不同养殖模式下生长迟缓与正常华南鲤肠道健康状况的比较






摘要:【目的】从肠道健康角度揭示华南鲤生长迟缓的原因,为深入探究鱼类生长迟缓现象与肠道健康的内在关联提供理论依据。【方法】取当年同批繁殖的华南鲤幼鱼,分别在循环水和池塘网箱2种养殖模式下养殖5个月,根据其体质量分为4组[循环水模式生长正常组(R-N),循环水模式生长迟缓组(R-GS),池塘网箱模式生长正常组(P-N),池塘网箱模式生长迟缓组(P-GS)],然后比较分析4组华南鲤的肠道组织形态、消化酶活性及菌群结构差异。【结果】2种养殖模式下,生长迟缓华南鲤的肠绒毛高度和肌层厚度均显著低于生长正常华南鲤(Plt;0.05,下同),肠道淀粉酶、胰蛋白酶和脂肪酶的活性也低于生长正常华南鲤,其中胰蛋白酶活性差异达显著水平。池塘网箱模式下华南鲤肠道菌群Alpha多样性指标显著高于循环水模式下的华南鲤;但同一养殖模式下,生长迟缓与正常华南鲤的肠道菌群Alpha多样性差异不显著(Pgt;0.05,下同)。相对于生长正常华南鲤,循环水模式生长迟缓华南鲤肠道变形菌门相对丰度显著降低,而脱硫杆菌门、厚壁菌门和放线菌门的相对丰度显著升高;池塘网箱模式生长迟缓华南鲤肠道梭杆菌门和厚壁菌门的相对丰度显著下降,而绿弯菌门、蓝藻门和酸杆菌门的相对丰度显著升高,脱硫杆菌门、放线菌门、浮霉菌门和变形菌门的相对丰度也有所升高,但差异不显著。在属分类水平上,循环水模式生长迟缓华南鲤肠道的未分类脱硫弧菌科、红球菌属、弧菌属和希瓦氏菌属相对丰度较生长正常华南鲤显著升高;池塘网箱模式生长迟缓华南鲤肠道的unclassified Pirellulaceae和Fimbriiglobus相对丰度较生长正常华南鲤显著升高。KEGG信号通路分析结果表明,2种养殖模式下生长迟缓与正常华南鲤肠道丰度差异菌群均显著富集在内分泌系统通路上。【结论】无论是在池塘网箱模式还是循环水模式下,生长迟缓华南鲤的肠道健康指标均发生明显变化,具体表现为消化酶活性降低,对食物消化能力减弱,即肠道健康水平下降可能是分化出生长迟缓华南鲤的主要原因之一。
关键词:华南鲤;生长迟缓;肠道健康;消化酶;菌群结构
中图分类号:S965.116文献标志码:A文章编号:2095-1191(2024)10-3147-13
Comparison of intestinal health status in growth-stunted andnormal Cyprinus carpio rubrofuscus under differentaquaculture modes
GENG Guo-hua1,2,ZHU Hua-ping2,3*,MA Dong-mei2,3,ZHONG Zai-xuan2,3,ZHAO Shu-hao1,2,FAN Jia-jia2,3,TIANYuan-yuan2,3,HAN Fang1,LIU Xian-de1*
(1Fisheries College,Jimei University,Xiamen,Fujian 361021,China;2Pearl River Fisheries Research Institute,Chi-nese Academy of Fishery Sciences/Key Laboratory of Tropical and Subtropical Fishery Resources Application and Cultiva-tion,Ministry of Agriculture and Rural Affairs,Guangzhou,Guangdong 510380,China;3Guangdong Provincial KeyLaboratory of Aquatic Animal Immunology and Sustainable Aquaculture,Guangzhou,Guangdong 510380,China)
Abstract:【Objective】To elucidate the causes of stunted growth in Cyprinus carpio rubrofuscus from the perspectiveof intestinal health,which provided theoretical reference for further investigation of the intrinsic relationship between thefish stunted growth and intestinal health.【Method】C.carpiorubrofuscus juveniles bred in the same batch in the same year were cultured in 2 culture modes for 5 months:recirculating water system and pond netting.They were then divided into 4 groups according to their body weight,with the following designations:normal group in recirculating water system(R-N);growth-stunted group in recirculating water system(R-GS);normal group in pond netting(P-N);growth-stunted group in pond netting(P-GS).The differences in intestinal tissue morphology,digestive enzyme activities and bacterialflora structure of C.carpiorubrofuscus among the 4 groups were compared and analyzed.【Result】In the 2 culture modes,the height of intestinal villi and the thickness of muscularis propria of growth-stunted C.carpiorubrofuscus were signifi-cantly lower than those of normal C.carpiorubrofuscus(Plt;0.05,the same below),and the activities of intestinal amy-lase,trypsin and lipase were lower than those of normal C.carpiorubrofuscus,with the difference in the activity of tryp-sin reaching a significant level.The Alpha diversity of the intestinal flora of C.carpiorubrofuscus in the pond netting mode was significantly higher than that of C.carpiorubrofuscus in the recirculating water system mode;however,the difference in the Alpha diversity of intestinal flora of growth-stunted and normal-growing C.carpiorubrofuscus in thesame culture mode was not significant(Pgt;0.05,the same below).Compared with the normal-growing C.carpiorubrofus-cus,the relative abundance of growth-stunted C.carpiorubrofuscus intestinal Proteobacteria was significantly decreasedintherecirculating water system mode,while the relative abundance of Desulfobacterota,Firmicutes and Actinobacteria were significantly increased;the relative abundance of growth-stunted C.carpiorubrofuscus intestinal Fusobacteria and Firmicutes in the pond netting mode was significantly decreased,while the relative abundance of Chloroflexi,Cyanobac-teria and Acidobacteria were significantly increased,and Desulfobacterota,Actinobacteria,Planctomycetes and Protebac-teria also increased in relative abundance,but the differences were not significant.At the genus level,the relative abun-dance of unclassified Desulfovibrionaceae,Rhodococcus,Vibrio,Shewanella in the intestine of growth-stunted C.carpiorubrofuscus in therecirculating water system mode was significantly increased compared with that of normal C.carpioru-brofuscus;the relative abundance of unclassified Pirellulaceae and Fimbriiglobus in the intestine of growth-stunted C.car-piorubrofuscusin the pond netting mode was significantly increased compared with that of normal C.carpiorubrofuscus.The results of KEGG signaling pathway analysis showed that intestinal abundance differential flora in 2 modes of culturein growth-stunted and normal C.carpiorubrofuscus all enriched in the endocrine system pathway.【Conclusion】Both inthe pond netting andrecirculating water system modes,the intestinal health indexes of growth-stunted C.carpiorubrofus-cus change greatly,which is manifested by the reduced activity of digestive enzymes and weakened ability to digest food.In other words,the reduced level of intestinal health is one of the main reasons for the differentiation of growth-stunted C.carpiorubrofuscus.
Key words:Cyprinus carpio rubrofuscus;stunted growth;intestinal health;digestive enzyme;flora structure
Foundation items:National Key Research and Development Program of China(2023YFD2400203);National Natu-ral Science Foundation of China(32072971);Guangdong Rural Revitalization Strategy Special Project(2022-SPY-00-019);Fujian Science and Technology Plan Project(2023N0011)
0引言
【研究意义】华南鲤(Cyprinus carpio rubrofus-cus)是我国云南省元江流域、珠江流域广东和广西段及海南省岛内水域的土著鱼类,属于鲤(Cyprinus carpio Linnaeus)的4个亚种之一(朱华平等,2018)。在池塘或稻田人工养殖模式下,部分同批繁殖的华南鲤养殖至5月龄后会分化为生长迟缓型和生长正常型2种群体,二者形体差异明显,其体质量差异在25%左右。肠道健康对鱼类的健康至关重要,健康的肠道具备完善的机械保护屏障、化学保护屏障、免疫保护屏障和微生物保护屏障,在维持内环境平衡及阻碍外来病原微生物入侵方面发挥重要作用(Vancamelbeke and Vermeire,2017;Zhang et al.,2020;陈秀梅等,2022;蒋鑫涛等,2023)。鱼类肠表面是机械保护屏障的重要组成部分,其完整性和表面积是评估鱼类肠道吸收功能、生长性能及健康水平的重要指标(Chen et al.,2018;Bai etal.,2019);消化酶是鱼类健康的化学保护屏障,消化酶活性升高可增强对食物的消化能力,有效抵御外来有害物质的侵害(Hu etal.,2014);肠道菌群是鱼类的微生物保护屏障,适宜的菌群组成及比例可为鱼类提供维生素等营养物质,抑制肠道致病菌生长和促进鱼体快速生长(Tran et al.,2018;罗君等,2022)。因此,明确生长迟缓华南鲤肠道健康状况对揭示其生长迟缓的原因具有重要意义。【前人研究进展】肠道是动物消化器官中最长的部分,主要的消化和吸收作用均在肠道内完成,尤其是肠道上皮细胞不仅具有营养物质消化和吸收的功能,还发挥屏障保护和免疫防御的作用(徐革锋等,2009)。肠道病变可引起肝胰腺等器官的损伤及功能障碍,进而影响鱼类的整体健康水平(米海峰等,2015)。经济鱼类常见的肠道健康评估指标包括肠道内壁组织形态、消化酶活性、肠道微生物组成及其多样性(Hooper and Macpher-son,2010;米海峰等,2015;何琴等,2023),通过肠道内壁组织形态观察可测定肠道内的肠绒毛高度和宽度,而这2个指标与肠道表面积呈正相关,是评估肠道消化能力的重要指标之一(Caspary,1992;黄玉章等,2010)。鱼类肠道内消化酶具有消化分解食物和促进营养物质吸收的作用,其活性可反映鱼类消化吸收营养物质的能力及肠道健康状况(Dawood,2021),根据底物类型的不同,可分为蛋白酶、淀粉酶及脂肪酶等(张云龙等,2017)。鱼类肠道内定殖有种类丰富、数量庞大的菌群,在促进鱼类生长发育、营养代谢、维持宿主健康和免疫调控等方面发挥着重要作用(孟晓林等,2019;Xiong et al.,2019)。至今,关于异速生长鱼类个体生长速度与肠道菌群结构多样性及组成相关性的研究已有较多报道。李英英等(2017)研究表明,生长缓慢大黄鱼(Pseudos-ciaenacrocea)肠道菌群OTU数量及Chao1指数显著高于生长正常的大黄鱼;饶刘瑜等(2018)通过比较转生长激素基因鲤(Cyprinus carpio L.)和野生对照鲤不同发育阶段的肠道微生物群落结构差异,结果发现转基因鲤肠道中存在高丰度的厚壁菌门(Fir-micutes)细菌,而对照鲤中拟杆菌门(Bacteroidetes)细菌较多;王沈同(2023)研究证实,低体质量组草鱼(Ctenopharyngodonidella)的肠道菌群Alpha多样性指数高于高体质量组草鱼。此外,某些特殊菌属可能与鱼类生长速率相关,如快速生长达氏鳇(Huso dauricus)群体肠道中的鞘氨醇单胞菌属(Sphin-gomonas)相对丰度(67.0%)显著高于慢速生长达氏鳇群体(29.5%)(王若愚等,2023)。【本研究切入点】目前,有关异速生长鱼体肠道微生物种群特征的研究已有较多报道(Sun et al.,2009;Zhang et al.,2021),但系统揭示肠道健康状况影响鱼类生长的研究鲜见报道。【拟解决的关键问题】以华南鲤为研究对象,比较分析池塘网箱和循环水养殖模式下生长迟缓和正常华南鲤的肠道形态结构、消化酶活性及其菌群结构特征,以期从肠道健康角度揭示华南鲤生长迟缓的原因,为深入探究鱼类生长迟缓现象与肠道健康的内在关联提供理论依据。
1材料与方法
1.1试验鱼饲养管理与试验设计
取当年同批繁殖的华南鲤幼苗(平均体长5.0 cm),400尾养殖于珠江水产研究所室内循环水水泥池(长4.0 m,宽2.5 m,水深约0.8 m;水体约8.0 m3),400尾养殖于池塘网箱(在面积约2234m2的池塘中放置长4.0 m,宽3.0 m,水深约1.8 m的网箱),各养殖阶段根据鱼体大小投喂适口的人工配合饲料,循环水模式的投喂时间分别为8:30和18:00,池塘网箱模式的投喂时间分别为9:00和18:30,每日投饵量约为鱼体总体质量的3%。2种养殖模式在试验期间均持续充气增氧,循环水水池每周换1次曝气的自来水,换水量为养殖水量的1/2。养殖5个月后,2种养殖模式下的华南鲤均出现生长迟缓个体,检测其肠道健康状况,分析出现生长迟缓个体的原因。停止投饵15h后,从循环水模式中挑选30尾体质量高的华南鲤(85.35±15.21 g)设为循环水模式生长正常组(R-N),30尾体质量低的个体(17.51±3.43 g)设为循环水模式生长迟缓组(R-GS);从池塘网箱模式中挑选30尾体质量高的个体(93.09±13.78 g)设为池塘网箱模式生长正常组(P-N),30尾体质量低的个体(18.74±4.57 g)设为池塘网箱模式生长迟缓组(P-GS)。动物试验由中国水产科学研究院珠江水产研究所实验动物管理和使用伦理委员会批准,批准号LAEC-PRFRI-2023-06-35。
1.2肠道样品采集
华南鲤经MS-222麻醉后称重,每组随机取18尾,用75%酒精擦洗体表,无菌操作剪开腹腔和肠道,取出肠道内容物,将每3尾鱼的肠道内容物制成1个混合样品,放入2 mL冻存管中,每组采集6个样品,液氮速冻后置于-80℃超低温冰箱保存。每组另取6尾,按肠道生理弯曲部位在中肠相同位置分别截取一小段,4%多聚甲醛固定保存,用于制作肠道组织切片;其他中肠部分经液氮速冻后放入-80℃超低温冰箱保存,用于测定消化酶活性。
1.3肠道组织切片制备及观察
4%多聚甲醛固定24h的肠道样品经梯度浓度乙醇脱水、二甲苯透明及石蜡包埋后,截面横切5μm厚的切片,苏木精—伊红染色,置于光学显微镜下观察拍照。通过ImageJ比较分析肠道组织结构差异,测量记录各组华南鲤的肠绒毛高度、宽度和肌层厚度(n=50),并沿肠道横截面的黏膜曲线画线测量黏膜长度(n=5),根据对应黏膜上的绒毛数量计算肠绒毛密度:
肠绒毛密度(根/mm)=肠绒毛数量/对应黏膜长度
1.4肠道消化酶活性测定
将用于消化酶活性测定的肠道样品剪开,PBS洗去肠道内容物,吸水纸吸干,称重,然后按照重量(g)∶体积(mL)=1∶9的比例加入预冷生理盐水,置于冰上匀浆,2500 r/min离心10 min,取上清液用于肠道消化酶活性测定。采用紫外比色法测定胰蛋白酶(Trypsin)活性,使用淀粉—碘比色法测定淀粉酶(Amylase)活性,以比色法测定脂肪酶(Lipase)活性,所有消化酶活性检测试剂盒均购自南京建成生物工程研究所。
1.5水环境样品采集
为分析水环境菌群与鱼类肠道菌群间的关联性,采用五点取样法分别对循环水模式(R-W)和池塘网箱模式(P-W)进行水样采集,共5 L水样,分别重复取3次水样。采集的水样先用0.45μm大孔径滤膜抽滤去除杂质,然后以0.22µm滤膜过滤收集水体中的微生物,液氮速冻保存。
1.6高通量测序分析
采用十六烷基三甲基溴化铵(CTAB)法提取4组华南鲤24个肠道内容物样品及6个水环境微生物样品的基因组DNA,经1.0%琼脂糖凝胶电泳和紫外分光光度计检测合格后,以基因组DNA为模板,使用微生物16S rDNA序列V3~V4可变区特异性引物(341F:5'-CCTACGGGNGGCWGCAG-3';805R:5'-GACTACHVGGGTATCTAATCC-3')进行PCR扩增(Logue et al.,2015)。PCR扩增产物经纯化、定量和回收后,委托上海百趣生物医学科技有限公司在NovaSeq 6000测序仪上完成2×250 bp双端测序。测序获得的原始数据采用Cutadapt去除接头和引物序列,以FLASH对双端测序得到的每对Reads进行拼接,再用Fqtrim(v0.94)过滤掉长度小于100 bp或不确定碱基含量大于5%的低质量Reads,运用Vsearch(v2.3.4)去除嵌合体,然后利用DADA2进行长度过滤及去噪,最终获得高质量的扩增子序列变异体(Ampliconsequence variants,ASVs)(张小明等,2024)。在SILVA数据库(https://www.arbsilva.de/documenta‐tion/release 138/)中对获得的ASVs进行比对分析及物种注释,采用Qiime计算每个样品的物种丰度指数(Observed species)、Shannon指数、Simpson指数、Chao1指数和Pieloue指数,以明确华南鲤肠道微生物群落Alpha多样性,并通过主坐标分析(PCoA)评估肠道微生物群落Beta多样性。
1.7统计分析
试验数据采用SPSS 24.0进行单因素方差分析(One-way ANOVA)和Duncanʼs多重比较,并以t检验对不同样本的微生物群落结构及物种组成进行差异显著性分析。
2结果与分析
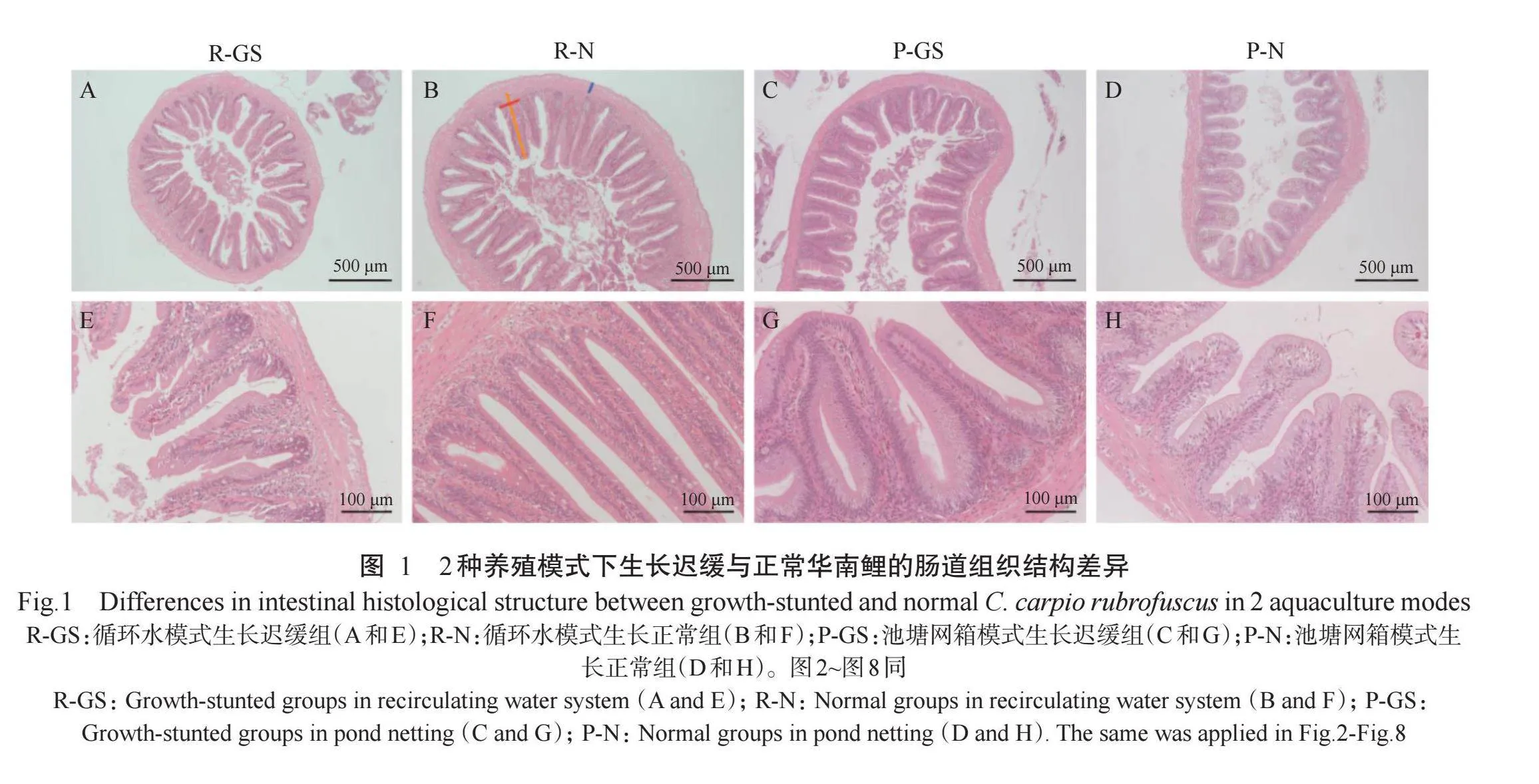
2.1 2种养殖模式下华南鲤肠道组织结构差异
2种养殖模式下,华南鲤的中肠组织结构如图1所示。在显微镜观察的基础上,对华南鲤肠绒毛的高度、宽度、肌层厚度及肠绒毛密度4个形态指标进行统计,结果(图2)显示,在池塘网箱模式下,P-GS组华南鲤的肠绒毛高度、肌层厚度和肠绒毛密度均显著低于P-N组华南鲤(Plt;0.05,下同),肠绒毛宽度也低于P-N组华南鲤,但差异不显著(Pgt;0.05,下同);在循环水模式下,R-GS组华南鲤的肠绒毛高度、宽度和肌层厚度均显著低于R-N组华南鲤,但肠绒毛密度与R-N组华南鲤无显著差异。
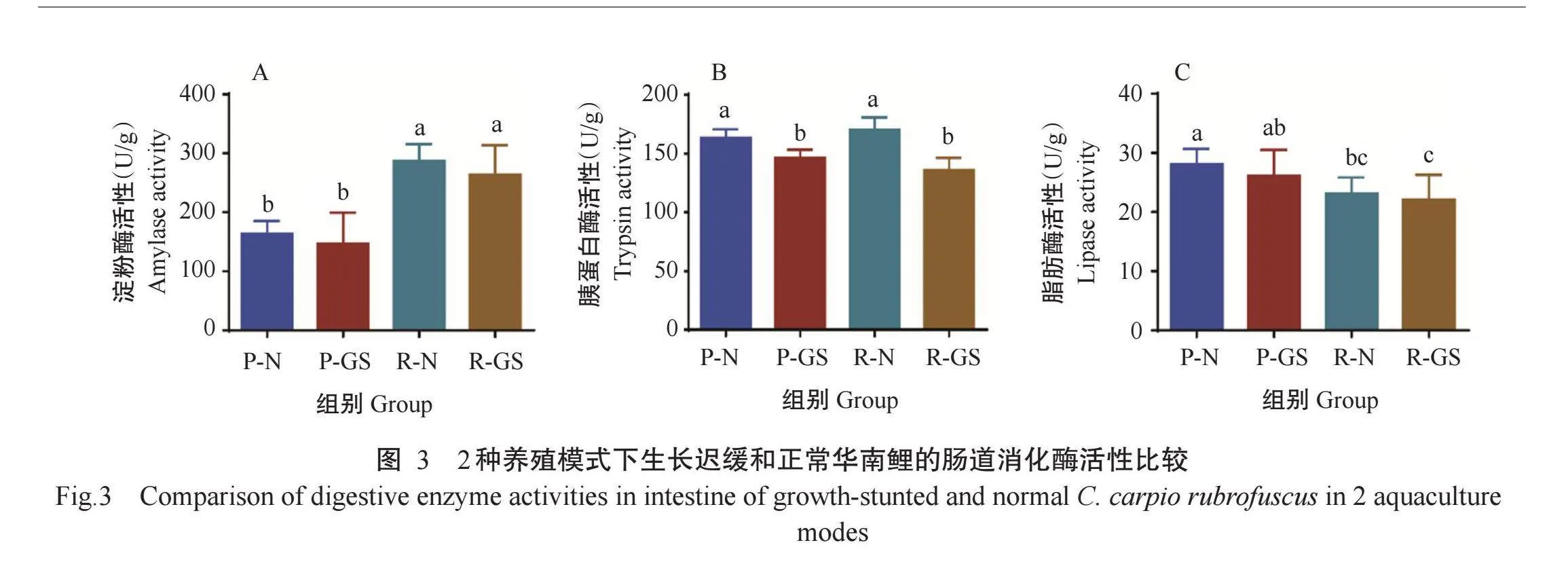
2.2 2种养殖模式下华南鲤肠道消化酶活性差异
由图3可看出,无论是池塘网箱模式还是循环水模式,生长迟缓华南鲤肠道的胰蛋白酶活性均显著低于生长正常华南鲤,淀粉酶活性和脂肪酶活性也低于生长正常华南鲤,但差异不显著。在4组华南鲤中,以R-N组华南鲤肠道的胰蛋白酶活性和淀粉酶活性最高。
2.3养殖水体菌群与华南鲤肠道菌群的相关性
2.3.1养殖水体和华南鲤肠道菌群多样性基于微生物16S rDNA序列V3~V4可变区对4组华南鲤24个肠道内容物样品及2种养殖模式6个水环境微生物样品进行高通量测序,并计算各样品的菌群多样性指数,结果(表1)显示,循环水模式养殖水体及其养殖的华南鲤肠道菌群Alpha多样性(物种丰度指数、Shannon指数、Simpson指数、Chao1指数和Pieloue指数)均显著低于池塘网箱模式下对应的各组样品。同一养殖模式下,生长迟缓与正常华南鲤肠道菌群Alpha多样性指数的差异均不显著,说明环境对不同生长速度华南鲤肠道微生物组成具有相似的影响。
基于PCoA分析评估养殖水体和华南鲤肠道菌群Beta多样性,结果如图4所示。在未加权距离矩阵的PCoA分析中,第一主坐标(PCoA1)、第二主坐标(PCoA2)的贡献率分别为34.55%和15.29%;在加权距离矩阵的PCoA分析中,PCoA1、PCoA2的贡献率分别为47.29%和22.18%。2种PCoA分析获得的结果基本相似,循环水模式下的样品聚集于第三象限,池塘网箱模式下的样品聚集于第四象限;2种养殖模式下的华南鲤肠道内容物样品明显分开,同一养殖模式下生长迟缓与正常华南鲤的肠道内容物样品聚集在一起,表明养殖模式对华南鲤肠道菌群组成有明显影响。
2.3.2养殖水体和华南鲤肠道菌群结构特征基于布雷柯蒂斯(Bray-Curtis)距离的UPGMA聚类分析结果(图5)显示,在2种养殖模式下均表现为生长迟缓华南鲤肠道内容物样品先聚为一支,再与生长正常华南鲤肠道内容物样品聚类在一起,即生长迟缓与正常华南鲤的肠道菌群结构存在差异,二者间可有效区分开。
通过绘制Venn图展示各组样品间的共有或特有ASV数目,直观反映不同样品间ASV组成的相似性及重叠情况。由图6-A可看出,所有样品的共有ASV数目为19个,R-W样品、P-W样品、P-N样品、P-GS样品、R-N样品、R-GS样品的特有ASV数目分别为1244、2572、3124、3847、1367和641个。由图6-B~图6-D可看出,池塘网箱模式下养殖华南鲤肠道内容物样品及其养殖水体的共有ASV数目明显高于循环水模式下对应的各组样品,表明池塘网箱模式养殖水体的菌群多样性较循环水模式养殖水体更丰富,使得池塘网箱模式下的生长迟缓华南鲤肠道菌群结构可能更复杂,涉及的菌群种类更多。此外,2种养殖模式下的生长迟缓华南鲤肠道内容物样品共有ASV数目为131个(图6-B),说明2种养殖模式下生长迟缓华南鲤肠道菌群结构存在相似性。
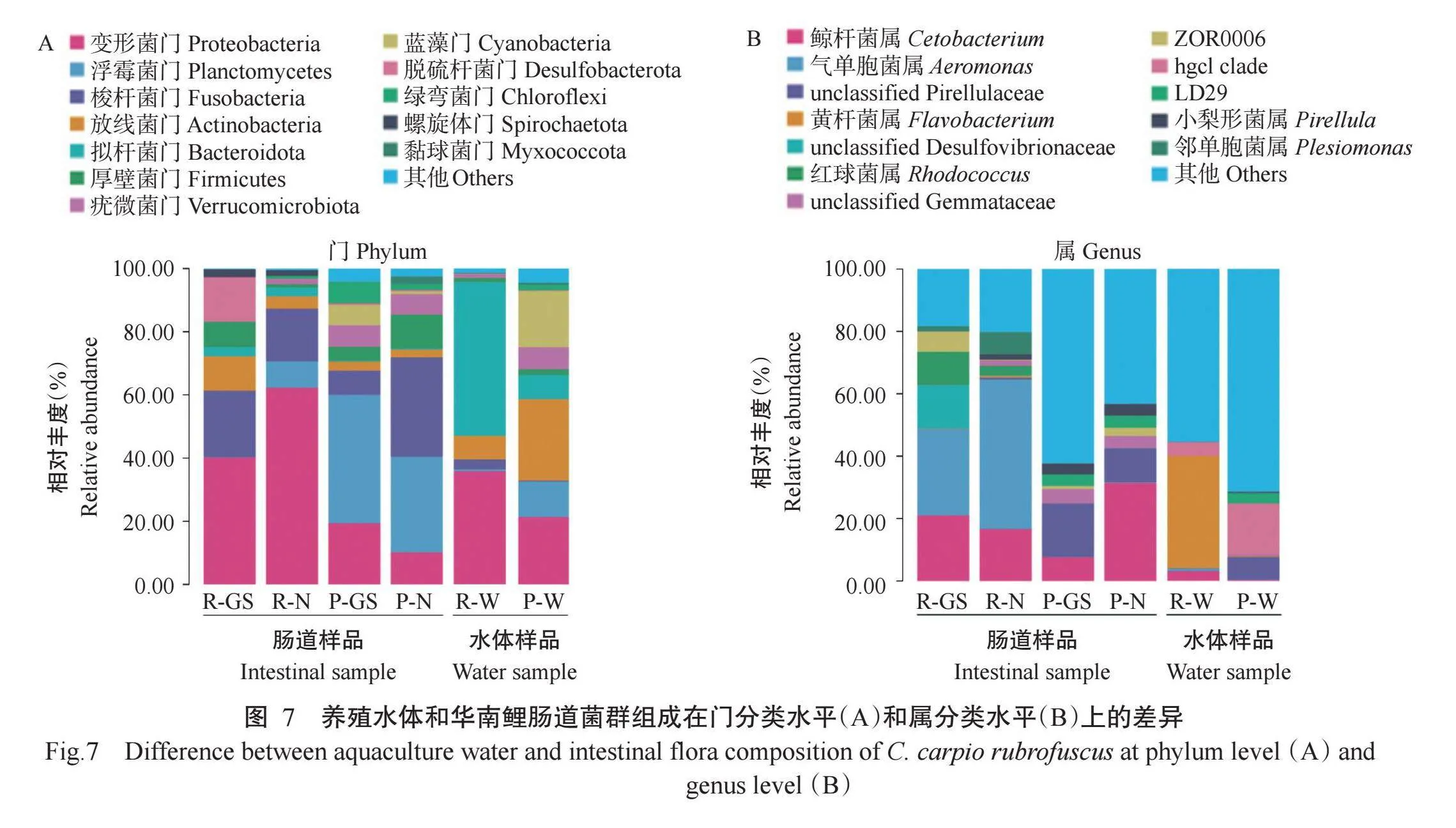
2.3.3生长迟缓与正常华南鲤肠道菌群组成差异如图7-A所示,循环水模式养殖水体的菌群组成以拟杆菌门和变形菌门(Proteobacteria)为主,其相对丰度分别为48.63%和35.81%;池塘网箱模式养殖水体的菌群组成多样性更高,主要包括放线菌门(Actinobacteria,25.79%)、变形菌门(21.30%)、蓝藻门(Cyanobacteria,17.88%)和浮霉菌门(Planctomy-cetes,11.27%)等;变形菌门是2种养殖模式下的共有优势菌门。循环水模式下,华南鲤肠道菌群主要由变形菌门、梭杆菌门(Fusobacteria)和放线菌门组成,相对于生长正常华南鲤,生长迟缓华南鲤肠道变形菌门相对丰度显著降低,而脱硫杆菌门(Desulfo-bacterota)、厚壁菌门和放线菌门的相对丰度显著升高。池塘网箱模式下,华南鲤肠道菌群优势菌门由浮霉菌门、梭杆菌门和变形菌门组成,与生长正常华南鲤相比,生长迟缓华南鲤肠道梭杆菌门和厚壁菌门的相对丰度显著下降,而绿弯菌门(Chloroflexi)、蓝藻门和酸杆菌门(Acidobacteriota)的相对丰度显著升高,脱硫杆菌门、放线菌门、浮霉菌门和变形菌门的相对丰度也有所升高,但差异不显著。
如图7-B所示,在属分类水平上2种养殖水体的菌群组成存在明显差异,循环水模式养殖水体菌群结构组成中相对丰度排名前3的属分别是黄杆菌属(Flavobacterium,35.97%)、Limnohabitans(16.47%)和Emticicia(6.03%),池塘网箱模式养殖水体菌群结构组成中相对丰度排名前3的属分别为hgcI clade(16.35%)、双色藻属(Cyanobium PCC-6307,12.80%)和unclassified Pirellulaceae(7.26%)。循环水模式下,生长迟缓华南鲤肠道的优势菌属包括气单胞菌属(Aeromonas,27.69%)、鲸杆菌属(Cetobacterium,20.99%)、未分类脱硫弧菌科(unclassified Desulfovi-brionaceae,13.99%)、红球菌属(Rhodococcus,10.65%)、弧菌属(Vibrio,6.65%)和短螺旋体属(Brevinema,2.48%),生长正常华南鲤肠道的优势菌属包括气单胞菌属(47.96%)、鲸杆菌属(16.66%)、邻单胞菌属(Plesiomonas,7.12%)和短螺旋体属(1.78%);相对于生长正常华南鲤,生长迟缓华南鲤的未分类脱硫弧菌科、红球菌属、弧菌属和希瓦氏菌属(Shewanella)相对丰度显著升高。池塘网箱模式下,生长迟缓华南鲤肠道的优势菌属有小梨形菌属(Pirellula,17.14%)、鲸杆菌属(7.62%)和unclassified Gemmataceae(4.58%),生长正常华南鲤肠道的优势菌属包括鲸杆菌属(31.45%)和unclassified Pirellulaceae(10.85%);与生长正常华南鲤相比,生长迟缓华南鲤的unclassified Pirellulaceae和Fimbriiglobus相对丰度显著升高。
2.4华南鲤肠道菌群功能预测结果
采用PICRUSt 2预测华南鲤肠道菌群功能,结果(图8)显示,池塘网箱模式下,生长迟缓与正常华南鲤肠道丰度差异菌群显著富集在心血管疾病(Cardiovascular disease)、消化系统(Digestive sys-tem)、能量代谢(Energy metabolism)、内分泌系统(Endocrine system)及信号分子与相互作用(Signal molecules and interaction)等KEGG信号通路上;循环水模式下,生长迟缓与正常华南鲤肠道丰度差异菌群则显著富集在外源性物质生物降解与代谢(Xenobiotics biodegradation and metabolism)、细胞运动(Cell motility)、循环系统(Circulatory system)、折叠/分类/降解(Folding,sorting and degradation)及内分泌系统等KEGG信号通路上。其中,内分泌系统是2种养殖模式下生长迟缓与正常华南鲤肠道丰度差异菌群均显著富集到的KEGG信号通路,说明无论是在池塘网箱模式还是循环水模式下,华南鲤肠道健康水平下降和生长迟缓现象可能都与内分泌相关通路异常存在潜在关联。
3讨论
肠道健康对于鱼类的健康养殖至关重要,其中,肠绒毛高度和密度的增加可扩大肠道消化吸收食物的表面积,肌层厚度增加可增强肠道收缩蠕动的能力,因此肠道形态结构指标可从侧面反映鱼体的消化能力(翟少伟等,2016;Cao et al.,2022)。本研究结果表明,在循环水和池塘网箱模式下,生长迟缓华南鲤的肠绒毛高度和肌层厚度均显著低于生长正常华南鲤,与李英英等(2015)对大黄鱼、麦浩彬等(2020)对珍珠龙胆石斑幼鱼(Epinephelus lanceolatu♂×E.fuscoguttatus♀)的研究结果相似,即生长缓慢型个体的肠道肌层较薄、肠绒毛高度和密度较低,而生长快速型个体的肠绒毛高度和密度均高于正常个体。由此推测,2种养殖模式下生长迟缓华南鲤的肠道健康均出现异常,肠绒毛高度和密度降低致使肠表面积相应减小,肠道消化吸收能力下降,进而出现鱼体生长缓慢现象。消化酶具有催化食物分解的作用,其活性水平可反映鱼体对营养成分的消化和吸收利用能力,是衡量鱼类肠道健康及生长状况的重要指标之一(田宏杰等,2006;Comabella et al.,2006;刘敏和张辉,2008;余友斌等,2023)。本研究结果显示,无论是池塘网箱模式还是循环水模式,生长迟缓华南鲤肠道淀粉酶、胰蛋白酶和脂肪酶的活性均低于生长正常华南鲤,其中胰蛋白酶活性差异达显著水平。消化酶活性与经济鱼类的生长性能间存在高度相关性,钱永生等(2019)研究发现快速生长的瓯江彩鲤具有较高的蛋白酶活性和脂肪酶活性,袁禹惠等(2023)研究表明生长性能最高的斜带石斑鱼(E.coioides)稚鱼肠道胰蛋白酶活性显著高于其他组别的斜带石斑鱼稚鱼。生长迟缓华南鲤消化酶活性降低,导致鱼体肠道对食物的消化能力减弱,也是肠道健康水平降低的表现。
鱼类肠道内定殖有种类丰富、数量庞大的菌群(Eckburg et al.,2005;Roeselers et al.,2011),与宿主经过长期进化选择后共同形成一个在调节生理生化反应、促进食物消化、介导宿主免疫应答及抵抗病原体侵染等方面发挥重要作用的微生态系统(Sugita et al.,1996;Pérez et al.,2010;Ghanbari et al.,2015;Wang et al.,2018)。鱼类肠道菌群Alpha多样性可反映其健康状况和代谢能力(Clarke et al.,2014;Luan et al.,2023)。本研究结果表明,池塘网箱模式养殖水体菌群Alpha多样性指标显著高于循环水模式养殖水体,与之对应的是,池塘网箱模式下华南鲤肠道菌群Alpha多样性指标均显著高于循环水模式下的华南鲤,说明鱼体肠道菌群多样性与养殖水环境中的菌群多样性呈正相关,与李存玉等(2015)的研究结果基本一致,即池塘养殖牙鲆(Paralichthys olivaceus)肠道菌群多样性高于工厂化养殖牙鲆,且水环境中的菌群可能直接影响牙鲆肠道菌群结构及其稳态。在同一养殖模式下,生长迟缓与正常华南鲤的肠道菌群Alpha多样性差异不显著,说明华南鲤肠道菌群Alpha多样性与其生长性状间无显著相关性,与Chapagain等(2019)的研究结果一致,即快速生长与慢速生长虹鳟(Oncorhynchus mykiss)间的肠道菌群Alpha多样性无显著差异;但Zhao等(2023)对祁连山裸鲤(Gymnocyprischilianensis)的研究发现,较高体质量个体的肠道微生物群落丰度和均匀度均高于较低体质量个体。可见,鱼类生长差异与肠道菌群多样性的关联尚未明确。
除了菌群Alpha多样性外,肠道菌群结构组成也是衡量肠道健康的重要指标(王飞飞等,2022)。本研究结果显示,2种养殖模式下华南鲤肠道菌群结构中相对丰度排名前5的门均包括变形菌门、厚壁菌门、浮霉菌门、梭杆菌门和放线菌门,与Wu等(2013)、Eichmiller等(2016)、王蕾(2017)的研究结果相似。2种养殖模式下,生长迟缓与正常华南鲤的肠道菌群结构组成虽然在门和属分类水平上相似,但各菌群的相对丰度存在差异,与工厂化和网箱养殖模式下黄条鰤(Seriola lalandi)幼鱼(周鹤庭等,2022)、稻田和池塘养殖模式下建鲤(Cyprinus car-piovar.Jian)(赵柳兰等,2021)的肠道菌群研究结果相似,说明不同养殖模式下鱼类具有稳定的核心菌群,但肠道菌群结构组成比例会随着养殖水体的变化而改变。此外,无论是池塘网箱模式还是循环水模式,生长迟缓华南鲤肠道菌群中放线菌门、脱硫杆菌门和蓝藻门的相对丰度较生长正常华南鲤均呈升高趋势,其中,放线菌门和脱硫杆菌门相对丰度在循环水模式下呈显著升高趋势,蓝藻门相对丰度在池塘网箱模式下呈显著升高趋势。放线菌门相对丰度的变化趋势与王悦等(2021)的研究结果相似,故推测肠道菌群中放线菌门的相对丰度与华南鲤生长性能存在一定相关性。在属分类水平上,循环水模式下生长迟缓华南鲤肠道菌群中希瓦氏菌属的相对丰度较生长正常华南鲤显著升高。希瓦氏菌属具有适应性极强的代谢系统,可产生硫化氢(H2S)(商宝娣等,2015)。哺乳动物体内的H2S浓度升高,会破坏肠道屏障而引起肠道炎症反应(Dordevićet al.,2021;卢歌雪,2023)。张燕玉等(2019)研究证实,海藻希瓦氏菌(Shewanella algae)能引起半滑舌鳎肠绒毛出现溶解、脱落等组织损伤,且伴有大量炎性细胞浸润于肌肉层和黏膜下层;Choi等(2021)研究发现,希瓦氏菌在低体质量皱纹盘鲍(Haliotis discus han-nai)肠道中的相对丰度显著升高。由此推测,华南鲤肠道菌群中希瓦氏菌属相对丰度较高,可产生高浓度的H2S而破坏肠道屏障,引起肠道损伤及消化能力减弱,最终导致华南鲤出现生长缓慢现象。
4结论
无论是在池塘网箱模式还是循环水模式下,生长迟缓华南鲤的肠道健康指标均发生明显变化,具体表现为消化酶活性降低,对食物消化能力减弱,即肠道健康水平下降是分化出生长迟缓华南鲤的主要原因之一。
参考文献(References):
陈秀梅,王桂芹,单晓枫,钱爱东.2022.鱼类肠道屏障损伤与肠道炎症发生发展关系的研究进展[J].河南农业科学,51(5):1-9.[Chen X M,Wang G Q,Shan X F,Qian A D.2022.Research progress on the relationship between intes‐tinal barrier damage and intestinal inflammation develop‐ment in fish[J].Journal of Henan Agricultural Sciences,51(5):1-9.]doi:10.15933/j.cnki.1004-3268.2022.05.001.
何琴,王利,段荟芹,苟小兰.2023.枯草芽孢杆菌和粪肠球菌对鲫鱼生长性能、血清学指标和肠道微生物多样性的影响[J].江苏农业学报,39(1):142-147.[He Q,Wang L,Duan H Q,Gou X L.2023.Effects of Bacillus subtilis and Enterococcus faecalis on growth performance,serum bio‐chemical indices and intestinal microflora of Carassius auratus[J].Jiangsu Journal of Agricultural Sciences,39(1):142-147.]doi:10.3969/j.issn.1000-4440.2023.01.017.
黄玉章,林旋,王全溪,谢建强,陈佳铭,赵堇,林树根.2010.黄芪多糖对罗非鱼肠绒毛形态结构及肠道免疫细胞的影响[J].动物营养学报,22(1):108-116.[Huang Y Z,Lin X,Wang Q X,Xie J Q,Chen J M,Zhao J,Lin S G.2010.Effects of Astragalus polysaccharide on structure of intestinal villus and intestinal immunocyte of tilapia[J].Chinese Journal of Animal Nutrition,22(1):108-116.]doi:10.3969/j.issn.1006-267x.2010.01.017.
蒋鑫涛,陈有铭,黄鉴鹏,欧光海,温震威,李豫,马骞,陈刚.2023.复合益生菌对杂交石斑鱼生长性能、抗氧化能力和肠道健康的影响[J].广东海洋大学学报,43(5):81-91.[Jiang X T,Chen Y M,Huang J P,Ou G H,Wen Z W,Li Y,Ma Q,Chen G.2023.Effects of compound probio-tics on growth performance,antioxidant capacity and intes‐tinal health of hybrid grouper(Epinephelusfuscogutatus♀×Epinephelus polyphekadion♂)[J].Journal of Guang‐dong Ocean University,43(5):81-91.]doi:10.3969/j.issn.1673-9159.2023.05.011.
李存玉,徐永江,柳学周,杨洪军,史宝,史学营,朱学武.2015.池塘和工厂化养殖牙鲆肠道菌群结构的比较分析[J].水产学报,39(2):245-255.[Li C Y,Xu Y J,Liu X Z,Yang H J,Shi B,Shi X Y,Zhu X W.2015.Comparative analysis of composition,diversity and origin of intestinal bacterial community in pond-and indoor-tank-culture Japa‐nese flounder(Paralichthys olivaceus)[J].Journal of Fis-heries of China,39(2):245-255.]doi:10.3724/SP.J.1231.2015.59484.
李英英,陈曦,李素一,李盼,李艳虹,宋铁英.2015.肠道消化吸收相关因子对大黄鱼生长速度的影响[J].大连海洋大学学报,30(3):271-275.[Li Y Y,Chen X,Li S Y,Li P,Li Y H,Song T Y.2015.Influence of factors related to the intestinal digestion and absorption on growth of cultured large yellow croaker Pseudosciaenacrocea[J].Journal of Dalian Ocean University,30(3):271-275.]doi:10.16535/j.cnki.dlhyxb.2015.03.007.
李英英,陈曦,宋铁英.2017.不同生长速度的大黄鱼肠道菌群结构的差异[J].大连海洋大学学报,32(5):509-513.[Li YY,Chen X,Song T Y.2017.Differences in intestinalflora of cultured large yellow croaker Pseudosciaenacro-cea with different growth rates[J].Journal of Dalian Ocean University,32(5):509-513.]doi:10.16535/j.cnki.dlhyxb.2017.05.002.
刘敏,张辉.2008.鱼类消化酶的研究进展[J].渔业经济研究,(6):6-10.[Liu M,Zhang H.2008.Research progress on the digestive enzyme of the fish[J].Fisheries Economy Research,(6):6-10.]doi:10.3969/j.issn.1674-9189.2008.06.002.
卢歌雪.2023.肝硬化患者肠道脱硫弧菌分离鉴定及其比较研究[D].无锡:江南大学.[Lu G X.2023.Isolation,iden-tification and comparison of intestinal Desulfovibrio in patients with liver cirrhosis[D].Wuxi:Jiangnan Univer-sity.]doi:10.27169/d.cnki.gwqgu.2023.000629.
罗君,付伟杰,杨二军,黄建盛,谢瑞涛,陈刚.2022.槲皮素对杂交石斑鱼生长性能、抗氧化能力和肠道菌群的影响[J].广东海洋大学学报,42(4):13-22.[Luo J,Fu W J,Yang E J,Huang J S,Xie R T,Chen G.2022.Effects of quercetin on growth performance,antioxidant capacity andintestinal microflora of hybrid grouper(Epinephelusfus-coguttatus♀×Epinephelus polyphekadion♂)[J].Journal of Guangdong Ocean University,42(4):13-22.]doi:10.3969/j.issn.1673-9159.2022.04.002.
麦浩彬,郭鑫伟,王金港,迟淑艳,董晓慧,杨奇慧,刘泓宇,章双.2020.摄食不同水平饲料蛋白质对珍珠龙胆石斑鱼幼鱼肠道组织形态和菌群组成的影响[J].大连海洋大学学报,35(1):63-70.[Mai H B,Guo X W,Wang J G,Chi S Y,Dong X H,Yang Q H,Liu H Y,Zhang S.2020.Effects of dietary protein levels on intestinal tract histomor-phology and microflora composition in juvenile pearl gen-tian grouper(Epinephelus lanceolatu♂×E.fuscoguttatus♀)[J].Journal of Dalian Ocean University,35(1):63-70.]doi:10.16535/j.cnki.dlhyxb.2019-123.
孟晓林,李文均,聂国兴.2019.鱼类肠道菌群影响因子研究进展[J].水产学报,43(1):143-155.[Meng X L,Li W J,Nie G X.2019.Effect of different factors on the fish intes-tinal microbiota[J].Journal of Fisheries of China,43(1):143-155.]doi:10.11964/jfc.20181011476.
米海峰,孙瑞健,张璐,李宝圣,王武刚,吴业阳,王用黎.2015.鱼类肠道健康研究进展[J].中国饲料,(15):19-22.[Mi H F,Sun R J,Zhang L,Li B S,Wang W G,Wu Y Y,Wang Y L.2015.Research progress of fish intestinal health[J].China Feed,(15):19-22.]doi:10.15906/j.cnki.cn11-2975/s.20151505.
钱永生,陈红林,杜金星,刘至治,王成辉.2019.4种体色瓯江彩鲤的生长、摄食和呼吸特性差异及其相关性分析[J].中国水产科学,26(4):695-702.[Qian Y S,Chen H L,Du J X,Liu Z Z,Wang C H.2019.Comparison of growth,feeding and respiration characteristics and their correlation among four color patterns in Oujiang color carp[J].Jour-nal of Fishery Sciences of China,26(4):695-702.]doi:10.3724/SP.J.1118.2019.18367.
饶刘瑜,李学梅,李星浩,朱文根,余育和,颜庆云.2018.转基因鲤鱼与对照鲤肠道微生物群落差异研究[J].水生生物学报,42(2):349-355.[Rao LY,Li X M,Li X H,Zhu W G,Yu Y H,Yan Q Y.2018.Comparison between the intes-tinal bacterial communities of the transgenic common carp and the controls[J].Acta Hydrobiologica Sinica,42(2):349-355.]doi:10.7541/2018.044.
商宝娣,杨星,李正友,张效平.2015.希瓦氏菌的研究进展[J].福建农业,(7):152-154.[Shang B D,Yang X,Li Z Y,Zhang X P.2015.Research progress on Shewanella[J].Fujian Agriculture,(7):152-154.]
田宏杰,庄平,高露姣.2006.生态因子对鱼类消化酶活力影响的研究进展[J].海洋渔业,28(2):158-162.[Tian H J,Zhuang P,Gao L J.2006.Advances on the studies of the effect of ecological factors on activities of digestive enzymes of fish[J].Marine Fisheries,28(2):158-162.]doi:10.3969/j.issn.1004-2490.2006.02.013.
王飞飞,王夏雯,金倩,张智慧,王泽平,田胜营,王信海.2022.温度对克氏原螯虾肠道菌群结构的影响[J].江苏农业学报,38(1):157-164.[Wang F F,Wang X W,Jin Q,Zhang Z H,Wang Z P,Tian S Y,Wang X H.2022.Effects of temperature on gut microbiota structure of Procambarus clarkii[J].Jiangsu Journal of Agricultural Sciences,38(1):157-164.]doi:10.3969/j.issn.1000-4440.2022.01.019.
王蕾.2017.鲤鱼早期发育阶段肠道菌群的分析及其免疫相关性研究[D].济南:山东师范大学.[Wang L.2017.Analysis of gut microflora in early developmental stage and its relationship with immunity in common carp,Cypri-nus carpio[D].Jinan:Shandong Normal University.]
王若愚,孙博,曹顶臣,孙志鹏,王念民,胡炜,张颖,许式见.2023.达氏鳇和施氏鲟生长差异群体肠道菌群特征研究[J].中国水产科学,30(9):1093-1101.[Wang R Y,Sun B,Cao D C,Sun Z P,Wang N M,Hu W,Zhang Y,Xu S J.2023.Gut microbiota of Huso dauricus and Acipenser schrencki populations with different growth rates[J].Jour-nal of Fishery Sciences of China,30(9):1093-1101.]doi:10.12264/JFSC2023-0208.
王沈同.2023.草鱼生长差异肠道菌群鉴定及短链脂肪酸受体基因功能研究[D].上海:上海海洋大学.[Wang S T.2023.Identification of intestinal microbiotas in grass carp with different growth and functional research of short-chain fatty acid receptor gene[D].Shanghai:Shang-hai Ocean University.]doi:10.27314/d.cnki.gsscu.2021.000531.
王悦,赵盼月,陈学豪,翟少伟.2021.精养池模式下不同生长速度花鳗鲡的肠道菌群比较研究[J].饲料工业,42(4):48-52.[Wang Y,Zhao P Y,Chen X H,Zhai S W.2021.The comparative research on intestinal flora of Anguilla marmorata with different growth rates under intensive cul-ture ponds condition[J].Feed Industry,42(4):48-52.]doi:10.13302/j.cnki.fi.2021.04.009.
徐革锋,陈侠君,杜佳,牟振波.2009.鱼类消化系统的结构、功能及消化酶的分布与特性[J].水产学杂志,22(4):49-55.[Xu G F,Chen X J,Du J,Mou Z B.2009.Fish diges-tive system:It’s structure,function and the distributions and characteristics of digestive enzymes[J].Chinese Jour-nal of Fisheries,22(4):49-55.]doi:10.3969/j.issn.1005-3832.2009.04.013.
余友斌,黄温赟,崔铭超.2023.养殖密度对大黄鱼生长、血清生化、营养成分、消化酶和代谢酶活力的影响[J].渔业现代化,50(3):64-71.[Yu Y B,Huang W Y,Cui M C.2023.Effects of stocking densities on growth performance,nutrient composition,serum biochemical,digestive and metabolic enzymes activities of large yellow croaker(Lari-michthyscrocea)[J].Fishery Modernization,50(3):64-71.]doi:10.3969/j.issn.1007-9580.2023.03.008.
袁禹惠,龚埜,黄岩,李松林.2023.饲料DHA与EPA质量比对斜带石斑鱼稚鱼生长性能、体组成及消化酶活力的影响[J].广东海洋大学学报,43(6):1-8.[Yuan Y H,Gong Y,Huang Y,Li S L.2023.Effects of dietary DHA/EPA mass ratio on growth performance,body composition and digestive enzyme activity of larval grouper(Epinephelus coioides)[J].Journal of Guangdong Ocean University,43(6):1-8.]doi:10.3969/j.issn.1673-9159.2023.06.001.
翟少伟,史庆超,陈学豪.2016.饲料中添加抗菌肽Surfactin对吉富罗非鱼肠道健康的影响[J].水生生物学报,40(4):823-829.[Zhai S W,Shi Q C,Chen X H.2016.Effect of dietary antimicrobial peptides-Surfactin supplementation on parameters of intestinal health indices of genetically improved farmed tilapia(Gift,Oreochromis niloticus)[J].Acta Hydrobiologica Sinica,40(4):823-829.]doi:10.7541/2016.106.
张小明,张婷婷,张贞贞,李菁菁,赵旺生.2024.不同饲养方式对南江黄羊肠道菌群结构及血清免疫指标的影响[J].南方农业学报,55(2):334-345.[Zhang X M,Zhang T T,Zhang Z Z,Li J J,Zhao W S.2024.Effects of different feeding methods on the intestinal flora structure and serum immune indexes of Nanjiang yellow goat[J].Journal of Southern Agriculture,55(2):334-345.]doi:10.3969/j.issn.2095-1191.2024.02.004.
张燕玉,韩卓然,孙敬锋,吕爱军,胡秀彩,刘军锋.2019.海藻希瓦氏菌感染对半滑舌鳎肠道菌群结构及相关功能基因表达的影响[J].南方农业学报,50(10):2300-2307.[Zhang Y Y,Han Z R,Sun J F,LüA J,Hu X C,Liu J F.2019.Effects of infection with Shewanella algae on the microbial communities and expression of related func-tional genes in the intestine of Cynoglossussemilaevis[J].Journal of Southern Agriculture,50(10):2300-2307.]doi:10.3969/j.issn.2095-1191.2019.10.21.
张云龙,张海龙,王凌宇,顾贝易,樊启学.2017.鱼类早期发育阶段异速生长及核酸、消化酶变化的研究进展[J].中国水产科学,24(3):648-656.[Zhang Y L,Zhang H L,Wang LY,Gu B Y,Fan Q X.2017.Allometric growth and ontogenetic changes in nucleic acids and digestive enzy-mes during the early life stage in fish species[J].Journal of Fishery Sciences of China,24(3):648-656.]doi:10.3724/SP.J.1118.2017.16210.
赵柳兰,龙亚男,罗杰,刘巧,周剑,杜军,周亚,杨佰维,杨淞.2021.池塘和稻田两种养殖模式下建鲤肠道菌群、免疫酶活性及肌肉氨基酸比较分析[J].中国水产科学,28(1):48-56.[Zhao L L,Long Y N,Luo J,Liu Q,Zhou J,Du J,Zhou Y,Yang B W,Yang S.2021.Analysis and com-parison of intestinal microbiota,immune enzyme activi-ties,and muscle flavor of Jian carp in two culture modes[J].Journal of Fishery Sciences of China,28(1):48-56.]doi:10.12264/JFSC2020-0148.
周鹤庭,徐永江,姜燕,崔爱君,王滨,柳学周.2022.两种养殖模式下黄条鰤幼鱼消化道菌群对生长的微生态调控作用[J].中国水产科学,29(10):1437-1448.[Zhou H T,XuY J,Jiang Y,Cui A J,Wang B,Liu X Z.2022.Micro eco-logical regulation of gastrointestinal microflora in the growth of yellowtail kingfish(Seriola lalandi)juveniles under indoor tank culture and cage culture modes[J].Jour-nal of Fishery Sciences of China,29(10):1437-1448.]doi:10.12264/JFSC2022-0185.
朱华平,苏换换,马冬梅,黄樟翰.2018.华南鲤选育品种与地方品种的遗传多样性比较分析[J].农业生物技术学报,26(8):1371-1381.[Zhu H P,Su H H,Ma D M,Huang Z H.2018.Comparative analysis of genetic diversity in Cyp-rinus carpio rubrofuscus among selective-breeding popula-tion and landraces[J].Journal of Agricultural Biotechno-logy,26(8):1371-1381.]doi:10.3969/j.issn.1674-7968.2018.08.010.
Bai N,Gu M,Liu M J,Jia Q,Pan S H,Zhang Z Y.2019.Corn gluten meal induces enteritis and decreases intestinal immunity and antioxidant capacity in turbot(Scophthal-mus maximus)at high supplementation levels[J].PLoS One,14(3):e213867.doi:10.1371/journal.pone.0213867.
Cao K L,Wang Y Y,Li M L,Zhang C Y,Lahaye L,Kabir Chowdhury M A,Li X Q,Leng X J.2022.Supplementa-tion of a multienzyme complex,an organic acid-essential oil complex,and prebiotic alone or in combination affects growth,nutrient utilization,and immune function of rain-bow trout(Oncorhynchus mykiss)[J].Aquaculture Nutri-tion,(1):1068537.doi:10.1155/2022/1068537.
Caspary W F.1992.Physiology and pathophysiology of intesti-nal absorption[J].The American Journal of Clinical Nutri-tion,55(1):299S-308S.doi:10.1093/ajcn/55.1.299s.
Chapagain P,Arivett B,Cleveland B M,Walker D M,Salem M.2019.Analysis of the fecal microbiota of fast-and slow-growing rainbow trout(Oncorhynchus mykiss)[J].BMC Genomics,20:788.doi:10.1186/s 12864-019-6175-2.
Chen K,Zhou X Q,Jiang W D,Wu P,Liu Y,Jiang J,Kuang S Y,Tang L,Tang W N,Zhang YA,Feng L.2018.Impaired intestinal immune barrier and physical barrier function by phosphorus deficiency:Regulation of TOR,NF-κB,MLCK,JNK and Nrf2 signalling in grass carp(Ctenopharyngodonidella)after infection with Aeromonas hydrophila[J].Fishamp;Shellfish Immunology,74:175-189.doi:10.1016/j.fsi.2017.12.060.
Choi M J,Oh Y D,Kim Y R,Lim H K,Kim J M.2021.Intesti-nal microbial diversity is higher in pacific abalone(Halio-tis discus hannai)with slower growth rates[J].Aquacul-ture,537:736500.doi:10.1016/j.aquaculture.2021.736500.Clarke S F,Murphy E F,O'Sullivan O,Lucey A J,HumphreysM,Hogan A,Hayes P,O'Reilly M,Jeffery I B,Wood-Martin R,Kerins D M,Quigley E,Ross R P,O'Toole PW,Molloy M G,Falvey E,Shanahan F,Cotter P D.2014.
Exercise and associated dietary extremes impact on gut microbial diversity[J].Gut,63(12):1913-1920.doi:10.1136/gutjnl-2013-306541.
Comabella Y,Mendoza R,Aguilera C,Carrillo O,Hurtado A,García-Galano T.2006.Digestive enzyme activity duringearly larval development of the cubangarAtractosteus tris-toechus[J].Fish Physiology and Biochemistry,32:147-157.doi:10.1007/s 10695-006-0007-4.
Dawood M A O.2021.Nutritional immunity of fish intestines:Important insights for sustainable aquaculture[J].Reviews in Aquaculture,13(1):642-663.doi:10.1111/raq.12492.
DordevićD,JančíkováS,VítězováM,Kushkevych I.2021.Hydrogen sulfide toxicity in the gut environment:Meta-analysis of sulfate-reducing and lactic acid bacteria in inflammatory processes[J].Journal of Advanced Research,27:55-69.doi:10.1016/j.jare.2020.03.003.
Eckburg P B,Bik E M,Bernstein C N,Purdom E,Dethlefsen L,Sargent M,Gill S R,Nelson K E,Relman D A.2005.Diversity of the human intestinal microbial flora[J].Scien-ce,308(5728):1635-1638.doi:10.1126/science.1110591.
Eichmiller J J,Hamilton M J,Staley C,Sadowsky M J,Sorensen P W.2016.Environment shapes the fecal micro‐biome of invasive carp species[J].Microbiome,4:44.doi:10.1186/s40168-016-0190-1.
Ghanbari M,Kneifel W,Domig K J.2015.A new view of the fish gut microbiome:Advances from next-generation sequencing[J].Aquaculture,448:464-475.doi:10.1016/j.aquaculture.2015.06.033.
Hooper L V,Macpherson A J.2010.Immune adaptations that maintain homeostasis with the intestinal microbiota[J].Na-ture Reviews Immunology,10(3):159-169.doi:10.1038/nri2710.
Hu C H,Xiao K,Jiao L F,Song J.2014.Effects of zinc oxidesupported on zeolite on growth performance,intestinal bar‐rier function and digestive enzyme activities of Nile tilapia[J].Aquaculture Nutrition,20(5):486-493.doi:10.1111/anu.12101.
Logue J B,Stedmon C A,Kellerman AM,Nielsen N J,Anders‐son A F,Laudon H,Lindström E S,Kritzberg E S.2015.Experimental insights into the importance of aquatic bacte‐rial community composition to the degradation of dis‐solved organic matter[J].The ISME Journal,10(3):533-545.doi:10.1038/ismej.2015.131.
Luan Y Y,Li M,Zhou W,Yao YY,Yang Y L,Zhang Z,RingøE,Erik Olsen R,Liu Clarke J,Xie S Q,Mai K S,Ran C,Zhou Z G.2023.The fish microbiota:Research progress and potential applications[J].Engineering,29:137-146.doi:10.1016/j.eng.2022.12.011.
Pérez T,Balcázar J L,Ruiz-Zarzuela I,Halaihel N,Vendrell D,de Blas I,Múzquiz J L.2010.Host-microbiota interactions within the fish intestinal ecosystem[J].Mucosal Immuno-logy,3(4):355-360.doi:10.1038/mi.2010.12.
Roeselers G,Mittge E K,Stephens W Z,Parichy D M,Cava-naugh C M,Guillemin K,Rawls J F.2011.Evidence for a core gut microbiota in the zebrafish[J].The ISME Jour‐nal,5(10):1595-1608.doi:10.1038/ismej.2011.38.
Sugita H,Shibuya K,Shimooka H,Deguchi Y.1996.Antibacte‐rial abilities of intestinal bacteria in freshwater cultured fish[J].Aquaculture,145(1-4):195-203.doi:10.1016/S0044-8486(96)01319-1.
Sun Y Z,Yang H L,Ling Z C,Chang J B,Ye J D.2009.Gut microbiota of fast and slow growing grouper Epinephelus coioides[J].African Journal of Microbiology Research,3(11):713-720.
Tran N T,Zhang J,Xiong F,Wang G T,Li W X,Wu S G.2018.Altered gut microbiota associated with intestinal di-sease in grass carp(Ctenopharyngodonidellus)[J].World Journal of Microbiologyamp;Biotechnology,34:71.doi:10.1007/s 11274-018-2447-2.
Vancamelbeke M,Vermeire S.2017.The intestinal barrier:A fundamental role in health and disease[J].Expert Review of Gastroenterologyamp;Hepatology,11(9):821-834.doi:10.1080/17474124.2017.1343143.
Wang A R,Ran C,RingøE,Zhou Z G.2018.Progress in fish gastrointestinal microbiota research[J].Reviews in Aqua‐culture,10(3):626-640.doi:10.1111/raq.12191.
Wu S G,Tian J Y,Gatesoupe F J,Li W X,Zou H,Yang B J,Wang G T.2013.Intestinal microbiota of gibel carp(Carassius auratus gibelio)and its origin as revealed by 454 pyrosequencing[J].World Journal of Microbiology and Biotechnology,29(9):1585-1595.doi:10.1007/s11274-013-1322-4.
Xiong J B,Nie L,Chen J.2019.Current understanding on the roles of gut microbiota in fish disease and immunity[J].Zoological Research,40(2):70-76.doi:10.24272/j.issn.2095-8137.2018.069.
Zhang H L,Ran C,Teame T,Ding Q W,Hoseinifar S H,Xie M X,Zhang Z,Yang Y L,Olsen R E,Gatlin D M,RingøE,Duan M,Zhou Z G.2020.Research progress on gut health of farmers teleost fish:A viewpoint concerning the intestinal mucosal barrier and the impact of its damage[J].Reviews in Fish Biology and Fisheries,30(4):569-586.doi:10.1007/s 11160-020-09614-y.
Zhang Y,Wen B,David M A,Gao J Z,Chen Z Z.2021.Com‐parative analysis of intestinal microbiota of discus fish(Symphysodonharaldi)with different growth rates[J].Aquaculture,540:736740.doi:10.1016/j.aquaculture.2021.736740.
Zhao Z M,Zhao H,Zhang L,Huang Z P,Ke H Y,Liu Y,Duan Y L,Li H D,Wang X Y,Li Q.2023.Integrated analysis of how gender and body weight affect the intestinal microbial diversity of Gymnocyprischilianensis[J].Scientific Re-ports,13:8811.doi:10.1038/s41598-023-35600-y.
(责任编辑兰宗宝)

