] SCHREBLER R S, BALLESTEROS L, BURGOS A, et al. Electrodeposited nanostructured α-Fe2O3photoanodes for solar water splitting: effect of surface Co-modification on photoelectrochemical performance [J]. Journal of the Electrochemical Society, 2011, 158 (8): D500-D505.
[42] UDDIN M T, NICOLAS Y, OLIVIER C, et al. Nanostructured SnO2–ZnO heterojunction photocatalysts showing enhanced photocatalytic activity for the degradation of organic dyes [J]. Inorganic Chemistry, 2012, 51 (14): 7764-7773.
[43] DU Y L, ZHANG N, WANG C M. Photo-catalytic degradation of trifluralin by SnO2-doped Cu2O crystals [J]. Catalysis Communications, 2010, 11 (7): 670-674.
[44] RAHMAN G, JOO O-S. Photoelectrochemical water splitting at nanostructured α-Fe2O3electrodes [J]. International Journal of Hydrogen Energy, 2012, 37 (19): 13989-13997.
[45] SHEWALE P S, SIM K U, KIM Y-B, et al. Structural and photoluminescence characterization of SnO2:F thin films deposited by advanced spray pyrolysis technique at low substrate temperature [J]. Journal of Luminescence, 2013, 139: 113-118.
[46] XIA J, LU X H, GAO W, et al. Hydrothermal growth of Sn4+-doped FeS2cubes on FTO substrates and its photoelectrochemical properties [J]. Electrochimica Acta, 2011, 56 (20): 6932-6939.
[47] ZENG Q Y, BAI J, LI J H, et al. A novel in situ preparation method for nanostructured α-Fe2O3films from electrodeposited Fe films for efficient photoelectrocatalytic water splitting and the degradation of organic pollutants [J]. Journal of Materials Chemistry A, 2015, 3 (8): 4345-4353.
[48] LEE T-H, RYU H H, LEE W-J. Photoelectrochemical properties of iron (III)-doped TiO2nanorods [J]. Ceramics International, 2015, 41 (6): 7582-7589.
[49] RAMKUMAR S, RAJARAJAN G. Effect of Fe doping on structural, optical and photocatalytic activity of WO3nanostructured thin films [J]. Journal of Materials Science: Materials in Electronics, 2016, 27 (2): 1847-1853.
[ 编辑:温靖邦 ]
Photoelectrochemical property of FTO thin film under visible light enhanced by electrochemical doping of Fe //
XIAO Shi-qing, HAO Li-hua, XIE Meng-yang, MA Rong-wei, NIU Zhen-jiang*
A visible-light-responsive Fe-doped FTO (F-doped SnO2) thin film was prepared by cathodic polarization of FTO conductive glass in a FeSO4solution followed by thermal oxidation in air at 500 °C. The morphology, structure and surface composition of the thin film were characterized by scanning electron microscopy (SEM), X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS). The photocurrent vs. time curve of the thin film was measured in 1.0 mol/L NaOH solution under visible light and zero bias. The results showed that the electrochemically modified FTO thin film contains about 1.0at% of Fe, comprises two structural phases of tetragonal and orthorhombic SnO2, and presents a nanoporous morphology. The photocurrent density of the Fe-doped FTO thin film is 0.5 μA/cm2, which is significantly higher than that of the undoped FTO thin film (0.019 μA/cm2).
fluorine doped tin dioxide; iron doping; cathodic polarization; visible light; photocurrent
Institute of Physical Chemistry, Zhejiang Normal University, Jinhua 321004, China
O611.4
A文献标志码::1004 – 227X (2017) 09 – 0455 – 07
10.19289/j.1004-227x.2017.09.004
2017–03–17
2017–05–16
国家自然科学基金(21173196)。
肖仕清(1989–),男,湖南祁东人,在读硕士研究生,主要研究方向为电化学。
牛振江,教授,(E-mail) nzjiang@zjnu.cn。

