EGCG对热应激诱导猪骨骼肌卫星细胞凋亡的保护作用








摘要:【目的】探究表没食子儿茶素没食子酸酯(EGCG)对热应激诱导猪骨骼肌卫星细胞凋亡的影响及其作用机制,为缓解猪热应激反应和促进猪肌肉生长提供理论参考。【方法】41.5℃处理2 d构建猪骨骼肌卫星细胞热应激模型,使用CCK-8法检测热应激和EGCG对细胞生长水平的影响,利用Hoechst 33258染色和流式细胞仪检测热应激和EGCG对细胞凋亡水平的影响,采用实时荧光定量PCR检测Bcl-2、BAX、Caspase-3、Caspase-9和Cytc基因相对表达量,探究EGCG对热应激细胞凋亡相关基因表达的影响,并通过Western blotting检测Bcl-2和BAX蛋白相对表达量及Bax/Bcl-2比值,探究EGCG对热应激细胞凋亡相关蛋白表达的影响。【结果】41.5℃处理2 d极显著降低猪骨骼肌卫星细胞生长水平(Plt;0.01,下同)并极显著提高细胞凋亡率;热应激能显著提高Caspase-3和Cytc基因及BAX蛋白的相对表达量(Plt;0.05,下同),极显著提高BAX和Caspase-9基因的相对表达量,极显著降低Bcl-2基因的相对表达量,显著降低Bcl-2蛋白的相对表达量。EGCG能缓解热应激诱导的猪骨骼肌卫星细胞生长水平下降和凋亡水平升高,并降低促凋亡相关基因BAX、Caspase-3、Caspase-9和Cytc及BAX蛋白的相对表达量,提高抗凋亡基因Bcl-2和蛋白Bcl-2的相对表达量。【结论】EGCG能缓解热应激诱导的猪骨骼肌卫星细胞生长水平下降,并通过调控Bcl-2、BAX、Caspase-3、Cas-pase-9和Cytc等抗/促凋亡相关基因及其蛋白的相对表达量来缓解热应激引起的细胞凋亡。
关键词:猪;EGCG;热应激;骨骼肌;卫星细胞;细胞凋亡
中图分类号:S828.1文献标志码:A文章编号:2095-1191(2024)10-3190-09
Protective effects of EGCG on heat stress-induced apoptosis in porcine skeletal muscle satellite cells
YANG Bao1,WANG Qian1,LIYin1,JIANG Qin-yang1,2,HUANG Yan-na1,2*
(1College of Animal Science and Technology,Guangxi University,Nanning,Guangxi 530004,China;2Guangxi KeyLaboratory of Animal Breeding,Disease Control and Prevention,Nanning,Guangxi 530004,China)
Abstract:【Objective】This study aimed to investigate the effects and mechanisms of epigallocatechingallate(EGCG)on apoptosis of porcine skeletal muscle satellite cells induced by heat stress,providing theoretical reference for alleviating heat stress responses and promoting muscle growth in pigs.【Method】A heat stress model of porcine skeletal musclesate-llite cells was established by treating cells at 41.5°C for 2 d.The effects of heat stress and EGCG on cell growth level were assessed using the CCK-8 assay.Hoechst 33258 staining and flow cytometry were used to detect effects of heat stress and EGCG on cell apoptosis levels.Followed by real-time fluorescence quantitative PCR to measure the relative expression levels of Bcl-2,BAX,Caspase-3,Caspase-9 and Cytc genes,exploring the effects of EGCG on apoptosis related gene expression under heat stress.Western blotting was performed to detect the relative expression levels of Bcl-2 and BAX proteins and the BAX/Bcl-2 ratio,investigating the effects of EGCG on apoptosis related protein expression under heat stress.【Result】Treatment at 41.5°C for 2 d extremely significantly decreased cell growth level(Plt;0.01,the same below)and extremely significantly increased apoptosis rate.Heat stress significantly increased the relative expression of Caspase-3 and Cytc genes and BAX protein(Plt;0.05,the same below)and extremely significantly elevated the relative expression of BAX and Caspase-9 genes,while extremely significantly reduced the relative expression of Bcl-2 gene andsignificantly reduced the relative expression of Bcl-2 protein.EGCG alleviated the decrease in cell growth level and the in-crease in apoptosis level of porcine skeletal muscle satellite cells induced by heat stress,reduced the relative expression of pro-apoptotic genes(BAX,Caspase-3,Caspase-9 and Cytc)and BAX protein,and increased the relative expression of the anti-apoptotic gene Bcl-2 and Bcl-2 protein.【Conclusion】EGCG mitigates the decline in growth level of porcine skele-tal muscle satellite cells induced by heat stress,and alleviates apoptosis by regulating the relative expression of anti-and pro-apoptotic genes and proteins,including Bcl-2,BAX,Caspase-3,Caspase-9 and Cytc.
Key words:pig;EGCG;heat stress;skeletal muscle;satellite cells;apoptosis
Foundation items:National Natural Science Foundation of China(32360839,31760672);Guangxi Natural Science Foundation(2022GXNSFAA035525)
0引言
【研究意义】猪生长发育的最佳温度为16~22℃(Huynh et al.,2005),大规模集约化养殖易造成环境温度升高,致使猪群产生热应激反应,进而降低其生产性能和繁殖性能。热应激会导致猪的采食量、生长速度和繁殖率下降(Pearce et al.,2013;Morales et al.,2014),并影响其肌肉生长速度(Locke and Celotti,2014),而猪的肌肉生长速度由骨骼肌细胞决定(Gao et al.,2015)。因此,探究热应激对猪骨骼肌细胞生长和凋亡的影响及其缓解机制,对缓解猪热应激反应及促进猪肌肉生长具有重要意义。【前人研究进展】持续的高温环境会影响育肥猪的肉品质,导致pH、滴水损失和肌内脂肪含量等指标显著下降(杨培歌,2014),究其原因是高温环境引起育肥猪产生了热应激反应,并导致蛋白合成降低、脂肪沉积增加及活性氧积累(Ma etal.,2010;Pearce et al.,2011;王泽平等,2022;Bejaouiet al.,2023);环境温度升高还会导致猪的日增重降低(马现永等,2015)。骨骼肌卫星细胞能通过细胞增殖和分化为骨骼肌纤维的形成提供物质基础,因此其增殖和分化能力对猪的瘦肉产量和生长效率至关重要(Ren et al.,2024)。研究表明,高温环境能引起骨骼肌卫星细胞内活性氧积累,进而激活细胞内氧化应激和凋亡相关信号通路(Ganesan et al.,2017)。表没食子儿茶素没食子酸酯(Epigallocatechin-3-gallate,EGCG)是一种天然多酚类物质,具有抗病毒(Ohno et al.,2013)、抗氧化(Shanmugametal.,2016)、抗菌(Lee et al.,2017)、抗炎(Song et al.,2019)及抗癌(Maleki Dana et al.,2022)等功能,已广泛用于保健产品开发和医药领域研究。Othman等(2017)研究发现,EGCG能通过调节线粒体途径中抗/促凋亡信号蛋白平衡来降低心肌组织细胞凋亡水平,进而预防心肌受损;Zhao等(2021)研究表明,EGCG通过Keap1/Nrf2信号通路提高肉鸡的抗氧化能力,而缓解热应激对鸡肉品质的影响;Zhou等(2022)研究发现,EGCG能通过抑制氧化应激而缓解排卵后猪卵母细胞的衰老和凋亡水平(Zhou et al.,2022);Raoofi等(2023)研究证实,小鼠日粮中补充EGCG可显著降低经慢性阴囊热诱导的小鼠睾丸组织中细胞凋亡水平,进而缓解热应激引起的睾丸功能障碍。【本研究切入点】至今,有关猪热应激的研究已有较多报道(Morales et al.,2014;马现永等,2015;崔艳军,2016),但鲜见探究EGCG对热应激猪骨骼肌卫星细胞保护作用的研究报道。因此探究EGCG对热应激诱导猪骨骼肌卫星细胞凋亡的保护作用对开发缓解热应激的功能性饲料添加剂具有重要意义。【拟解决的关键问题】构建猪骨骼肌卫星细胞热应激模型,探究EGCG对热应激诱导的猪骨骼肌卫星细胞凋亡的影响及其作用机制,为实际生产中缓解猪热应激反应和促进猪肌肉生长提供理论参考。
1材料与方法
1.1试验材料
猪骨骼肌卫星细胞购自上海基斯德喏生物科技有限公司。EGCG(纯度≥98%)购自西安万方生物科技有限公司;CCK-8试剂盒购自北京索莱宝科技有限公司;TRIzol、反转录试剂盒和实时荧光定量PCR试剂盒购自南京诺唯赞生物科技股份有限公司;ECL发光显影液和Hoechst 33258染色液购自上海碧云天生物技术股份有限公司;细胞凋亡检测试剂盒购自杭州科联工程技术有限公司;PBS、DMEM高糖培养基和胎牛血清购自美国Gibco公司;BAX Rabbit pAb、Bcl-2 Rabbit pAb、β-Actin Rabbit pAb和HRP Goat Anti-Rabbit购自武汉爱博泰克生物科技有限公司。主要仪器设备:M200 PRO酶标仪购自德国TECAN公司;倒置荧光显微镜购自日本Nikon公司;荧光定量PCR仪和ChemiDocTM MP成像系统购自美国Bio-Rad公司;流式细胞仪购自美国Ther-moFisher Scientific公司。
1.2试验方法
1.2.1猪骨骼肌卫星细胞培养猪骨骼肌卫星细胞以1×105个/mL的密度接种在60 mm细胞培养皿中,补充含10%胎牛血清的细胞培养基,置于37.0℃、5%CO2培养箱中培养。待细胞汇合度达80%~90%时弃培养基,PBS清洗2次,加入1 mL胰蛋白酶消化,待细胞逐渐变圆后,弃胰蛋白酶,加入2 mL细胞培养基终止消化。将细胞吹打悬浮,1000 r/min离心5 min,弃上清液。加入适量细胞培养基并吹打混匀,接种于60mm细胞培养皿或6孔细胞培养板中,置于37.0℃、5%CO2培养箱中培养。
1.2.2猪骨骼肌卫星细胞生长水平检测试验设37.0℃、37.0℃+EGCG、41.5℃和41.5℃+EGCG组。取生长状态良好的猪骨骼肌卫星细胞消化并调整密度至5×104个/mL,96孔细胞培养板每孔加入100.0μL细胞悬液,培养24 h后弃上清液,加入100.0μL含20μmol/L EGCG或不含EGCG的细胞培养基(20μmol/L为预试验筛选所得的最适浓度),在37.0和41.5℃培养箱中分别培养1、2、3、4和5 d。根据CCK-8试剂盒说明测定猪骨骼肌卫星细胞生长水平,每组设6个重复。
1.2.3 Hoechst 33258染色将猪骨骼肌卫星细胞接种至24孔细胞培养板中,按照37.0℃、37.0℃+EGCG、41.5℃和41.5℃+EGCG分组处理2 d;去除细胞培养基,PBS洗涤1次;4%多聚甲醛固定20min,PBS洗涤3次,每次5 min;每孔加入250.0μL Hoechst 33258染色液,置于摇床上染色5min;染色结束后,PBS洗涤3次,每次5min;倒置显微镜下采集图像。
1.2.4流式细胞仪检测将猪骨骼肌卫星细胞以5×104个/孔的密度接种于6孔细胞培养板中;41.5℃培养箱培养2 d后收集细胞;1×Binding Buffer重悬细胞,并根据细胞凋亡检测试剂盒说明加入PI和Annexin-FITC;涡旋混匀,避光孵育5 min;经流式细胞仪检测,用FlowJo_V10进行分析。
1.2.5 RNA提取与实时荧光定量PCR检测使用TRIzol试剂提取猪骨骼肌卫星细胞总RNA,再以反转录试剂盒反转录合成cDNA。引物委托南宁捷尼斯生物科技有限公司合成,扩增引物序列见表1。实时荧光定量PCR反应体系10.0μL:2×PerfectStart®Green qPCR SuperMix 5.0μL,上、下游引物各0.25μL,ddH2O 2.5μL,cDNA模板2.0μL。扩增程序:95℃预变性30 s;95℃5 s,60℃30 s,进行45个循环。以18S rRNA为内参基因,采用2-ΔΔCt法计算目的基因相对表达量。
1.2.6 Western blotting检测收集细胞沉淀,PBS洗涤1次;1500r/min离心5min,弃上清液;加入RIPA裂解液,于冰上裂解30 min;4℃下12000 r/min离心10 min,收集上清液;加入5×Loading Buffer,充分混匀后95℃水浴10 min;-20℃保存备用。取10.0μL蛋白样品进行10%SDS-PAGE检测(100 V至溴酚蓝染液到达分离胶底部)后,在200 mA下转膜90min;5%脱脂牛奶室温封闭2 h,加入一抗Bcl-2(兔抗,1∶1000稀释)、BAX(兔抗,1∶1000稀释)和β-Actin(兔抗,1∶2000稀释),4℃下孵育12 h;TBST洗涤后加入二抗(羊抗兔,1∶10000稀释),室温下孵育50 min;加入适量ECL发光显影液,在凝胶显影仪下曝光观察。以β-Actin为内参,使用ImageJ对图像中的条带进行灰度分析。
1.3统计分析
试验数据通过正态分布和方差齐性检验后,采用SPSS 25.0进行单因素方差分析(One-way ANOVA)。
2结果与分析
2.1热应激对猪骨骼肌卫星细胞生长水平的影响
猪骨骼肌卫星细胞经37.0和41.5℃分别处理1、2、3、4和5 d后,通过CCK-8试剂盒检测细胞生长水平。结果(图1)显示,与37.0℃组相比,41.5℃组猪骨骼肌卫星细胞生长水平在第1 d时开始显著下降(Plt;0.05,下同),第2、3、4和5 d时极显著下降(Plt;0.01,下同),其中第4和5 d,37.0℃组和41.5℃组的猪骨骼肌卫星细胞生长水平均趋于平缓。表明热应激对猪骨骼肌卫星细胞生长水平呈时间依赖性抑制,且随着细胞密度的升高,抑制作用逐渐趋于平缓。因此,选择41.5℃处理2 d构建猪骨骼肌卫星细胞热应激模型。
2.2热应激对猪骨骼肌卫星细胞凋亡的影响
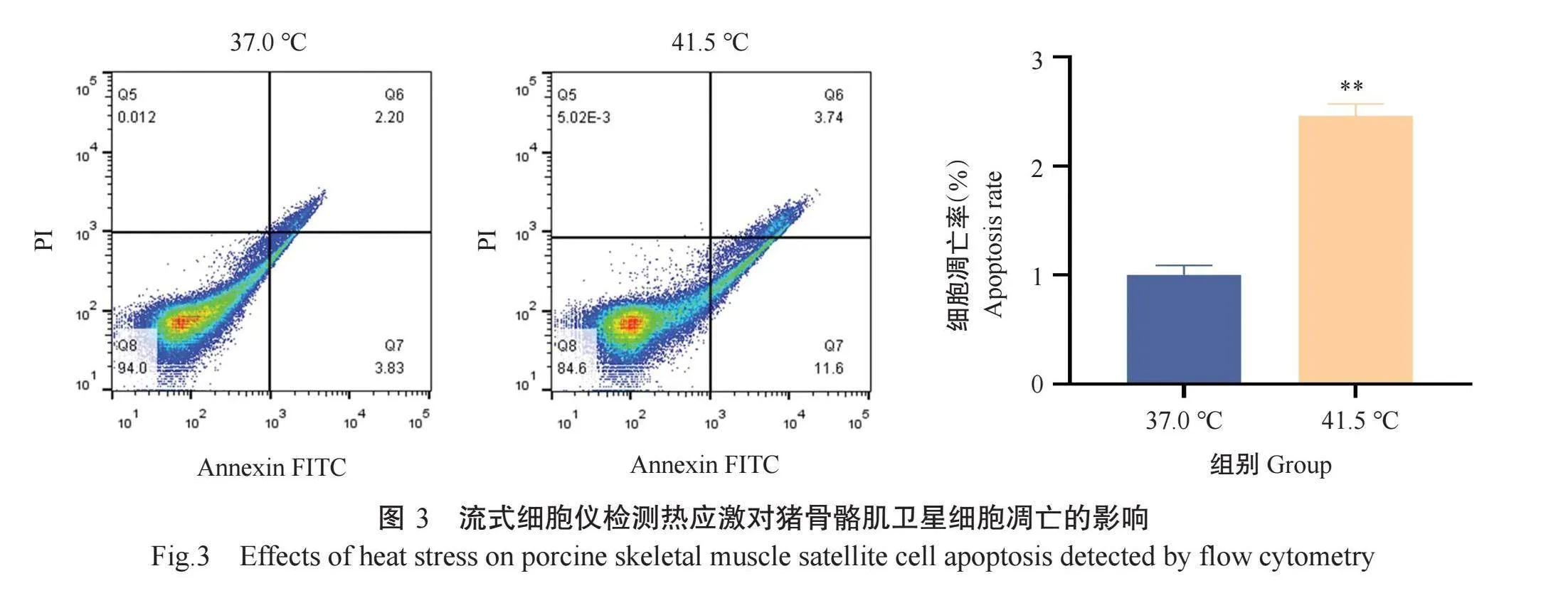
Hoechst 33258是可穿透细胞膜的蓝色荧光染料,染色后活细胞的细胞核呈弥散均匀荧光,而凋亡细胞的胞质或细胞核呈浓染致密的块状荧光。Hoechst 33258染色结果(图2)显示,41.5℃组出现大量浓染致密的块状荧光,而37.0℃组仅出现少量浓染致密的块状荧光。流式细胞仪检测结果(图3)也显示,41.5℃组的猪骨骼肌卫星细胞凋亡率极显著高于37.0℃组,约是37.0℃组的2.46倍。表明热应激诱导了猪骨骼肌卫星细胞凋亡。
2.3热应激对猪骨骼肌卫星细胞凋亡相关基因和蛋白相对表达量的影响
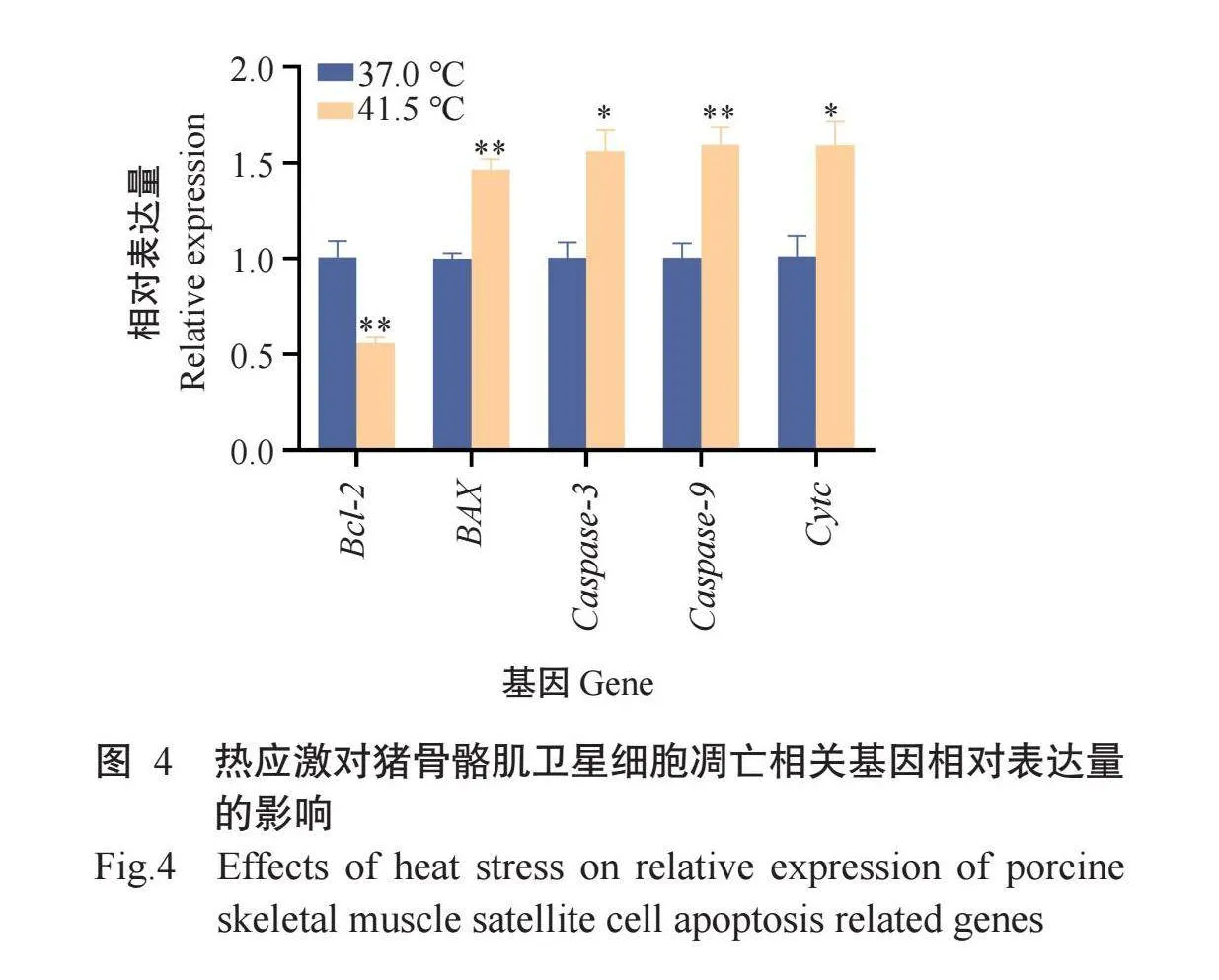
采用实时荧光定量PCR和Western blotting分别检测猪骨骼肌卫星细胞凋亡相关基因及其蛋白相对表达量,结果(图4和图5)显示,与37.0℃组相比,41.5℃组Bcl-2基因相对表达量极显著降低,Bcl-2蛋白相对表达量显著降低;BAX基因相对表达量极显著升高,BAX蛋白相对表达量显著升高;Cas-pase-3和Cytc基因相对表达量显著升高;Caspase-9基因相对表达量极显著升高;BAX/Bcl-2比值显著升高,进一步证实热应激促进了猪骨骼肌卫星细胞凋亡。
2.4 EGCG对热应激猪骨骼肌卫星细胞生长水平的影响
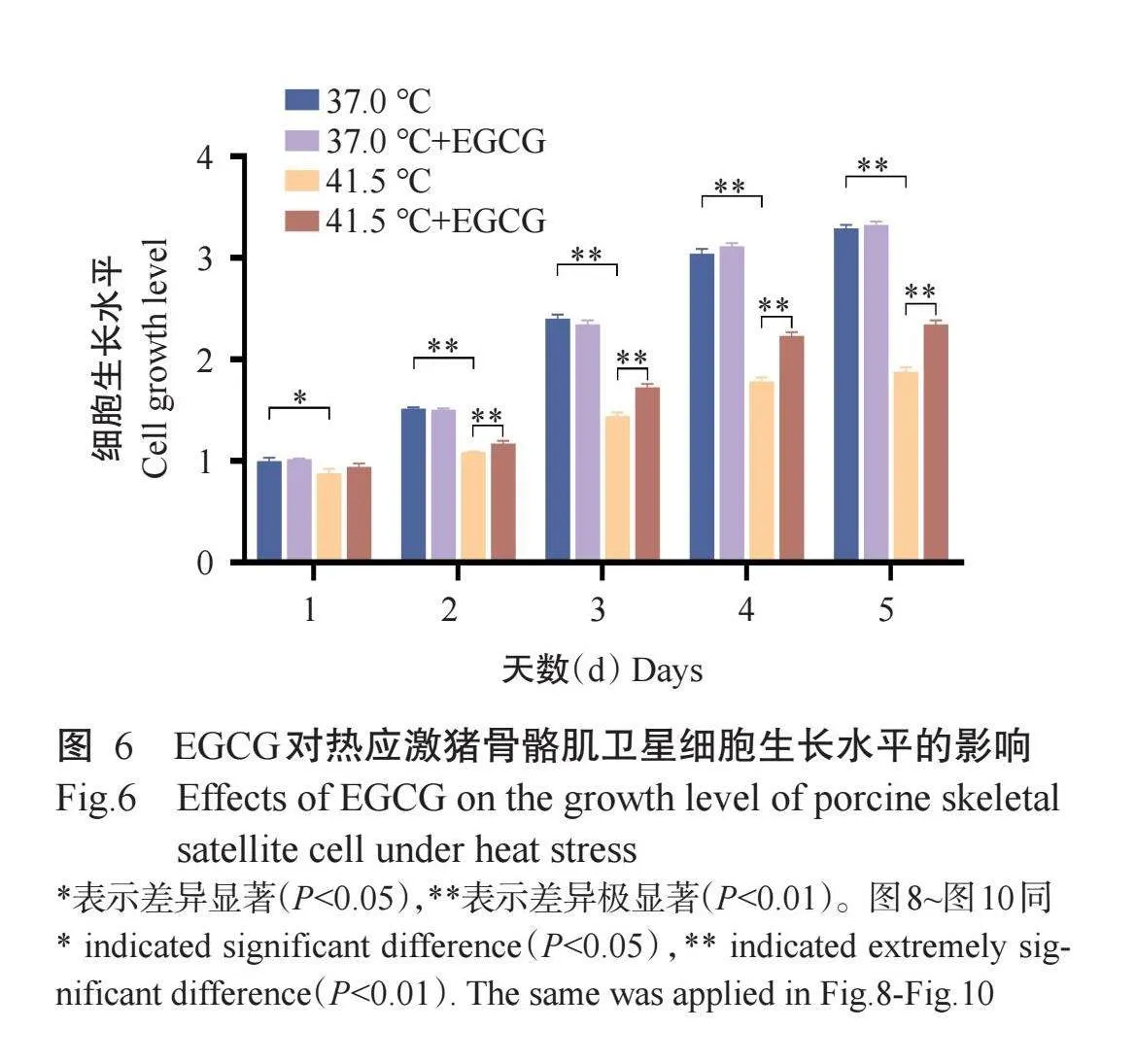
CCK-8试剂盒检测结果(图6)显示,在第1~5 d,与37.0℃组相比,37.0℃+EGCG组猪骨骼肌卫星细胞生长水平均无显著变化(Pgt;0.05,下同);与41.5℃组相比,41.5℃+EGCG组猪骨骼肌卫星细胞生长水平在第1 d时无显著变化,第2、3、4和5 d时极显著升高。表明EGCG能缓解热应激诱导的猪骨骼肌卫星细胞生长水平下降。
2.5 EGCG对热应激猪骨骼肌卫星细胞凋亡的影响
Hoechst 33258染色结果(图7)显示,与37.0℃组相比,37.0℃+EGCG组浓染致密的块状荧光数量无明显变化;与41.5℃组相比,41.5℃+EGCG组浓染致密的块状荧光数量有所减少。流式细胞仪检测结果(图8)也显示,与37.0℃组相比,37.0℃+EGCG组猪骨骼肌卫星细胞凋亡率无显著变化;与41.5℃组相比,41.5℃+EGCG组猪骨骼肌卫星细胞凋亡率极显著降低。表明EGCG能缓解热应激引起的猪骨骼肌卫星细胞凋亡。
2.6 EGCG对热应激猪骨骼肌卫星细胞凋亡相关基因和蛋白相对表达量的影响
实时荧光定量PCR和Western blotting检测结果(图9和图10)显示,与37.0℃组相比,37.0℃+EGCG组Bcl-2、BAX、Caspase-3、Caspase-9、Cytc基因相对表达量无显著变化;与41.5℃组相比,41.5℃+EGCG组Bcl-2基因及其蛋白相对表达量显著升高,BAX、Caspase-3、Caspase-9、Cytc基因和BAX蛋白相对表达量显著或极显著降低,Bax/Bcl-2比值极显著降低。可见,EGCG能通过调控Bcl-2、BAX等凋亡相关基因和蛋白的表达来缓解热应激诱导的猪骨骼肌卫星细胞凋亡。
3讨论
机体在高温环境下会产生热应激反应,而热应激会引起蛋白的异常折叠,并导致线粒体和细胞损伤,最终引起细胞内氧化自由基水平升高及抗氧化防御系统失衡(Khajavi et al.,2003;李述方和王海荣,2023)。坏死和凋亡是细胞走向死亡的过程,也是维持生命稳态的重要途径,骨骼肌细胞凋亡在肌肉生长发育中发挥着重要的调节作用(Wang et al.,2015)。研究表明,高温环境可直接引起育肥猪肝脏细胞凋亡(崔艳军,2016);高温环境还能引起小鼠骨骼肌中的活性氧积累和自由基水平升高,继而激活氧化应激及细胞凋亡相关信号通路(Ganesan et al.,2017)。细胞凋亡时细胞核浓缩,DNA降解形成凋亡小体,最后被溶酶体降解(Xu etal.,2019)。本研究通过Hoechst 33258染色和流式细胞仪检测发现,41.5℃处理2d后,猪骨骼肌卫星细胞核内出现大量浓染致密的块状荧光,处于凋亡期的细胞比例显著升高,表明热应激能诱导猪骨骼肌卫星细胞凋亡。
Bcl-2家族能通过线粒体途径调控细胞凋亡,抗凋亡因子Bcl-2与促凋亡因子BAX相互结合形成二聚体而发挥抑制细胞凋亡的作用,BAX过表达则能对抗Bcl-2的抗凋亡作用(Oltvai et al.,1993)。因此,BAX/Bcl-2比值常用于反映细胞凋亡水平。线粒体损伤时释放的Cytc能激活Caspase-3和Cas‐pase-9的表达,并诱导细胞凋亡(Wang et al.,2014)。研究表明,热应激猪肌内前体脂肪细胞Bcl-2/BAX相关的凋亡通路信号被激活而显著提高细胞凋亡水平(谢红月,2021);热应激还能通过上调BAX/Bcl-2比值及Caspase-3等基因的表达水平来诱导猪睾丸间质细胞凋亡(何芝凤等,2023)。本研究发现,热应激提高了促凋亡相关基因BAX、Caspase-3、Caspase-9和Cytc及BAX蛋白的相对表达量,抑制了抗凋亡基因Bcl-2和蛋白Bcl-2的相对表达量,且BAX/Bcl-2比值也显著升高,进一步表明热应激能促进猪骨骼肌卫星细胞凋亡。
EGCG能通过促进细胞间的连接互作而保护细胞(Sigler and Ruch,1993),且其结构中含有酚羟基,具备较强的抗氧化能力,可清除细胞内过量的自由基和活性氧,进而缓解热应激造成的细胞氧化损伤(de Oliveira et al.,2016)。Xiang等(2017)研究发现,EGCG能通过抑制内质网应激来缓解高葡萄糖诱导的小鼠足细胞凋亡;Butt(2020)研究证实,添加EGCG能明显改善热应激诱导的间充质干细胞形态异常和活力下降,并显著降低BAX基因表达水平和凋亡细胞数量;Raoofi等(2023)研究发现,日粮中添加EGCG能有效保护小鼠睾丸组织免受慢性热应激的影响,降低睾丸组织细胞凋亡水平。线粒体是调控细胞凋亡的重要细胞器,线粒体膜电位下降能通过Bcl-2家族引起细胞凋亡(刘冬梅等,2014;Fu et al.,2019)。谢红月(2021)研究表明,EGCG通过调节Bcl-2家族中Bcl-2和BAX的表达水平来缓解热应激引起的猪皮下前体脂肪细胞损伤。本研究发现,EGCG能缓解热应激诱导的猪骨骼肌卫星细胞生长水平下降及凋亡水平升高,并降低促凋亡相关基因BAX、Caspase-3、Caspase-9和Cytc的相对表达量,提高抗凋亡基因Bcl-2的相对表达量。可见EGCG是通过调控相关抗/促凋亡基因的表达来缓解热应诱导的猪骨骼肌卫星细胞凋亡。
4结论
EGCG能缓解热应激诱导的猪骨骼肌卫星细胞生长水平下降,并通过调控Bcl-2、BAX、Caspase-3、Caspase-9和Cytc等抗/促凋亡相关基因及其蛋白的相对表达量来缓解热应激引起的细胞凋亡。
参考文献(References):
崔艳军.2016.热应激和氧化应激对肥育猪骨骼肌代谢的影响及硫辛酸的调控作用[D].北京:中国农业科学院.[Cui Y J.2016.Effects of heat stress and oxidative stress on metabolism of skeletal muscle and protection of lipoic acid in finishing pigs[D].Beijing:Chinese Academy of Agricultural Sciences Dissertation.]
何芝凤,李芳芳,胡传活,赵欢欢,赵文婧,黄明光.2023.茶多酚对高温下猪睾丸间质细胞损伤及睾酮分泌异常的缓解作用[J].畜牧与兽医,55(11):59-64.[He Z F,Li F F,Hu C H,Zhao H H,Zhao W J,Huang M G.2023.Allevia-ting effects of tea polyphenols on damage and abnormal testosterone secretion of Leydig cells in boar under high temperature[J].Animal Husbandryamp;Veterinary Medi‐cine,55(11):59-64.]
李述方,王海荣.2023.热应激对绵羊机体氧化损伤及免疫功能的影响[J].江苏农业学报,39(7):1606-1612.[Li S F,Wang H R.2023.Effects of heat stress on oxidative dama-ge and immune function in sheep[J].Jiangsu Journal of Agricultural Sciences,39(7):1606-1612.]doi:10.3969/j.issn.1000-4440.2023.07.017.
刘冬梅,张晶,王晓非.2014.表没食子儿茶素没食子酸酯(EGCG)对ConA所致肝损伤小鼠Bcl-2/Bax表达的影响[J].热带医学杂志,14(6):745-747.[Liu D M,Zhang J,Wang X F.2014.Effect of epigallocatechin gallate(EGCG)on the expression of Bcl-2/Bax in mouse withliver injury[J].Journal of Tropical Medicine,14(6):745-747.]
马现永,蒋宗勇,师子彪,郑春田,王丽,胡友军,杨雪芬,高开国.2015.热应激对猪生产性能、肉品质的影响及作用机制研究进展[C]//中国畜牧兽医学会.中国猪业科技大会暨中国畜牧兽医学会2015年学术年会论文集:327.[Ma X Y,Jiang Z Y,Shi Z B,Zhen C T,Wang L,Hu Y J,Yang X F,Gao K G.2015.Research progress on effects of heat stress on performance and meat quality of pigs and its mechanism[C]//Chinese Association of Animal Science and Veterinary Medicine.China Pig Industry Science and Technology Conference and China Animal Husbandry and Veterinary Society 2015 Academic Annual Meeting:327.]
王泽平,沈婕,赵为民,付言峰,李碧侠,任守文,程金花,李辉.2022.热应激对猪颗粒细胞蛋白质表达谱的影响[J].江苏农业学报,38(6):1569-1577.[Wang Z P,Shen J,Zhao W M,Fu Y F,Li B X,Ren S W,Cheng J H,Li H.2022.Effects of heat stress on protein expression profilesin porcine granulosa cells[J].Jiangsu Journal of Agricul‐tural Sciences,38(6):1569-1577.]doi:10.3969/j.issn.1000-4440.2022.06.015.
谢红月.2021.热应激对猪皮下和肌内前体脂肪细胞脂肪沉积、脂肪代谢和细胞凋亡的影响及EGCG调控机制[D].南宁:广西大学.[Xie H Y.2021.Effects of heat stress on lipid deposition,lipid metabolism and apoptosis of por-cine subcutaneous and intramuscular preadipocytes and EGCG regulation mechanism[D].Nanning:Guangxi Uni-versity.]
杨培歌.2014.热应激对肥育猪肌肉品质及其代谢物的影响[D].北京:中国农业科学院.[Yang P G.2014.Effects of heat stress on meat quality and muscle metabolites of fat-tening pigs[D].Beijing:Chinese Academy of Agricultural Sciences.]
Bejaoui B,Sdiri C,Ben Souf I,Belhadj Slimen I,Ben Larbi M,Koumba S,Martin P,M’Hamdi N.2023.Physicochemical properties,antioxidant markers,and meat quality as affected by heat stress:A review[J].Molecules,28(8):3332.doi:10.3390/molecules28083332.
Butt H,Mehmood A,Ejaz A,Humayun S,Riazuddin S.2020.Epigallocatechin-3-gallate protects Wharton’s jelly derived mesenchymal stem cells against in vitro heat stress[J].European Journal of Pharmacology,872:172958.doi:10.1016/j.ejphar.2020.172958.
de Oliveira M R,Nabavi S F,Daglia M,Rastrelli L,Nabavi S M.2016.Epigallocatechin gallate and mitochondria—A story of life and death[J].Pharmacological Research,104:70-85.doi:10.1016/j.phrs.2015.12.027.
Fu Y R,Jin Y C,Zhao Y,Shan A S,Fang H T,Shen J L,Zhou C H,Yu H,Zhou Y F,Wang X,Wang J M,Li R H,Wang R,Zhang J.2019.Zearalenone induces apoptosis in bovine mammary epithelial cells by activating endoplasmic reticu-lum stress[J].Journal of Dairy Science,102(11):10543-10553.doi:10.3168/jds.2018-16216.
Ganesan S,Summers C M,Pearce S C,Gabler N K,ValentineR J,Baumgard L H,Rhoads R P,Selsby J T.2017.Short-term heat stress causes altered intracellular signaling in oxi-dative skeletal muscle[J].Journal of Animal Science,95(6):2438-2451.doi:10.2527/jas.2016.1233.
Gao C Q,Zhao Y L,Li H C,Sui W G,Yan H C,Wang X Q.2015.Heat stress inhibits proliferation,promotes growth,and induces apoptosis in cultured Lantang swine skeletalmuscle satellite cells[J].Journal of Zhejiang University.SCIENCE.B,16(6):549-559.doi:10.1631/jzus.B1400339.
Huynh T TT,Aarnink A J A,Gerrits W J J,Heetkamp M J W,Canh T T,Spoolder H A M,Kemp B,Verstegen M W A.2005.Thermal behaviour of growing pigs in response to high temperature and humidity[J].Applied Animal Beha-viour Science,91(1-2):1-16.doi:10.1016/j.applanim.2004.10.020.
Khajavi M,Rahimi S,Hassan Z M,Kamali M A,Mousavi T.2003.Effect of feed restriction early in life on humoral and cellular immunity of two commercial broiler strains underheat stress conditions[J].British Poultry Science,44(3):490-497.doi:10.1080/000071660310001598328.
Lee S,Al Razqan G S,Kwon D H.2017.Antibacterial acti-vity of epigallocatechin-3-gallate(EGCG)and its syner-gism withβ-lactam antibiotics sensitizing carbapenem-associated multidrug resistant clinical isolates of Acineto-bacter baumannii[J].Phytomedicine:International Jour-nal of Phytotherapy and Phytopharmacology,24:49-55.doi:10.1016/j.phymed.2016.11.007.
Locke M,Celotti C.2014.The effect of heat stress on skeletal muscle contractile properties[J].Cell Stressamp;Chapero-nes,19(4):519-527.doi:10.1007/s 12192-013-0478-z.
Ma X Y,Jiang Z Y,Lin Y C,Zheng C T,Zhou G L.2010.Dietary supplementation with carnosine improves antioxi-dant capacity and meat quality of finishing pigs[J].Jour-nal of Animal Physiology and Animal Nutrition,94(6):e286-e295.doi:10.1111/j.1439-0396.2010.01009.x.
Maleki Dana P,Sadoughi F,Asemi Z,Yousefi B.2022.The role of polyphenols in overcoming cancer drug resistance:A comprehensive review[J].Cellularamp;Molecular Biology Letters,27(1):1.doi:10.1186/s 11658-021-00301-9.
Morales A,Grageola F,García H,Arce N,Araiza B,Yáñez J,Cervantes M.2014.Performance,serum amino acid con-centrations and expression of selected genes in pair-fed growing pigs exposed to high ambient temperatures[J].Journal of Animal Physiology and Animal Nutrition,98(5):928-935.doi:10.1111/jpn.12161.
Ohno A,Kataoka S,Ishii Y,Terasaki T,Kiso M,Okubo M,Yamaguchi K,Tateda K.2013.Evaluation of Camellia sinensis catechins as a swine antimicrobial feed additive that does not cause antibiotic resistance[J].Microbes and Environments,28(1):81-86.doi:10.1264/jsme2.me12137.
Oltvai Z N,Milliman C L,Korsmeyer S J.1993.Bcl-2 heterodi-merizes in vivo with a conserved homolog,Bax,that acce-lerates programmed cell death[J].Cell,74(4):609-619.doi:10.1016/0092-8674(93)90509-o.
Othman A I,Elkomy M M,El-Missiry M A,Dardor M.2017.Epigallocatechin-3-gallate prevents cardiac apoptosis by modulating the intrinsic apoptotic pathway in isoproterenol-induced myocardial infarction[J].European Journal of Pharmacology,794:27-36.doi:10.1016/j.ejphar.2016.11.014.
Pearce S C,Gabler N K,Ross J W,Escobar J,Patience J F,Rhoads R P,Baumgard L H.2013.The effects of heatstress and plane of nutrition on metabolism in growing pigs[J].Journal of Animal Science,91(5):2108-2118.doi:10.2527/jas.2012-5738.
Pearce S C,Upah N C,Harris A,Gabler N K,Ross J W,Rhoads R P,Baumgard L H.2011.Effects of heat stress on energetic metabolism in growing pigs[J].Federation of American Societies for Experimental Biology,25(S1):1052.5.doi:10.1096/fasebj.25.1.
Raoofi A,Omraninava M,Javan R,Maghsodi D,Rustamzadeh A,Nasiry D,Ghaemi A.2023.Protective effects of epigal-locatechin gallate in the mice induced by chronic scrotal hyperthermia[J].Tissueamp;Cell,84:102165.doi:10.1016/j.tice.2023.102165.
Ren Z Y,Zhang S Y,Shi L Y,Zhou A,Lin X,Zhang J,Zhu X S,Huang L,Li K.2024.Integrated ATAC-seq and RNA-seq analysis of in vitro cultured skeletal muscle satellite cells to understand changes in cell proliferation[J].Cells,13(12):1031.doi:10.3390/cells 13121031.
Shanmugam T,Selvaraj M,Poomalai S.2016.Epigallocatechin gallate potentially abrogates fluoride induced lung oxida-tive stress,inflammation via Nrf2/Keap1 signaling path-way in rats:An in-vivo and in-silico study[J].International Immunopharmacology,39:128-139.doi:10.1016/j.intimp.2016.07.022.
Sigler K,Ruch R J.1993.Enhancement of gap junctional inter-cellular communication in tumor promoter-treated cells by components of green tea[J].Cancer Letters,69(1):15-19.doi:10.1016/0304-3835(93)90026-6.
Song J,Lei X,Luo J X,Everaert N,Zhao G P,Wen J,Yang Y.2019.The effect of epigallocatechin-3-gallate on small intestinal morphology,antioxidant capacity and anti-inflammatory effect in heat-stressed broilers[J].Journal of Animal Physiology and Animal Nutrition,103(4):1030-1038.doi:10.1111/jpn.13062.
Wang L I,Liu F D,Luo Y,Zhu L Q,Li G H.2015.Effect of acute heat stress on adrenocorticotropic hormone,cortisol,interleukin-2,interleukin-12 and apoptosis gene expression in rats[J].Biomedical Reports,3(3):425-429.doi:10.3892/br.2015.445.
Wang Y J,Zheng W L,Bian X J,Yuan Y,Gu J H,Liu X Z,Liu Z P,Bian J C.2014.Zearalenone induces apoptosis and cytoprotective autophagy in primary Leydig cells[J].Toxi-cology Letters,226(2):182-191.doi:10.1016/j.toxlet.2014.02.003.
Xiang C H,Xiao X Y,Jiang B,Zhou M K,Zhang Y D,Li H,Hu Z.2017.Epigallocatechin-3-gallate protects from high glucose induced podocyte apoptosis via suppressing endo-plasmic reticulum stress[J].Molecular Medicine Reports,16(5):6142-6147.doi:10.3892/mmr.2017.7388.
Xu X B,Lai Y Y,Hua Z C.2019.Apoptosis and apoptotic body:Disease message and therapeutic target potentials[J].Bioscience Reports,39(1):BSR20180992.doi:10.1042/BSR20180992.
Zhao F,Wang X C,Li Y,Chen X Y,Geng Z Y,Zhang C.2021.Effects of dietary supplementation with epigallocatechin gallate on meat quality and muscle antioxidant capacity of broilers subjected to acute heat stress[J].Animals,11(11):3296.doi:10.3390/ani 11113296.
Zhou D J,Sun M H,Jiang W J,Li X H,Lee S H,Heo G,Niu Y J,Ock S A,Cui X S.2022.Epigallocatechin-3-gallate pro-tects porcine oocytes against post-ovulatory aging through inhibition of oxidative stress[J].Aging,14(21):8633-8644.doi:10.18632/aging.204368.
(责任编辑兰宗宝)

