基于代谢组学分析鼠伤寒沙门氏菌感染对文昌鸡盲肠代谢的影响





摘要:【目的】明确鼠伤寒沙门氏菌感染后文昌鸡盲肠内容物中代谢物的变化,筛选出与沙门氏菌感染高度相关的代谢物,揭示禽沙门氏菌病对文昌鸡盲肠代谢的影响,为进一步了解及防治该病提供数据支撑。【方法】以8×109 CFU/mL的鼠伤寒沙门氏菌悬液经口灌胃感染14日龄雄性文昌鸡建立禽沙门氏菌病模型,同时设健康对照组和灭活菌对照组,感染后第8 d基于代谢组学对文昌鸡盲肠内容物进行代谢物检测分析,筛选出存在显著差异的代谢物,并对差异代谢物进行KEGG通路富集分析。【结果】从文昌鸡盲肠内容物样本共鉴定出577种代谢物,在正离子(ESI+)、负离子(ESI-)模式下鉴定到的代谢物分别为417和160种。以Plt;0.05且权重值(VIP)gt;1为筛选条件,从577种代谢物中筛选出32种差异代谢物。相对于健康对照组,感染鼠伤寒沙门组存在24种差异代谢物,灭活菌对照组存在25种差异代谢物。在感染鼠伤寒沙门氏菌组中,花生四烯酸、3-(3-羟基苯基)丙酸、D-核糖、N6-乙酰基-L-赖氨酸、鸟嘌呤核苷、邻苯二酚、黄体酮、3-甲基-L-酪氨酸和4-羟基苯乙烯等9种代谢物显著升高(Plt;0.05,下同),而L-苏氨酸、黄嘌呤、鸟嘌呤、次黄嘌呤、尿嘧啶和酪胺等15种代谢物显著降低,以花生四烯酸含量升高最明显,其与健康对照组相比的差异倍数为8.60;KEGG通路富集分析结果显示,24种差异代谢物富集于嘌呤代谢、ABC转运蛋白、嘧啶代谢及不饱和脂肪酸生物合成等20条通路上,其中嘌呤代谢和ABC转运蛋白2条通路的富集程度达显著水平。【结论】鼠伤寒沙门氏菌感染能引起文昌鸡机体代谢紊乱,尤其是盲肠内容物中的花生四烯酸及其代谢产物或许是介导禽沙门氏菌性肠炎的关键物质,可作为解析沙门氏菌致病机制的切入点。
关键词:文昌鸡;鼠伤寒沙门氏菌;盲肠内容物;代谢组学;花生四烯酸
中图分类号:S858.31文献标志码:A文章编号:2095-1191(2024)10-3117-10
Metabolomics-based analysis on the effects of Salmonellatyphimurium infection on cecum metabolism ofWenchang chickens
CHEN Sheng-hong,XIE Yao-chen,WEN Xiao-bo,RAN Xu-hua*
(School of Tropical Agriculture and Forestry,Hainan University,Haikou,Hainan 570228,China)
Abstract:【Objective】To clarify the changes of metabolites in cecum contents of Wenchang chickens after Salmonella typhimurium infection,to screen out metabolites highly correlated with Salmonella infection,to reveal the effects of avian salmonellosis on cecum metabolism of Wenchang chickens,and to provide data support for further understanding and prevention of the disease.【Method】A model of avian salmonellosis was established by infecting 14-day-old male Wenchang chickens with S.typhimurium suspension of 8×109 CFU/mL by oral gavage,and a healthy control group and an inactivated bacterial control group were simultaneously established.Metabolite detection and analysis based on metabolo-mics was performed on the cecum contents of Wenchang chickens on the 8th d after infection,and metabolites with signifi-cant differences were screened out,and the differential metabolites were analyzed by KEGG enrichment.【Result】A total of 577 metabolites were identified in cecum content samples of Wenchang chickens,and the number of metabolites identi-fied in positive ion(ESI+)mode and negative ion(ESI-)mode were 417 and 160 respectively.A total of 32 differential metabolites were screened from the 577 metabolites using Plt;0.05 and weight(VIP)gt;1 as the screening condition.Com-pared to the healthy control group,24 differential metabolites were present in the S.typhimurium infected group and 25 differential metabolites were present in the inactivated bacterial control group.In the group infected with S.typhimurium,9 metabolites including arachidonic acid,3-(3-hydroxyphenyl)propionic acid,D-ribose,N6-acetyl-L-lysine,guano-sine,catechol,progesterone,3-methyl-L-tyrosine and 4-hydroxystyrene exhibited significant increase(Plt;0.05,the same below),while 15 metabolites including L-threonine,xanthine,guanine,hypoxanthine,uracil and tyramine demon-strated significant decline.Arachidonic acid content increase was the most obvious,the its fold difference compared to the healthy control group was 8.60.KEGG pathway enrichment analysis revealed that 24 differential metabolites enriched in 20 pathways,including purine metabolism,ABC transport protein,pyrimidine metabolism and biosynthesis of unsatu-rated fatty acid.Of these,the purine metabolism and ABC transport protein pathways reached significant level of enrich-ment.【Conclusion】S.typhimurium infection can cause metabolic disorders in the body of Wenchang chickens.Inparticu-lar,arachidonic acid and its metabolites in the contents of the cecum may be the key substances mediating avian salmo-nellaenteritis.This can provide a perspective forth study of Salmonella pathogenesis.
Key words:Wenchang chicken;Salmonella typhimurium;cecum contents;metabolomics;arachidonic acid
Foundation items:Regional Project of National Natural Science Foundation of China(32360878);Hainan Natural Science Foundation(321RC1020);Hainan Local Chicken Industry Technical System Special Project(HNARS-06-G05)
0引言
【研究意义】禽沙门氏菌病是由沙门氏菌(Sal-monella)感染引起的一种禽类疾病,根据其血清型的不同可分为鸡白痢、禽伤寒和禽副伤寒(张珍等,2019)。鸡白痢由鸡白痢沙门氏菌引起,主要通过种蛋传播,严重影响种蛋孵化率和雏鸡成活率(Cariou et al.,2013);禽伤寒由鸡伤寒沙门氏菌引起,通常引起3周龄以上的家禽发病,在成年鸡群中有较高的发病率(崔宏晓,2022);禽副伤寒由肠炎沙门氏菌(S.enteritidis)、鼠伤寒沙门氏菌(S.typhimurium)等有鞭毛可运动的沙门氏菌引起,主要威胁2周龄左右的雏鸡,在成年鸡群中多表现为阴性感染或慢性疾病症状(Saad etal.,2018;李惠龙,2020)。沙门氏菌属于革兰氏阴性菌,已知的血清型超过2600种,最常见的血清型为肠炎沙门氏菌和鼠伤寒沙门氏菌(张珍等,2019;张璐,2021;程佳莹等,2023)。沙门氏菌感染会进一步引起禽类对其他病原菌感染的抵抗力下降,严重时引起病鸡死亡(Ramtahal et al.,2022)。沙门氏菌对雏鸡的高威胁性及其在成年鸡群中的隐性致病状态,给家禽养殖业带来巨大经济损失(Maroufetal.,2022),而深入了解沙门氏菌感染对禽类机体的影响是防治该病最根本的途径和手段。【前人研究进展】鼠伤寒沙门氏菌的黏附与侵袭,会导致宿主出现以腹泻为主的临床特征,同时诱发肠道炎症反应(李萍等,2022),进而引起宿主肠道菌群失调及机体代谢紊乱等(袁晓慧等,2020;Lee et al.,2021)。代谢物变化能反映细胞功能的变化,因此通过代谢组学可快速检测并鉴定禽沙门氏菌病所引起的代谢紊乱特征(Schrimpe-Rutledge et al.,2016),为其临床诊断、病因与病理机制研究提供数据支持,有利于深入了解沙门氏菌感染对宿主机体代谢的影响。Antunes等(2011)通过代谢组学分析发现,鼠伤寒沙门氏菌感染显著影响小鼠类固醇、类花生酸、胆汁酸、碳水化合物和嘌呤类等代谢通路;Gutiérrez等(2021)研究表明,鼠伤寒沙门氏菌通过破坏胰岛素信号传导,而损害巨噬细胞的防御功能。此外,肠道菌群衍生的代谢物对宿主免疫防御等也发挥重要作用(Wang et al.,2023)。例如,多胺能抑制促炎细胞因子产生,吲哚可增强上皮细胞的屏障功能(Postler and Ghosh,2017);短链脂肪酸能促进抗炎因子表达(Levy et al.,2017),以及调节糖代谢紊乱(Serino,2018)、胆固醇合成(Michael et al.,2020)和紧密连接(Xia et al.,2020)等。Sokol等(2008)研究发现,粪杆菌(Fecalbacterium)代谢产物能阻断NF-κB通路并抑制IL-8产生;Levy等(2017)研究表明,脆弱拟杆菌(Bacteroides fragilis)分泌的聚糖A能刺激Treg细胞生成;Hu等(2021)研究证实,初级胆汁酸被肠道微生物转化为次级胆汁酸后,可发挥抑制促炎细胞因子分泌的作用。鉴于机体代谢物及肠道微生物代谢物对宿主健康有着至关重要的影响,揭示沙门氏菌感染后鸡盲肠代谢物的变化情况,不仅能丰富人们对禽沙门氏菌病的认知,还有助于从分子层面寻求防治方法。【本研究切入点】禽类盲肠是沙门氏菌的易感肠段,也是微生物丰度和数量最高的肠段(张春善等,2009),但目前关于鼠伤寒沙门氏菌感染对鸡盲肠代谢影响的研究鲜见报道。【拟解决的关键问题】基于代谢组学检测分析鼠伤寒沙门氏菌感染后文昌鸡盲肠内容物中代谢物的变化,筛选出与沙门氏菌感染高度相关的代谢物,旨在揭示禽沙门氏菌病对文昌鸡盲肠代谢的影响,为进一步了解及防治该病提供数据支撑。
1材料与方法
1.1试验材料
供试菌种为鼠伤寒沙门氏菌ATCC 14028株,购自广东环凯微生物科技有限公司;14日龄文昌鸡购自海南(潭牛)文昌鸡股份有限公司;甲酸、甲醇和乙腈购自赛默飞世尔科技(中国)有限公司;2-氯-L-苯丙氨酸和甲酸铵购自上海阿拉丁生化科技股份有限公司。Q Exactive HF-X质谱仪和Vanquish超高效液相系统购自美国ThermoFisher Scientific公司。
1.2试验分组及样本采集
选取30羽14日龄雄性文昌鸡(文昌维拉德品系),随机分为3组。健康对照组(A组):正常饲喂;灭活菌对照组(B组):经口灌胃1 mL的8×109 CFU/mL灭活鼠伤寒沙门氏菌悬液(70℃灭活30min);攻活菌组(C组):经口灌胃1 mL的8×109 CFU/mL鼠伤寒沙门氏菌悬液。所有试验鸡提供相同的饲料与饮水条件,分别饲养于温湿度、光照等环境条件基本一致的不同房舍。试验开始计为第0 d,于第8 d采取颈椎脱臼法安乐处死所有试验鸡,在冰上无菌采集肠道内容物样品用于检测分析(n=3),代谢组学检测委托苏州帕诺米克生物医药科技有限公司完成。动物试验由海南大学伦理委员会批准,许可证号HNUAUCC-2023-00080。
1.3样本检测
准确称取适量盲肠内容物样本,置于含600μL甲醇(含4 mg/L 2-氯-L-苯丙氨酸)的2 mL离心管中,涡旋振荡30 s,4℃下12000 r/min离心10 min,取上清液,过0.22μm滤膜后用于液相色谱—质谱检测(LC-MS)(Turroni et al.,2016)。色谱条件:使用ACQUITYUPLC®HSS T3色谱柱(2.1 mm×150 mm,1.8μm,柱温40℃)在Vanquish超高效液相系统中,以0.25 mL/min的液体流速(进样量2μL)进行检测。正离子(ESI+)模式下,流动相为0.1%甲酸乙腈和0.1%甲酸水,并通过梯度洗脱方式使用0.1%甲酸乙腈和0.1%甲酸水进行洗脱;负离子(ESI-)模式下,流动相为乙腈和5 mmol/L甲酸铵水,同样按梯度洗脱方式以乙腈和5 mmol/L甲酸铵水进行洗脱(Zelena et al.,2009)。质谱条件:使用Q Exactive HF-X质谱仪对样本进行检测,在ESI+和ESI-模式下分别采集数据(Want et al.,2013)。
1.4数据处理
使用ProteoWizard(v3.0.8789)将原始质谱下机文件转换为mzXML格式(Smith et al.,2006),再用XCMS对数据进行峰检测、峰过滤及峰对齐处理(Navarro-Reig et al.,2015),得到物质定量列表;获得的物质定量列表在mzCloud和KEGG等数据库中进行物质鉴定(Ogata et al.,1999;Abdelrazig et al.,2020)。采用R软件的Ropls包对样本数据进行主成分分析(PCA)、偏最小二乘判别分析(PLS-DA)、正交偏最小二乘判别分析(OPLS-DA)及制图(Thévenot et al.,2015),然后根据PLS-DA降维方法计算变量权重值(VIP),以log2 Fold Change表示组间差异倍数,当Plt;0.05且VIPgt;1时,认为代谢物分子具有统计学意义。采用MetaboAnalyst v6.0对筛选出来的差异代谢分子进行功能通路富集分析(Pang et al.,2020)。在统计分析过程中,通过Unpaired t检验或Mann-Whitney U检验对非正态分布的样本进行差异显著性分析,涉及2组以上数据时则进行单因素方差分析(One-way ANOVA),使用SPSS 24.0进行非参数Kruskal-Wallis检验,并以GraphPad Prism 8.0进行统计分析。
2结果与分析
2.1数据可靠性检验结果
由基峰色谱图(图1)可看出,各处理组色谱峰的峰值强度和保留时间趋势基本相似,说明重复性良好,检测结果可靠。
2.2组间差异分析结果
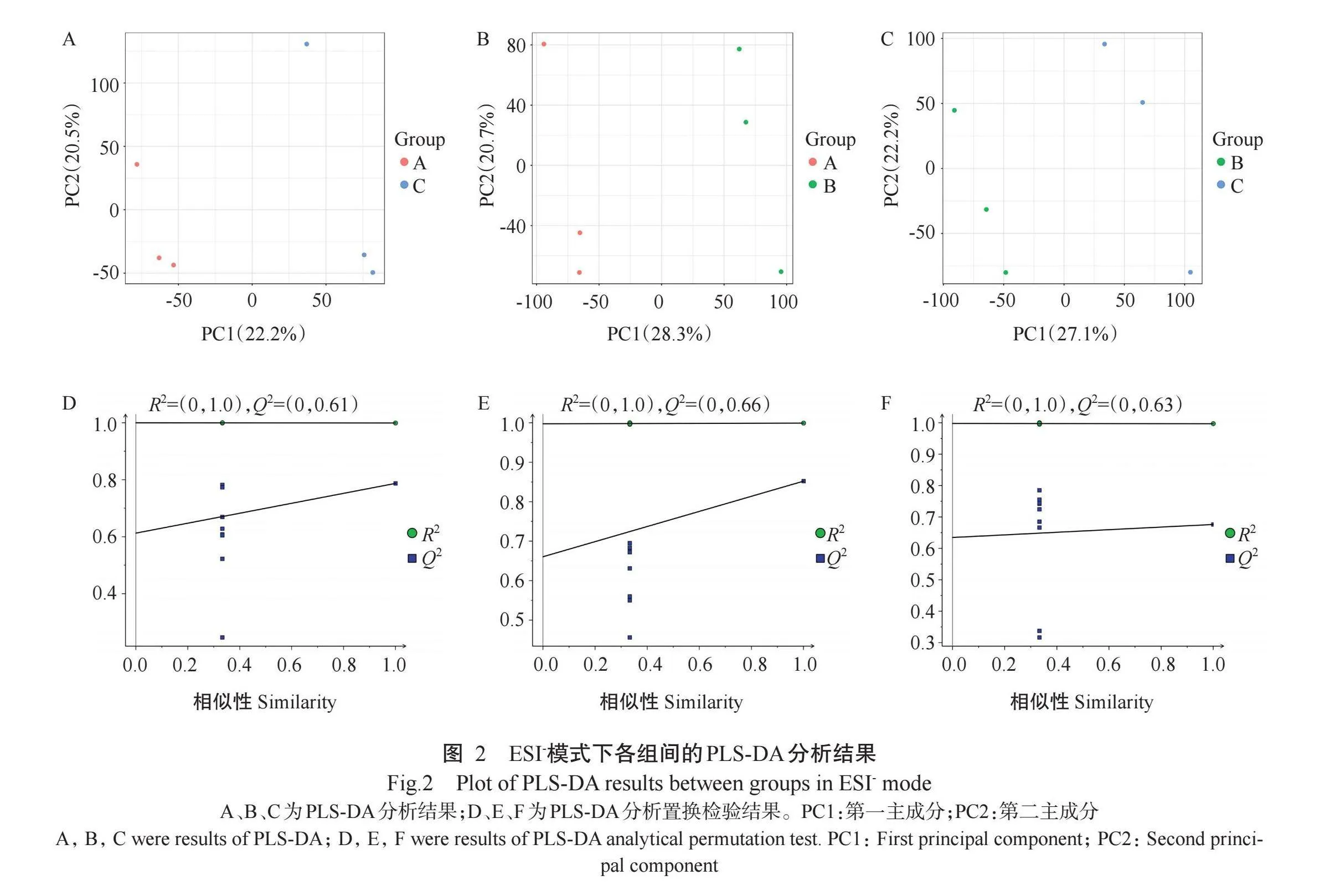
PLS-DA分析结果显示,在ESI-模式下不同比较组的样本点均分布在原点两端,界限清晰,各组间分离较明显,但组内均存在样本较分散现象(图2-A~图2-C)。模型置换检验可验证PLS-DA模型的可靠性,当R2(表示模型对数据的拟合程度)和Q2(表示建模后模型的预测能力)均低于1.0时,随着置换保留度的降低,随机模型Q2呈逐渐下降趋势(图2-D~图2-F),说明原模型不存在过拟合现象,模型稳健性良好,分析结果可靠。
2.3代谢物鉴定结果
所有文昌鸡盲肠内容物样本共鉴定出577种代谢物,在ESI+、ESI-模式下鉴定到的代谢物数量分别为417和160种。根据化学分类归属信息对鉴定到的所有代谢物进行分类统计,结果(图3)显示,有80种代谢物归属于羧酸及其衍生物(Carboxylic acids and derivatives),占13.86%;77种归属于脂肪酰基(Fatty acyls),占13.44%;42种归属于苯及其取代衍生物(Benzene and substituted derivatives),占7.28%;35种归属于类固醇及其衍生物(Steroids and steroid derivatives),占6.07%;31种归属于有机氧化合物(Organooxygen compounds),占5.37%;21种归属于肾上腺素脂质(Prenol lipids),占3.64%;13种归属于酚类(Phenols),占2.25%;10种归属于吡啶及其衍生物(Pyridines and derivatives),占1.73%。
2.4代谢物分析结果
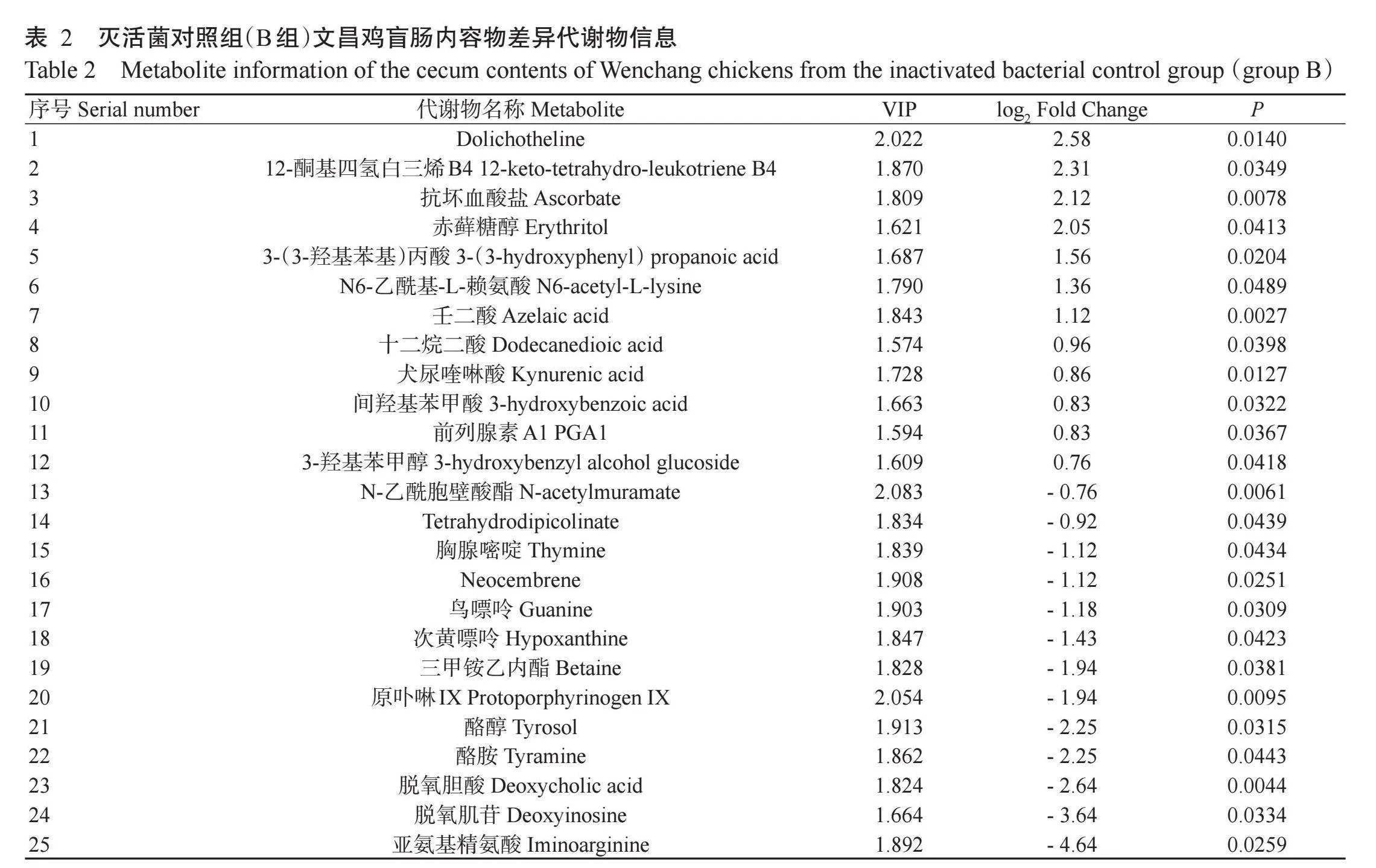
以rlt;0.05且VIPgt;1为筛选条件,从577种代谢物中筛选出32种差异代谢物。相对于A组,C组存在24种差异代谢物(表1),其中,花生四烯酸、3-(3-羟基苯基)丙酸、D-核糖、N6-乙酰基-L-赖氨酸、鸟嘌呤核苷、邻苯二酚、黄体酮、3-甲基-L-酪氨酸和4-羟基苯乙烯等9种代谢物显著升高(rlt;0.05,下同),而L-苏氨酸、黄嘌呤、鸟嘌呤、次黄嘌呤、尿嘧啶和酪胺等15种代谢物显著降低;以花生四烯酸的差异倍数最大,为8.60。相对于A组,B组存在25种差异代谢物(表2),其中,12-酮基四氢白三烯B4、抗坏血酸盐、赤藓糖醇、3-(3-羟基苯基)丙酸、N6-乙酰基-L-赖氨酸和壬二酸等12种代谢物显著升高,而N-乙酰胞壁酸酯、胸腺嘧啶、鸟嘌呤、次黄嘌呤和三甲铵乙内酯等13种代谢物显著降低。差异代谢物Z-socre分析结果(图4-A)及差异代谢物聚类分析结果(图4-B)均显示,B组和C组的差异代谢物与A组间存在明显差异,但B组与C组间存在较相似的代谢模式。
2.5差异代谢物KEGG通路富集分析结果
KEGG通路富集分析结果(图5)显示,C组中的24种差异代谢物主要富集于嘌呤代谢(Purine metabolism)、ABC转运蛋白(ABC transport proteins)、嘧啶代谢(Pyrimidine metabolism)及不饱和脂肪酸生物合成(Biosynthesis of unsaturated fatty acids)等20条通路(图5-A)上,其中嘌呤代谢和ABC转运蛋白2条通路的富集程度达显著水平;B组中的25种差异代谢物主要富集于嘌呤代谢、PPAR信号通路(PPAR signaling pathway)、ABC转运蛋白、酪氨酸代谢(Tyrosine metabolism)等16条通路(图5-B)上,其中嘌呤代谢和PPAR信号通路的富集程度达显著水平。
3讨论
禽类肠道菌群结构具有较高的丰度及多样性(Bjerrum etal.,2006;Stanley et al.,2014),能分解复杂的有机质,并产生多种具有益生作用的次级代谢产物(Sun et al.,2018;Michael et al.,2020),直接或间接参与宿主的多种代谢通路调节(Zhu et al.,2023;Ren etal.,2024)。本研究结果表明,文昌鸡感染鼠伤寒沙门氏菌后其盲肠内容物中的花生四烯酸含量差异倍数为8.60(r=0.007),灭活菌对照组文昌鸡盲肠内容物中的花生四烯酸差异倍数为6.87(r=0.09),差异代谢物聚类分析也发现B组与C组间存在较相似的代谢模式,可能是热灭活处理释放了细菌脂多糖所导致。值得注意的是,花生四烯酸属于不饱和脂肪酸,在生物体内主要是以磷脂的形式存在于细胞膜上,可在磷脂酶A2(PLA2)的作用下分解成游离形式,或在多种酶的作用下通过环氧合酶(COX)、脂氧合酶(LOX)和细胞色素P450(CYP450)代谢途径分解成具有生物活性的类花生酸(Zhang et al.,2023)。
花生四烯酸衍生的前列腺素(PGs)和白三烯(LTs)在机体肠道中发挥促炎与抗炎作用,且这些作用是由不同类型的G蛋白偶联受体(GPRs)介导(Stenson,2014;Kawahara et al.,2015;Yokomizo et al.,2018)。在促进炎症方面,前列腺素E2(PGE2)通过中性粒细胞和肿瘤相关成纤维细胞上的前列腺素E受体2(EP2)在多个步骤中促进炎症反应,从而形成结直肠癌的肿瘤微环境(Aoki and Narumiya,2017)。已有研究显示,抑制花生四烯酸衍生的类花生酸可降低Th17和Th1细胞介导的炎症反应,进而缓解结肠炎(Monk et al.,2014);而白三烯B4(LTB4)刺激树突状细胞上的高亲和力受体BLT1,导致促炎细胞因子IL-6、TNF-“和IL-12分泌合成,诱导Th1和Th17细胞增加TNBS诱导的结肠炎严重程度(Zhou et al.,2018)。此外,类花生酸可发挥抗炎作用,在炎症性肠病中PGE2通过其受体EP4的信号传导,增加上皮完整性而发挥保护作用(Kabashima et al.,2002;Jiang et al.,2007)。结肠炎患者的中性粒细胞表现出前列腺素D受体(DP)高水平表达(Sturm et al.,2014),而DP激动剂治疗可降低肠道中的髓过氧化物酶活性,说明中性粒细胞的迁移受PGD2-DP轴抑制(Ajueboretal.,2000),对炎症发挥控制效果。LTB4对12-羟基十七碳三烯酸(12-HHT)受体具有高亲和力,当缺乏12-HHT受体时会增加DSS诱导的结肠炎严重程度,进一步说明LTB4和12-HHT介导的信号传导具有抗炎特性(Iizuka et al.,2010)。LTB4还能通过其受体控制组织炎症期间的巨噬细胞迁移(Ermis et al.,2024)。可见,花生四烯酸的代谢产物既具有促炎作用,也具有抗炎作用。
炎症反应受多种复杂且庞大的细胞通路调控(殷斌等,2023)。已有研究表明,在巨噬细胞中ABC转运蛋白介导胆固醇外流,可减少TLR4引起的炎症信号(Sontagetal.,2010);巨噬细胞可通过调控嘌呤代谢产生细胞外腺苷,使巨噬细胞偏向M2极化(Ohradanova-Repic et al.,2018);PPAR可被多不饱和脂肪酸衍生物激活,进而调节炎症反应(Korbecki et al.,2019)。本研究的差异代谢物KEGG通路富集分析结果显示,经鼠伤寒沙门氏菌感染后文昌鸡盲肠中的多种差异代谢物富集于嘌呤代谢、ABC转运蛋白及PPAR信号通路上,故推测禽沙门氏菌性肠炎的发生受多种代谢通路调控。因此,通过代谢组学筛选可进一步明确与禽沙门氏菌性肠炎高度相关的分子,结合选择性育种在禽类抗病育种方面的应用(Berghofetal.,2019),有利于加速推进禽类抗沙门氏菌病育种进程。
综上所述,鼠伤寒沙门氏菌感染导致文昌鸡盲肠内容物中的花生四烯酸含量显著升高,而花生四烯酸的代谢产物广泛参与炎症反应,故推测花生四烯酸及其代谢产物介导了禽沙门氏菌性肠炎的发生与发展,因此,通过干预花生四烯酸的代谢途径有助于防治禽沙门氏菌性肠炎。
4结论
鼠伤寒沙门氏菌感染能引起文昌鸡机体代谢紊乱,尤其是盲肠内容物中的花生四烯酸及其代谢产物或许是介导禽沙门氏菌性肠炎的关键物质,可作为解析沙门氏菌致病机制的切入点。
参考文献(References):
程佳莹,肖梦诗,任昕淼,于颖,付晓丹,牟海津.2023.基于转录组学分析肠道菌群发酵褐藻胶寡糖对沙门氏菌的作用机制[J].河南农业科学,52(6):139-149.[Cheng J Y,Xiao M S,Ren X M,Yu Y,Fu X D,Mou H J.2023.Mechanism of alginate oligosaccharides fermented with gut microbiota inoculum against Salmonella enterica by transcriptomic analysis[J].Journal of Henan Agricultural Sciences,52(6):139-149.]doi:10.15933/j.cnki.1004-3268.2023.06.015.
崔宏晓.2022.沙门氏菌外膜囊泡对鸡单核吞噬细胞免疫激活的作用研究[D].杨凌:西北农林科技大学.[Cui H X.2022.Immune activation of Salmonella outer membrane vesicles on chicken mononuclear phagocytes[D].Yang-ling:Northwest Aamp;F University.]doi:10.27409/d.cnki.gxbnu.2022.000088.
李惠龙.2020.肠炎沙门氏菌感染后不同时间点鸡盲肠组织转录组分析[D].泰安:山东农业大学.[Li H L.2020.Temporal transcriptome following Salmonella enteritidis infection in chicken cecum[D].Taiʼan:Shandong Agricul‐tural University.]doi:10.27277/d.cnki.gsdnu.2020.000536.
李萍,苏佳丽,杜欣军,王硕.2022.大豆豆粕及赤小豆膳食补充对鼠伤寒沙门氏菌侵染小鼠肠道炎症的影响[J].天津科技大学学报,37(3):12-20.[Li P,Su J L,Du X J,WangS.2022.Effects of dietary supplementation of soybean and red bean on intestinal inflammation in mice infected with Salmonella enterica serotype Typhimurium[J].Journal of Tianjing University of Scienceamp;Technology,37(3):12-20.]doi:10.13364/j.issn.1672-6510.20210302.
殷斌,隽昌宁,秦雨,钟诚,赵增成,黄中利,沈涛,周慧爽,郑乾坤,林树乾.2023.基于系统生物学分析探究热应激诱导鸡肠道损伤的作用机制[J].南方农业学报,54(4):969-981.[Yin B,Juan C N,Qin Y,Zhong C,Zhao Z C,Huang Z L,Shen T,Zhou H S,Zheng Q K,Lin S Q.2023.Exploring the mechanism of heat stress-induced intestinal injury in chickens based on systems biology analysis[J].Journal of Southern Agriculture,54(4):969-981.]doi:10.3969/j.issn.2095-1191.2023.04.001.
袁晓慧,薛寒,张云增,潘志明,焦新安.2020.肠道菌群代谢产物在鼠伤寒沙门菌感染中的作用研究进展[J].中国微生态学杂志,32(8):966-970.[Yuan X H,Xue H,Zhang Y Z,Pan Z M,Jiao X A.2020.The role of gut microbiota metabolites in Salmonella enterica serovar Typhimurium infection:Research progress[J].Chinese Journal of Microe-cology,32(8):966-970.]doi:10.13381/j.cnki.cjm.20200 8021.
张春善,蒋燕侠,王博,王汝都,申红星.2009.铜、维生素A及互作效应对肉仔鸡肠壁组织结构、肠道微生物和血清生长激素的影响[J].中国农业科学,42(4):1485-1493.[Zhang C S,Jiang Y X,Wang B,Wang R D,Shen H X.2009.Influence of various dietary copper and vitamin Alevels on intestinal wall structure,cecal gut flora and GHin serum in broilers[J].Scientia Agricultura Sinica,42(4):1485-1493.]doi:10.3864/j.issn.0578-1752.2009.04.046.
张璐.2021.鸡源沙门氏菌血清型、耐药性及分子流行病学研究[D].北京:中国兽医药品监察所.[Zhang L.2021.Study on serotypes,antimicrobial resistance and molecular epidemiology of Salmonella spp.from chicken[D].Bei‐jing:China Institute of Veterinary Drug Control.]doi:10.27645/d.cnki.gzsys.2021.000004.
张珍,施开创,王孝德,黎宗强,尹彦文,屈素洁,陆文俊.2019.2015—2017年广西鸡源沙门氏菌耐药性与致病性的相关性分析[J].南方农业学报,50(10):2350-2358.[Zhang Z,Shi K C,Wang X D,Li Z Q,Yin Y W,Qu S J,Lu W J.2019.Correlation between antimicrobial resis‐tance and pathogenicity of Salmonella from chicken in Guangxi during 2015-2017[J].Journal of Southern Agri‐culture,50(10):2350-2358.]doi:10.3969/j.issn.2095-1191.2019.10.28.
Abdelrazig S,Safo L,Rance G A,Fay M W,Theodosiou E,Topham P D,Kim D H,Fernández-CastanéA.2020.Meta‐boliccharacterisation of Magnetospirillumgryphiswal-dense MSR-1 using LC-MS-based metabolite profiling[J].RSC Advances,10(54):32548-32560.doi:10.1039/d0ra 05326k.
Ajuebor M N,Singh A,Wallace J L.2000.Cyclooxygenase-2-derived prostaglandin D2 is an early anti-inflammatory signal in experimental colitis[J].American Journal of Physiology-Gastrointestinal and Liver Physiology,279(1):G238-G244.doi:10.1152/ajpgi.2000.279.1.G238.
Antunes L C M,Arena E T,Menendez A,Han J,Ferreira R B R,Buckner M M C,LolićP,Madilao L L,Bohlmann J,Borchers C H,Brett Finlay B.2011.Impact of Salmonella infection on host hormone metabolism revealed by metabo‐lomics[J].Infection and Immunity,79(4):1759-1769.doi:10.1128/iai.01373-10.
Aoki T,Narumiya S.2017.Prostaglandin E2-EP2 signaling as a node of chronic inflammation in the colon tumor microen‐vironment[J].Inflammation and Regeneration,37:4.doi:10.1186/s41232-017-0036-7.
Berghof T V L,Matthijs M G R,Arts J A J,Bovenhuis H,Dwars R M,van der Poel J J,Visker M H P W,Parmentier H K.2019.Selective breeding for high natural antibody level increases resistance to avian pathogenic Escherichia coli(APEC)in chickens[J].Developmentalamp;Compara‐tive Immunology,93:45-57.doi:10.1016/j.dci.2018.12.007.
Bjerrum L,Engberg R M,Leser T D,Jensen B B,Finster K,Pedersen K.2006.Microbial community composition of the ileum and cecum of broiler chickens as revealed by molecular and culture-based techniques[J].Poultry Science,85(7):1151-1164.doi:10.1093/ps/85.7.1151.
Cariou N,Christensen H,Salandre O,Albaric O,Bisgaard M,Malher X.2013.Genital form of pasteurellosis inbreeding turkeys infected during artificial insemination and isolation of an unusual strain of Pasteurella multocida[J].Avian Diseases,57(3):693-697.doi:10.1637/10471-121812-Case.1.
Ermis E,Nargis T,Webster K,Tersey S A,Anderson R M,Mirmira R G.2024.Leukotriene B4 receptor 2 governs macrophage migration during tissue inflammation[J].Jour‐nal of Biological Chemistry,300(1):105561.doi:10.1016/j.jbc.2023.105561.
Gutiérrez S,Fischer J,Ganesan R,Hos N J,Cildir G,Wolke M,Pessia A,Frommolt P,Desiderio V,Velagapudi V,Robin‐son N.2021.Salmonella typhimurium impairs glycolysis-mediated acidification of phagosomes to evade macro‐phage defense[J].PLoS Pathogens,17(9):e1009943.doi:10.1371/journal.ppat.1009943.
Hu J P,Wang C K,Huang X Y,Yi S L,Pan S,Zhang Y T,Yuan G X,Cao Q F,Ye X S,Li H.2021.Gut microbiota-mediated secondary bile acids regulate dendritic cells to attenuate autoimmune uveitis through TGR5 signaling[J].Cell Reports,36(12):109726.doi:10.1016/j.celrep.2021.109726.
Iizuka Y,Okuno T,Saeki K,Uozaki H,Okada S,Misaka T,Sato T,Toh H,Fukayama M,Takeda N,Kita Y,Shimizu T,Nakamura M,Yokomizo T.2010.Protective role of the leukotriene B4 receptor BLT2 in murine inflammatory coli‐tis[J].FASEB Journal,24(12):4678-4690.doi:10.1096/fj.10-165050.
Jiang G L,Nieves A,Im W B,Old D W,Dinh D T,Wheeler L.2007.The prevention of colitis by E prostanoid receptor 4 agonist through enhancement of epithelium survival and regeneration[J].The Journal of Pharmacology and Experi‐mental Therapeutics,320(1):22-28.doi:10.1124/jpet.106.111146.
Kabashima K,Saji T,Murata T,Nagamachi M,Matsuoka T,Segi E,Tsuboi K,Sugimoto Y,Kobayashi T,Miyachi Y,Ichikawa A,Narumiya S.2002.The prostaglandin receptor EP4 suppresses colitis,mucosal damage and CD4 cell acti‐vation in the gut[J].The Journal of Clinical Investigation,109(7):883-893.doi:10.1172/jci 14459.
Kawahara K,Hohjoh H,Inazumi T,Tsuchiya S,Sugimoto Y.2015.Prostaglandin E2-induced inflammation:Relevance of prostaglandin E receptors[J].Biochimica et Biophysica Acta-Molecular and Cell Biology of Lipids,1851(4):414-421.doi:10.1016/j.bbalip.2014.07.008.
Korbecki J,Bobiński R,Dutka M.2019.Self-regulation of the inflammatory response by peroxisome proliferator-activated receptors[J].Inflammation Research,68(6):443-458.doi:10.1007/s00011-019-01231-1.
Lee M,Hosseindoust A,Oh S M,Ko H S,Cho E S,Sa S,Kim Y I,Choi J W,Kim J S.2021.Impact of an anti-Salmonella typhimurium Bacteriophage on intestinal microbiota and immunity status of laying hens[J].Journal of Animal Phy-siology and Animal Nutrition,105(5):952-959.doi:10.1111/jpn.13424.
Levy M,Blacher E,Elinav E.2017.Microbiome,metabolites and host immunity[J].Current Opinion in Microbiology,35:8-15.doi:10.1016/j.mib.2016.10.003.
Marouf S,Ibrahim H M,El-Naggar M S,Swelum A A,Alqhtani A H,El-Saadony M T,El-Tarabily K A,Salem H M.2022.Inactivated pentavalent vaccine against myco‐plasmosis and salmonellosis for chickens[J].Poultry Scien-ce,101(11):102139.doi:10.1016/j.psj.2022.102139.
Michael O S,Dibia C L,Soetan O A,Adeyanju O A,Oyewole A L,Badmus O O,Adetunji C O,Soladoye A O.2020.Sodium acetate prevents nicotine-induced cardiorenal dys‐metabolism through uric acid/creatine kinase-dependent pathway[J].Life Sciences,257:118127.doi:10.1016/j.lfs.2020.118127.
Monk J M,Turk H F,Fan Y Y,Callaway E,Weeks B,Yang P Y,McMurray D N,Chapkin R S.2014.Antagonizing ara‐chidonic acid-derived eicosanoids reduces inflammatory Th17 and Th1 cell-mediated inflammation and colitis severity[J].Mediators of Inflammation,2014:917149.doi:10.1155/2014/917149.
Navarro-Reig M,Jaumot J,García-Reiriz A,Tauler R.2015.Evaluation of changes induced in rice metabolome by Cd and Cu exposure using LC-MS with XCMS and MCR-ALS data analysis strategies[J].Analytical and Bioanalyti‐cal Chemistry,407(29):8835-8847.doi:10.1007/s00216-015-9042-2.
Ogata H,Goto S,Sato K,Fujibuchi W,Bono H,Kanehisa M.1999.KEGG:Kyoto encyclopedia of genes and genomes[J].Nucleic Acids Research,27(1):29-34.doi:10.1093/nar/27.1.29.
Ohradanova-Repic A,Machacek C,Charvet C,Lager F,Le Roux D,Platzer R,Leksa V,Mitulovic G,Burkard T R,Zlabinger G J,Fischer M B,Feuillet V,Renault G,Blüml S,Benko M,Suchanek M,Huppa J B,Matsuyama T,Cavaco-Paulo A,Bismuth G,Stockinger H.2018.Extracel‐lular purine metabolism is the switchboard of immunosup‐pressive macrophages and a novel target to treat diseases with macrophage imbalances[J].Frontiers in Immuno-logy,9:852.doi:10.3389/fimmu.2018.00852.
Pang Z Q,Chong J,Li S Z,Xia J G.2020.MetaboAnalystR 3.0:Toward an optimized workflow for global metabolo‐mics[J].Metabolites,10(5):186.doi:10.3390/metabo 10 050186.
Postler T S,Ghosh S.2017.Understanding the holobiont:How microbial metabolites affect human health and shape the immune system[J].Cell Metabolism,26(1):110-130.doi:10.1016/j.cmet.2017.05.008.
Ramtahal M A,Amoako D G,Akebe A L K,Somboro A M,Bester L A,Essack S Y.2022.A public health insight into Salmonella in poultry in Africa:A review of the pastdecade:2010-2020[J].Microbial Drug Resistance,28(6):710-733.doi:10.1089/mdr.2021.0384.
Ren Y L,Shi X Y,Mu J,Liu S Y,Qian X,Pei W L,Ni S H,Zhang Z D,Li L,Zhang Z.2024.Chronic exposure to parabens promotes non-alcoholic fatty liver disease in asso‐ciation with the changes of the gut microbiota and lipid metabolism[J].Foodamp;Function,15(3):1562-1574.doi:10.1039/d3fo04347a.
Saad N J,Lynch V D,Antillón M,Yang C G,Crump J A,Pitzer V E.2018.Seasonal dynamics of typhoid and paratyphoid fever[J].Scientific Reports,8(1):6870.doi:10.1038/s41598-018-25234-w.
Schrimpe-Rutledge A C,Codreanu S G,Sherrod S D,McLean J A.2016.Untargeted metabolomics strategies-challenges and emerging directions[J].Journal of the American So-ciety for Mass Spectrometry,27(12):1897-1905.doi:10.1007/s 13361-016-1469-y.
Serino M.2018.Molecular paths linking metabolic diseases,gut microbiota dysbiosis and enterobacteria infections[J].Journal of Molecular Biology,430(5):581-590.doi:10.1016/j.jmb.2018.01.010.
Smith C A,Want E J,O’Maille G,Abagyan R,Siuzdak G.2006.XCMS:Processing mass spectrometry data for me-tabolite profiling using nonlinear peak alignment,matc-hing,and identification[J].Analytical Chemistry,78(3):779-787.doi:10.1021/ac051437y.
Sokol H,Pigneur B,Watterlot L,Lakhdari O,Bermúdez-Humarán L G,Gratadoux J J,Blugeon S,Bridonneau C,Furet J P,Corthier G,Grangette C,Vasquez N,Pochart P,Trugnan G,Thomas G,Blottière H M,DoréJ,Marteau P,Seksik P,Langella P.2008.Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients[J].Pro‐ceedings of the National Academy of Sciences of the United States of America,105(43):16731-16736.doi:10.1073/pnas.0804812105.
Sontag T J,Reardon C A,Getz G S.2010.ABC transporters:Lipid transport and inflammation[J].Current Opinion in Lipidology,21(2):159-160.doi:10.1097/MOL.0b013e32 83376910.
Stanley D,Hughes R J,Moore R J.2014.Microbiota of the chicken gastrointestinal tract:Influence on health,produc‐tivity and disease[J].Applied Microbiology and Biotech‐nology,98:4301-4310.doi:10.1007/s00253-014-5646-2.
Stenson W F.2014.The universe of arachidonic acid metabo‐lites in inflammatory bowel disease:Can we tell the goodfrom the bad?[J].Current Opinion inGastroenterology,30(4):347-351.doi:10.1097/mog.0000000000000075.
Sturm E M,Radnai B,Jandl K,StančićA,Parzmair G P,Högenauer C,Kump P,Wenzl H,Petritsch W,Pieber T R,Schuligoi R,Marsche G,Ferreirós N,Heinemann A,Schicho R.2014.Opposing roles of prostaglandin D2 receptors in ulcerative colitis[J].The Journal of Immuno-logy,193(2):827-839.doi:10.4049/jimmunol.1303484.
Sun L L,Xie C,Wang G,Wu Y,Wu Q,Wang X M,Liu J,Deng YY,Xia J L,Chen B,Zhang S Y,Yun C Y,Lian G,Zhang X J,Zhang H,Bisson W H,Shi J M,Gao X X,Ge P P,Liu C H,Krausz K W,Nichols R G,Cai J W,Rimal B,Patterson A D,Wang X,Gonzalez F J,Jiang C T.2018.Gut microbiota and intestinal FXR mediate the clinicalbenefits of metformin[J].Nature Medicine,24(12):1919-1929.doi:10.1038/s41591-018-0222-4.
Thévenot E A,Roux A,Xu Y,Ezan E,Junot C.2015.Analysis of the human adult urinary metabolome variations with age,body mass index,and gender by implementing a com‐prehensive workflow for univariate and OPLS statistical analyses[J].Journal of Proteome Research,14(8):3322-3335.doi:10.1021/acs.jproteome.5b00354.
Turroni S,Fiori J,Rampelli S,Schnorr S L,Consolandi C,Ba-rone M,Biagi E,Fanelli F,Mezzullo M,Crittenden A N,Henry A G,Brigidi P,Candela M.2016.Fecal metabo‐lome of the Hadza hunter-gatherers:A host-microbiome integrative view[J].Scientific Reports,6:32826.doi:10.1038/srep32826.
Wang X L,Niu L L,Wang Y X,Zhan S Y,Wang L J,Dai D H,Cao J X,Guo J Z,Li L,Zhang H P,Zhong T.2023.Com‐bining 16S rRNA sequencing and metabolomics data to decipher the interactions between gut microbiota,hostimmunity,and metabolites in diarrheic young small rumi‐nants[J].International Journal of Molecular Sciences,24(14):11423.doi:10.3390/ijms241411423.
Want E J,Masson P,Michopoulos F,Wilson I D,Theodoridis G,Plumb R S,Shockcor J,Loftus N,Holmes E,Nicholson J K.2013.Global metabolic profiling of animal and human tissues via UPLC-MS[J].Nature Protocols,8(1):17-32.doi:10.1038/nprot.2012.135.
Xia W R,Khan I,Li X A,Huang G X,Yu Z L,Leong W K,Han R X,Ho L T,Wendy Hsiao W L.2020.Adaptogenic flower buds exert cancer preventive effects by enhancing the SCFA-producers,strengthening the epithelial tight junction complex and immune responses[J].Pharmaco‐logical Research,159:104809.doi:10.1016/j.phrs.2020.104809.
Yokomizo T,Nakamura M,Shimizu T.2018.Leukotriene receptors as potential therapeutic targets[J].Journal of Clinical Investigation,128(7):2691-2701.doi:10.1172/jci97946.
Zelena E,Dunn W B,Broadhurst D,Francis-McIntyre S,Car‐roll K M,Begley P,O'Hagan S,Knowles J D,Halsall A,Consortium H,Wilson I D,Kell D B.2009.Development of a robust and repeatable UPLC-MS method for the long-term metabolomic study of human serum[J].Analytical Chemistry,81(4):1357-1364.doi:10.1021/ac8019366.
Zhang Y R,Liu Y X,Sun J,Zhang W,Guo Z,Ma Q.2023.Ara‐chidonic acid metabolism in health and disease[J].Med-Comm,4(5):e363.doi:10.1002/mco2.363.
Zhou J F,Lai W M,Yang W J,Pan J P,Shen H,Cai YY,Yang C X,Ma N J,Zhang Y,Zhang R,Xie X,Dong Z J,Gao Y,Du C S.2018.BLT1 in dendritic cells promotes Th1/Th17 differentiation and its deficiency ameliorates TNBS-induced colitis[J].Cellularamp;Molecular Immunology,15(12):1047-1056.doi:10.1038/s41423-018-0030-2.
Zhu J X,Liu W B,Bian Z,Ma Y M,Kang Z X,Jin J H,Li X Y,Ge S Y,Hao Y L,Zhang H X,Xie Y H.2023.Lactoba-cillus plantarum Zhang-LL inhibits colitis-related tumori‐genesis by regulating arachidonic acid metabolism and CD22-mediated B-cell receptor regulation[J].Nutrients,15(21):4512.doi:10.3390/nu 15214512.
(责任编辑兰宗宝)

