One-step Solvothermal Synthesis of Strontium-doped Ultralong Hydroxyapatite Nanowires
SUN Tuanwei, ZHU Yingjie
One-step Solvothermal Synthesis of Strontium-doped Ultralong Hydroxyapatite Nanowires
SUN Tuanwei1,2, ZHU Yingjie1,2
(1. Shanghai Institute of Ceramics, Chinese Academy of Sciences, Shanghai 200050, China; 2. Center of Materials Science and Optoelectronics Engineering, University of Chinese Academy of Sciences, Beijing 100049, China)
Ultralong hydroxyapatite nanowires (UHANWs) exhibit great potential in constructing different kinds of biomaterials such as the highly flexible biomedical paper and elastic porous scaffolds for various biomedical applications. Moreover, strontium (Sr), a trace elementin human body, plays an important role in bone metabolism. In this study, Sr-doped UHANWs (Sr-UHANWs) with various Sr/(Sr+Ca) molar ratios have been successfully prepared by the one-step oleate precursor solvothermal method. The effects of the Sr/(Sr+Ca) molar ratio on the morphology and crystal phase of the Sr-UHANWs were investigated. The as-prepared Sr-UHANWs exhibit high flexibility and ultralong 1D nanostructure. Moreover, the energy dispersive spectroscopy, X-ray powder diffraction, and Fourier transform infrared spectroscopy of the as-prepared samples reveal that Sr element has been successfully incorporated in UHANWs. The preparation method developed in this work may be suitable for the synthesis of Sr-UHANWs with Sr/(Sr+Ca) molar ratios ranging from 0 to 100 %, which may enlarge the biomedical applications of UHANWs such as bone and teeth defect repair.
hydroxyapatite; strontium; nanowire; solvothermal; biomaterials
The synthetic hydroxyapatite (HAP, Ca10(PO4)6(OH)2) is chemically similar to the inorganic component of bone and teeth, which endows it with excellent biocompatibility, good osteoconductivity and osteoinductivity[1-2]. HAP-based biomaterials have been intensively investigated and applied in various biomedical fields, such as bone and teeth defect repair, and drug delivery[3-4]. Strontium (Sr) is an important trace element in human body and essential in bone metabolism by regulating bone formation and resorption[5-6]. It was reported that Sr2+ions could stimulate the differentiation of osteoblasts and inhibit the differentiation of osteoclast[7]. Previous reports indicated that the incorporation of Sr2+ions into HAP could promote bone regeneration and bone defect repair[5,8].
Compared with other HAP nanostructured materials such as HAP nanoparticles, HAP microspheres, and 1D HAP nanorods[2-3,9-10], ultralong hydroxyapatite nanowires (UHANWs) with lengths of several hundred micrometres have high flexibility and ultralong 1D nanostructure, showing great potential in constructing different types of multifunctional biomaterials such as the highly flexible biomedical paper and elastic porous scaffolds[11-16]. Moreover, the Sr-doped UHANWs (Sr-UHANWs) can combine excellent advantages of both strontium and UHANWs, which will remarkably enhance the bioactivity and enlarge the application potentials of UHANWs- based biomaterials in various biomedical fields.
Previously, Xu[17]synthesized Sr-doped HAP whiskers using acetamide as a homogeneous precipitation reagent by hydrothermal treatment. Zhang,[18]hydrothermally synthesized Sr and Si co-doped HAP nanowires using Sr-containing calcium silicate as the precursor. However, to the best of our knowledge, the synthesis of Sr-doped ultralong HAP nanowires by the oleate precursor solvothermal method has not been reported in the literature.
In this study, the Sr-UHANWs with different Sr/(Sr+Ca) molar ratios have been synthesized by the one-step oleate precursor solvothermal method. The as-prepared Sr-UHANWs exhibit ultralong 1D nanostructure and high flexibility. Importantly, the preparation method developed in this work is simple, and may be applicable for the synthesis of other metal ions-doped UHANWs.
1 Experimental section
1.1 Materials
Oleic acid and SrCl2·6H2O were purchased from Aladdin Industrial Co. Ltd., and other chemicals were purchased from Sinopharm Chemical Reagent Co. Ltd. All chemicals were used as received without further purification.
1.2 Synthesis of Sr-UHANWs
The strontium-doped ultralong hydroxyapatite nanowires (Sr-UHANWs) with different Sr/(Sr+Ca) molar ratios were prepared by a facile one-step oleate precursor solvothermal method[19-21]. In a typical experiment for the synthesis of Sr-UHANWs with a Sr/(Sr+Ca) molar ratio of 0.4, a mixture of deionized water (135 mL), methanol (60 mL) and oleic acid (105 mL) was prepared under vigorous mechanical stirring in an ice-water bath. Then, 150 mL of NaOH (10.500 g) aqueous solution, 120 mL of aqueous solution containing 1.998 g of CaCl2and 3.199 g of SrCl2·6H2O, and 180 mL of NaH2PO4·2H2O (9.360 g) aqueous solution were separately added into the above mixture. After continuous stirring for 30 min, the resulting suspension was transferred into a Teflon-lined stainless steel autoclave (1000 mL), sealed and heated at 180 ℃ for 24 h. After cooling to room temperature, the product was washed with ethanol and deionized water 3 times, and dried, respectively. The as-prepared Sr-UHANWs with a Sr/(Sr+Ca) molar ratio of 0.4 is labeled as Sr40-UHANWs.
Similarly, the undoped UHANWs and Sr100-UHANWswere prepared under the same conditions but using 3.330 g of CaCl2and 7.999 g of SrCl2·6H2O, respectively. The Sr5-UHANWs, Sr20-UHANWs and Sr90-UHANWs were also prepared using the above method.
1.3 Characterization
Scanning electron microscopy (SEM) images and energy-dispersive spectroscopy (EDS) elemental mapping patterns of the as-prepared Sr-UHANWs with different Sr/(Sr+Ca) molar ratios were recorded with a field- emission scanning electron microscope (FEI Magellan 400, USA). The X-ray powder diffraction (XRD) patterns of Sr-UHANWs with different Sr/(Sr+Ca) molar ratios were recorded with an X-ray diffractometer (Rigaku D/max 2550 V, Cu Kαradiation,=0.154178 nm). Fourier transform infrared (FT-IR) spectra of Sr-UHANWs with different Sr/(Sr+Ca) molar ratios were taken using a FT-IR spectrometer (FTIR-7600, Lambda Scientific, Australia).
2 Results and discussion
2.1 Crystal phase analysis of Sr-UHANWs
Fig. 1 shows the XRD patterns of the as-prepared Sr-UHANWs with different Sr/(Ca+Sr) molar ratios. The XRD pattern of the undoped UHANWs can be indexed to a single crystal phase of hydroxyapatite with a hexagonal structure (Ca10(PO4)6(OH)2, JCPDS 09-0432). Moreover, the XRD pattern of Sr100-UHANWs can be indexed to strontium phosphate (Sr3(PO4)2, JCPDS 24-1008) and strontiumapatite (Sr10(PO4)6(OH)2, JCPDS 33-1348) with a hexagonal structure. The XRD patterns of Sr-UHANWs with different Sr/(Sr+Ca) molar ratios can be indexed to a hexagonal apatite crystal phase (Fig. 1(b-e)). Compared with the XRD pattern of the undoped UHANWs, all diffraction peaks of Sr-UHANWs with different Sr/(Sr+Ca) molar ratios shift to a lower diffraction angle, and the shift becomes larger as the Sr2+substitution ratio increases. The diffraction peak shift indicates the crystal lattice expansion in Sr-UHANWs, which is caused by the substitution of smaller Ca2+ions with larger Sr2+ions[22]. These experimental results demonstrate that the as-prepared Sr-UHANWs have a chemical compositions of Ca10–xSr(PO4)6(OH)2(where 0≤≤10).
2.2 Morphologies of Sr-UHANWs
As shown in Fig. 2(a-d), the morphology of the undoped UHANWs is similar to that of Sr40-UHANWs. Both undoped UHANWs and Sr40-UHANWs have highly flexible 1D nanostructure and ultrahigh aspect ratios. The energy-dispersive spectroscopy (EDS) elemental mapping patterns of Sr40-UHANWs (Fig. 2(e)) further confirm that the Sr, Ca, P, and O elements are homogeneously distributed in the Sr40-UHANWs, and Sr2+ions have been successfully doped into the UHANWs.
As shown in Fig. 3, the effect of Sr/(Sr+Ca) molar ratios on the morphology of the as-prepared Sr-UHANWs was further investigated. Interestingly, Sr5-UHANWs, Sr20-UHANWs, Sr90-UHANWs, and Sr100-UHANWs exhibit ultralong 1D nanostructure, which is similar to that of undoped UHANWs and Sr40-UHANWs, indicating that the oleate precursor solvothermal method reported herein may be suitable for the synthesis of Sr-UHANWs with Sr/(Ca+Sr) molar ratios ranging from 0 to 100%. High-magnification SEM images of Sr-UHANWs display that the surface of Sr-UHANWs is smooth, and in many cases Sr-UHANWs can self-assemble into nanowire bundles along the longitudinal direction. SEM images in Figs. 2 and 3, display that many Sr-UHANWs can bend owing to their high flexibility and ultralong nanostructure.
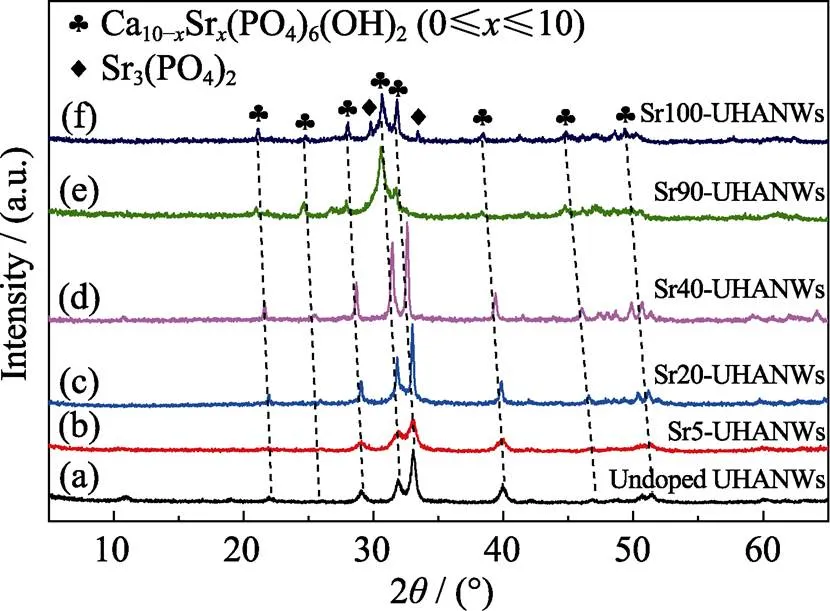
Fig. 1 XRD patterns of the as-prepared Sr-UHANWs with different Sr/(Sr+Ca) molar ratios
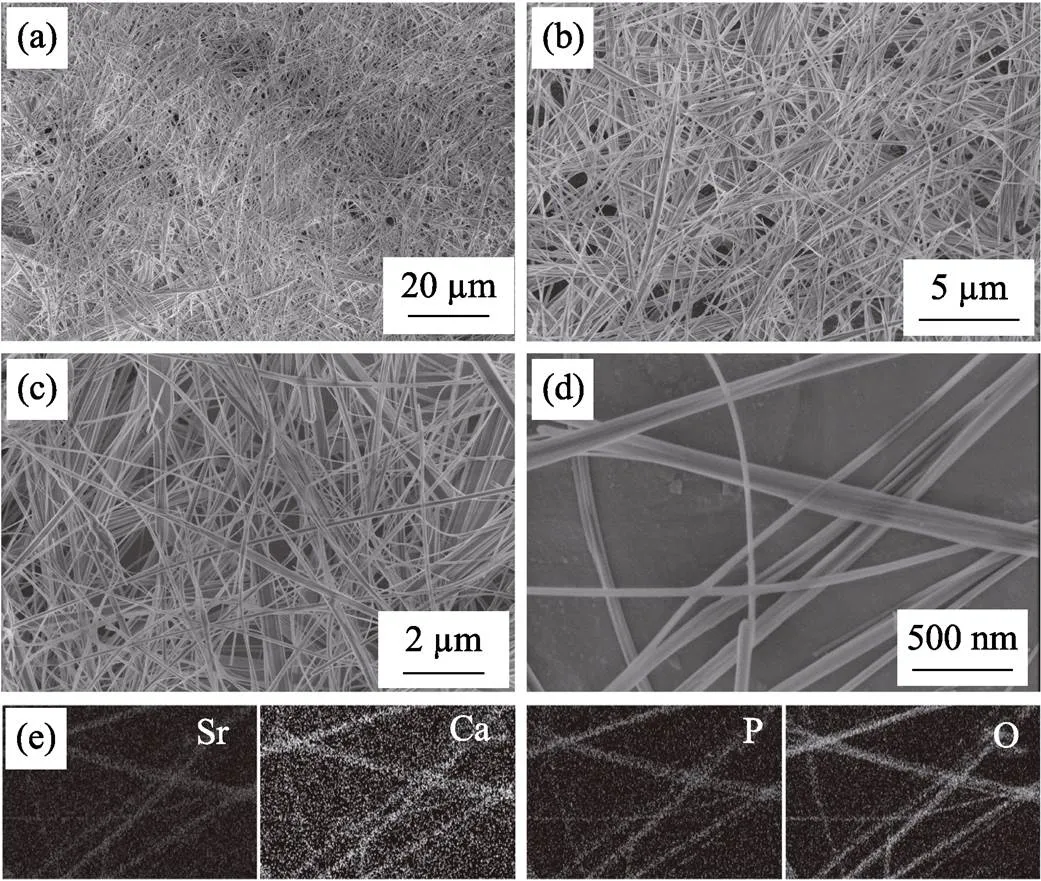
Fig. 2 SEM images of undoped UHANWs (a, b) and Sr40-UHANWs (c, d), and energy-dispersive spectroscopy (EDS) elemental mapping of Sr, Ca, P and O elements in Sr40-UHANWs (e)
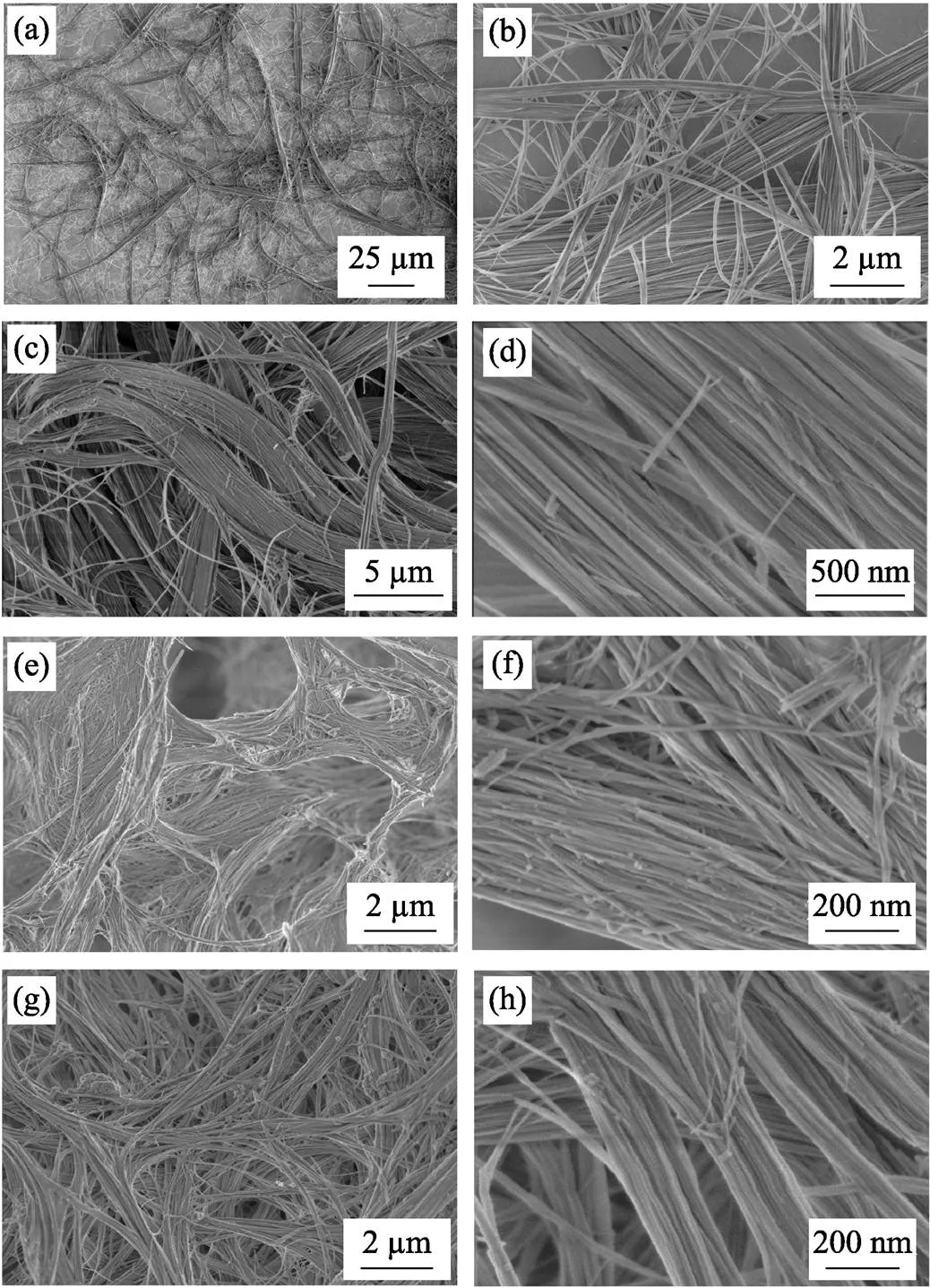
Fig. 3 SEM images of strontium-doped UHANWs with different Sr/(Sr+Ca) molar ratios(a, b) Sr5-UHANWs; (c, d) Sr20-UHANWs; (e, f) Sr90-UHANWs; (g, h) Sr100-UHANWs
According to our previously reported formation mechanism of undoped UHANWs[12,14,19], we propose that the formation of Sr-UHANWs is an oleate precursor solvothermal process. The precursors of calcium oleate and strontium oleate are first formed in the reaction system after the addition of oleic acid, NaOH, CaCl2, and SrCl2·6H2O. Then, the precursors of calcium oleate and strontium oleate transform to Sr-UHANWs after the addition of NaH2PO4·2H2O under the solvothermal conditions.
2.3 FT-IR analysis of Sr-UHANWs
The FTIR analysis was performed to characterize the Sr-UHANWs samples. As shown in Fig. 4, the broad absorption peak of all the samples at around 3440 cm−1derives from the adsorbed water in the samples. The Sr2+substitution obviously influences the absorption peaks of hydroxyl (−OH) in Sr-UHANWs. As the Sr2+substitution ratio increases, the absorption peaks of the stretching mode (3572 cm−1) and librational mode (633 cm−1) of −OH in Sr-UHANWs decrease in intensity and broaden, indicating that the Sr2+substitution leads to the loss of −OH groups and structural disorder[23]. The absorption peaks at around 1097, 1032, 962, 602, and 561 cm−1belong to the PO43−group in the samples[24]. However, the absorption peaks of the PO43−group in the range of 1150–950 cm−1slightly shift to lower wave numbers as the Sr2+substitution ratio of Sr-UHANWs increases, which also demonstrates the increase of disorder around phosphate sites caused by the Sr incorporation[23]. The above experimental results are consistent with the XRD results, and further confirm that the Sr2+ions have been successfully incorporated into the HAP crystal structure.
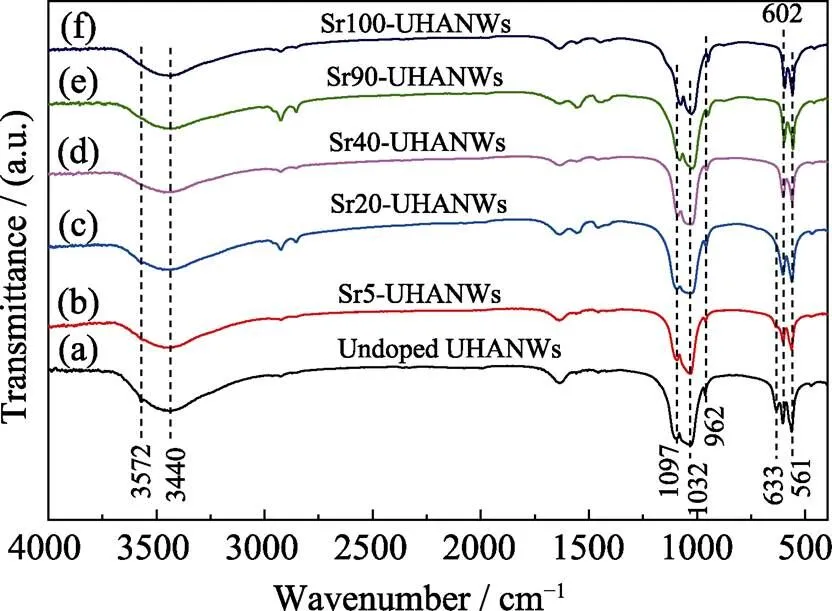
Fig. 4 FT-IR spectra of the as-prepared Sr-UHANWs with different Sr/(Sr+Ca) molar ratios
3 Conclusions
In summary, the strontium-doped ultralong hydroxyapatite nanowires (Sr-UHANWs) with different Sr/(Sr+Ca) molar ratios have been successfully synthesized using the one-step oleate precursor solvothermal method. The as-prepared Sr-UHANWs exhibit the ultralong 1D nanostructure and high flexibility. In addition, the EDS elemental mapping, XRD and FT-IR analyses confirm the successful incorporation of Sr2+ions into the crystal structure of UHANWs. Thus, the one-step oleate precursor solvothermal method developed in this study may be suitable for the synthesis of Sr-UHANWs with Sr/(Sr+Ca) molar ratios ranging from 0 to 100%, which may broaden the biomedical applications of UHANWs such as bone and teeth defect repair.
[1] QI C, ZHU Y J, LU B Q,. Hydroxyapatite hierarchically nanostructured porous hollow microspheres: rapid, sustainable microwave-hydrothermal synthesis by using creatine phosphate as an organic phosphorus source and application in drug delivery and protein adsorption., 2013, 19(17): 5332–5341.
[2] YU W L, SUN T W, DING Z Y,. Copper-doped mesoporous hydroxyapatite microspheres synthesized by a microwave-hydrothermal method using creatine phosphate as an organic phosphorus source: application in drug delivery and enhanced bone regeneration., 2017, 5(5): 1039–1052.
[3] LU B Q, ZHU Y J. One-dimensional hydroxyapatite materials: preparation and applications., 2017, 95(11): 1091–1102.
[4] YU W L, SUN T W, QI C,. Enhanced osteogenesis and angiogenesis by mesoporous hydroxyapatite microspheres-derived simvastatin sustained release system for superior bone regeneration., 2017, 7: 44129.
[5] YANG F, YANG D Z, TU J,. Strontium enhances osteogenic differentiation of mesenchymal stem cells andbone formation by activating Wnt/catenin signaling., 2011, 29(6): 981–991.
[6] LIN K L, XIA L G, LI H Y,. Enhanced osteoporotic bone regeneration by strontium-substituted calcium silicate bioactive ceramics., 2013, 34(38): 10028–10042.
[7] BONNELYE E, CHABADEL A, SALTEL F,. Dual effect of strontium ranelate: stimulation of osteoblast differentiation and inhibition of osteoclast formation and resorption., 2008, 42(1): 129–138.
[8] HABIBOVIC P, BARRALET J E. Bioinorganics and biomaterials: bone repair., 2011, 7(8): 3013–3026.
[9] KANE R J, WEISS-BILKA H E, MEAGHER M J,. Hydroxyapatite reinforced collagen scaffolds with improved architecture and mechanical properties., 2015, 17: 16–25.
[10] ZHENG X Y, HUI J F, LI H,. Fabrication of novel biodegradable porous bone scaffolds based on amphiphilic hydroxyapatite nanorods., 2017, 75: 699–705.
[11] SUN T W, YU W L, ZHU Y J,. Hydroxyapatite nanowire@magnesium silicate core-shell hierarchical nanocomposite: synthesis and application in bone regeneration., 2017, 9(19): 16435–16447.
[12] SUN T W, ZHU Y J, CHEN F. Highly flexible multifunctional biopaper comprising chitosan reinforced by ultralong hydroxyapatite nanowires., 2017, 23(16): 3850–3862.
[13] SUN T W, ZHU Y J, CHEN F,. Ultralong hydroxyapatite nanowires/collagen scaffolds with hierarchical porous structure, enhanced mechanical properties and excellent cellular attachment., 2017, 43(17): 15747–15754.
[14] SUN T W, ZHU Y J, CHEN F,. Ultralong hydroxyapatite nanowire/collagen biopaper with high flexibility, improved mechanical properties and excellent cellular attachment., 2017, 12(6): 655–664.
[15] SUN T W, YU W L, ZHU Y J,. Porous nanocomposite comprising ultralong hydroxyapatite nanowires decorated with zinc-containing nanoparticles and chitosan: synthesis and application in bone defect repair., 2018, 24(35): 8809–8821.
[16] SUN T W, ZHU Y J, CHEN F. Hydroxyapatite nanowire/collagen elastic porous nanocomposite and its enhanced performance in bone defect repair., 2018, 8(46): 26133–26144.
[17] XU J Q, YANG Y Q, WAN R,. Hydrothermal preparation and characterization of ultralong strontium-substituted hydroxyapatite whiskers using acetamide as homogeneous precipitation reagent., 2014, 2014: 863137.
[18] ZHANG N, ZHAI D, CHEN L,. Hydrothermal synthesis and characterization of Si and Sr co-substituted hydroxyapatite nanowires using strontium containing calcium silicate as precursors., 2014, 37: 286–291.
[19] LU B Q, ZHU Y J, CHEN F. Highly flexible and nonflammable inorganic hydroxyapatite paper., 2014, 20(5): 1242–1246.
[20] ZHANG Y G, ZHU Y J, CHEN F,. Ultralong hydroxyapatite nanowires synthesized by solvothermal treatment using a series of phosphate sodium salts., 2015, 144: 135–137.
[21] JIANG Y Y, ZHU Y J, CHEN F,. Solvothermal synthesis of submillimeter ultralong hydroxyapatite nanowires using calcium oleate precursor in a series of monohydroxy alcohols., 2015, 41(4): 6098–6102.
[22] KAVITHA M, SUBRAMANIAN R, NARAYANAN R,. Solution combustion synthesis and characterization of strontium substituted hydroxyapatite nanocrystals., 2014, 253: 129–137.
[23] TERRA J, DOURADO E R, EON J G,. The structure of strontium-doped hydroxyapatite: an experimental and theoretical study., 2009, 11(3): 568–577.
[24] QI C, ZHU Y J, LU B Q,. Hydroxyapatite nanosheet-assembled porous hollow microspheres: DNA-templated hydrothermal synthesis, drug delivery and protein adsorption., 2012, 22(42): 22642–22650.
一步溶剂热法合成锶掺杂羟基磷灰石超长纳米线
孙团伟1,2, 朱英杰1,2
(1. 中国科学院 上海硅酸盐研究所, 上海 200050; 2. 中国科学院大学材料与光电研究中心, 北京 100049)
羟基磷灰石超长纳米线可用于构建不同种类的生物材料, 如高柔性生物医用纸和弹性多孔骨缺损修复支架, 在生物医学领域具有良好的应用前景。锶元素作为一种微量元素, 在骨代谢过程中起着重要作用。本研究通过一步溶剂热法合成了具有不同锶掺杂量的羟基磷灰石超长纳米线; 研究了不同锶掺杂量对羟基磷灰石超长纳米线的形貌和物相的影响。所制备的锶掺杂羟基磷灰石超长纳米线具有高柔韧性和超长一维纳米结构。能量色散谱、X射线粉末衍射和傅里叶变换红外光谱分析表明, 锶元素成功地掺杂到了羟基磷灰石超长纳米线中。本研究发展的制备方法可以制备锶/(锶+钙)摩尔比从0到100%任一比例的锶掺杂羟基磷灰石超长纳米线, 大幅拓展了羟基磷灰石超长纳米线在骨缺损修复和牙科修复等生物医学领域中的应用。
羟基磷灰石; 锶; 纳米线; 溶剂热; 生物材料
TQ174
A
date:2019-08-04;
date: 2019-09-23
National Natural Science Foundation of China (21875277)
SUN Tuanwei (1989–), male, PhD. E-mail: stwcsu@163.com
孙团伟(1989–), 男, 博士. E-mail: stwcsu@163.com
Corresponding author:ZHU Yingjie, professor. E-mail: y.j.zhu@mail.sic.ac.cn
朱英杰, 研究员. E-mail: y.j.zhu@mail.sic.ac.cn
1000-324X(2020)06-0724-05
10.15541/jim20190398

