新型红霉素类大环内酯衍生物的合成及抗菌活性研究
李立晶 高蕾 孙静 李晶 陈华 张雷,*
(1 华南理工大学 生物科学与工程学院,广州510006;2 广东省药品检验所,广州 510180)
大环内酯类抗生素是一类分子中含有14~16元大环内酯结构的抗生素,因其抗菌谱广、组织分布广泛、副作用较少且价格低廉,在医药领域和农药领域都有着广泛的应用[1-3]。第一代大环内酯类抗生素以红霉素为代表(图1),主要包括红霉素及其衍生物,如琥乙红霉素、硬脂酸红霉素等。该代抗生素抗菌谱广、组织分布广泛、药动学性质良好,但在胃酸等酸性条件下不稳定,易降解失活,并导致胃肠道不良反应[4]。第二代大环内酯类抗生素包括克拉霉素、氟红霉素、阿奇霉素等(图1)。分别针对红霉素的C-6、C-8和C-9等不同位点进行结构改造,极大地增强了酸稳定性、延长了半衰期、减缓了胃肠道不良反应[5]。 然而,随着耐药菌的不断出现,对新型抗生素的需求更加迫切。1977年,Allen等[6]发现,C-3上的克拉定糖并非大环内酯类抗生素发挥抗菌活性的必需基团,相反的,它还与诱导细菌产生耐药性相关,因此围绕C-3脱克拉定糖后进行结构修饰的第三代大环内酯类抗生素(主要包括酰内酯与酮内酯)应运而生。泰利霉素是目前唯一上市的第三代大环内酯类抗生素,它不仅拓宽了大环内酯类抗生素的抗菌谱,而且还降低了细菌耐药性,大大增强了抗菌活性。但由于其严重的肝毒性,美国食品药品监督管理局(FDA)不得不对其适应症进行限制[7-9]。
基于大环内酯类抗生素的构效关系,本文设计两类大环内酯类衍生物:①以红霉素为原料,先合成氟红霉素,并去除C-3位克拉定糖,以期降低耐药性,对产生的C-3位羟基进行酯化反应弥补可能的抗菌活性的降低,同时C-11、C-12环合成碳酸酯增强抗菌活性[10],合成了衍生物5a~5k(图2);②以克拉霉素为原料去除C-3的克拉定糖,氧化为羰基,降低耐药性并提高对erm基因介导的耐药菌的抑制活性[11],并对C-11、C-12位进行环合衍生化[12-13],获得衍生物12a~12f(图2)。
1 实验部分
1.1 仪器与试剂
AscendTM400型核磁共振仪(德国Bruker公司),MAT-95XP型高分辨质谱仪(德国Bruker公司),FLUOstar®OPTIMA型多功能酶标仪(德国BMG Labtecg公司)。
1-氯甲基-4-氟-1,4-重氮化二环2.2.2辛烷双(四氟硼酸盐)(selectflour)采购自上海鹰谷信息科技有限公司,红霉素和克拉霉素由东阳光研究院提供,其它试剂除特殊说明外均为市售分析纯试剂。
1.2 化合物5a~5k的合成(图3)
红霉素(100.1g, 136.4 mmol)和1-氯甲基-4-氟-1,4-重氮化二环2.2.2辛烷双(四氟硼酸盐)(50.0g, 141.1mmol)溶解在400mL冰醋酸中,室温下搅拌,TLC监控反应,6h后基本反应完全。反应液用6mol/L的氢氧化钠溶液调节pH=10,二氯甲烷萃取,有机相依次用饱和碳酸氢钠溶液、饱和食盐水洗涤,无水硫酸钠干燥,过滤后减压浓缩得淡黄色固体,乙醇重结晶,得白色固体1(70.0g,收率68 %)。1H NMR(400MHz, DMSO-d6) δ(ppm): 4.81(d, J=5.8Hz, 1H), 4.77(dd, J=11.2, 2.4Hz, 1H), 4.72(d, J=4.3Hz, 1H), 4.39(s, 1H), 4.26~4.21(m, 2H), 4.18(d, J=7.3Hz, 1H), 4.08(s, 1H), 4.05~3.98(m, 3H), 3.54~3.48(m, 1H), 3.44(dd, J=7.0, 5.1Hz, 1H), 3.19(s, 3H), 3.14~3.08(m, 1H), 2.94~2.88(m, 1H), 2.87~2.85(m, 1H), 2.85~2.80(m, 1H), 2.71(dd, J=7.2, 4.9Hz, 1H), 2.47~2.42(m, 1H), 2.30~2.25(m, 1H), 2.22(s, 6H), 2.03~1.97(m, 1H), 1.94~1.89(m, 1H), 1.80~1.72(m, 1H), 1.66~1.60(m, 1H), 1.56(s, 3H), 1.48~1.45(m, 1H), 1.40(s, 3H), 1.35(s, 1H), 1.18~1.15(m, 3H), 1.14(m, 1H), 1.12(s, 3H), 1.10(s, 3H), 1.10 ~1.08(m, 6H), 1.07~1.05(m, 3H), 1.04~1.02(m, 3H), 0.78(t, J=7.3Hz, 3H)。

图1 红霉素、氟红霉素和克拉霉素的化学结构Fig.1 Chemical structures of erythromycin, flurithromycin and clarithromycin
图2 5a~5k及12a~12f的结构通式Fig.2 General structures of derivatives 5a~5k and 12a~12f
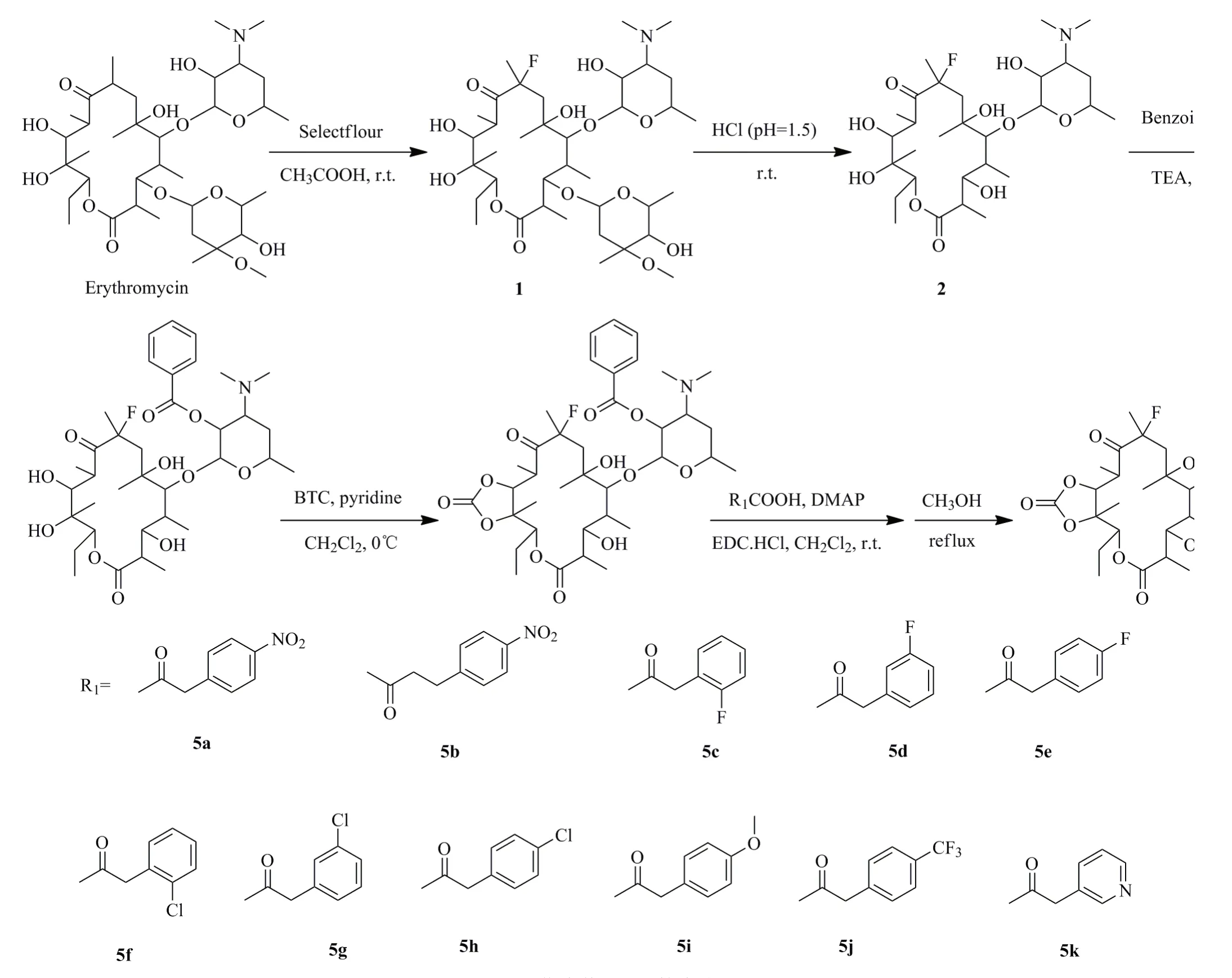
图3 化合物5a~5k的合成Fig.3 Syntheses of compounds 5a~5k
化合物1(70.0g, 93.0mmol)溶解在1200mL稀盐酸(pH=1.5)中,室温搅拌反应,TLC监控,24h后原料基本反应完全。反应液用氨水调节至pH=9。乙酸乙酯萃取,有机相依次用饱和碳酸氢钠溶液、饱和食盐水洗涤,无水硫酸钠干燥。过滤,滤液减压浓缩得白色固体,柱层析(V(二氯甲烷)/V(甲醇)=20:1)得白色固体2(40.0g,收率72%)。1H NMR(400MHz, DMSO-d6) δ(ppm): 5.12(s, 1H), 5.10(s, 1H), 4.48(d, J=7.3Hz, 1H), 4.12(s, 1H), 4.04(s, 1H), 3.69(d, J=10.6Hz, 2H), 3.56(s, 1H), 3.47~3.38(m, 1H), 3.20(dd, J=9.0, 8.3Hz, 1H), 3.09~2.99(m, 2H), 2.83(s, 3H), 2.22(s, 6H), 1.99(s, 1H), 1.98~1.90(m, 1H), 1.88~1.77(m, 2H), 1.75(s, 3H), 1.61(d, J=12.3Hz, 1H), 1.43~1.30(m, 2H), 1.19(s, 3H), 1.13(d, J=6.0Hz, 3H), 1.10~1.03(m, 9H), 1.01(d, J=6.5Hz, 3H), 0.73(t, J=7.1Hz, 3H)。
化合物2(40.0g,67.3mmol)溶解在250mL四氢呋喃中,0℃下加入苯甲酸酐(18.5g, 81.8mmol),搅拌溶解后在0.5h内缓慢滴加三乙胺9.50mL,加入DMAP(0.10g, 0.81mmol),搅拌0.5h后升至室温,TLC监控反应,48h后加入饱和碳酸氢钠溶液100mL,继续搅拌1h,静置分层,有机层依次用饱和碳酸氢钠溶液和饱和食盐水洗涤,无水硫酸钠干燥。过滤,滤液减压浓缩得白色固体,柱层析(V(二氯甲烷)/V(甲醇)=40:1)得白色固体3(42.0g,收率89 %)。1H NMR(400MHz, DMSO-d6) δ(ppm): 7.97(d, J=7.1Hz, 2H), 7.67~7.60(m, 1H), 7.53(t, J=7.6Hz, 2H), 5.25(d, J=6.8Hz, 1H), 5.06(dd, J=11.1, 2.3Hz, 1H), 4.86~4.81(m, 2H), 4.46(s, 1H), 4.08(s, 1H), 3.77(s, 1H), 3.58~3.48(m, 1H), 3.30(d, J=3.0Hz, 1H), 3.23~3.14(m, 1H), 2.88~2.78(m, 2H), 2.42~2.35(m, 1H), 2.16(s, 6H), 2.04~1.99(m, 1H), 1.96~1.87(m, 1H), 1.77(dd, J=14.4, 6.7Hz, 2H), 1.68(d, J=15.3Hz, 1H), 1.48~1.37(m, 4H), 1.35~1.26(m, 5H), 1.20~1.16(m, 3H), 1.06(d, J=6.6Hz, 3H), 1.02(d, J=6.7Hz, 3H), 0.87(s, 3H), 0.69(t, J=7.4Hz, 3H), 0.61(d, J=7.4Hz, 3H)。
化合物3(42.0g,60.3mmol)溶解在250mL二氯甲烷中,在0℃下加入吡啶2.1mL,缓慢滴加三光气(21.5g, 72.4mmol)的二氯甲烷溶液(50mL),缓慢滴加三乙胺30mL。继续搅拌5.5h,向反应瓶中加入少量的碎冰块猝灭反应。有机相依次用饱和碳酸氢钠溶液、饱和食盐水洗涤,无水硫酸钠干燥。过滤,滤液减压浓缩得黄色固体,柱层析(V(石油醚)/V(乙酸乙酯)=1:1)得淡黄色固体4(35.0g,收率80%)。1H NMR(400MHz, CDCl3) δ(ppm): 8.09(d, J=7.8Hz, 2H), 7.68(t, J=7.5Hz, 1H), 7.51(t, J=7.3Hz, 2H), 5.34~5.24(m, 1H), 4.90(d, J=9.3Hz, 1H), 4.86(s, 1H), 4.74(d, J=6.8Hz, 1H), 3.86~3.68(m, 2H), 3.63(s, 1H), 3.24(s, 1H), 2.79(dd, J=25.1, 3.8Hz, 6H), 2.60(t, J=13.4Hz, 1H), 2.41~2.30(m, 1H), 2.23(dd, J=23.0, 13.8Hz, 1H), 2.05(s, 1H), 1.99~1.91(m, 1H), 1.88~1.76(m, 2H), 1.76~1.69(m, 1H), 1.68~1.59(m, 6H), 1.57(s, 3H), 1.46(s, 3H), 1.36(d, J=5.3Hz, 3H), 1.12(d, J=6.6Hz, 3H), 0.88~0.79(m, 6H), 0.76(d, J=7.0Hz, 3H)。
化合物4(1.38mmol)溶解在二氯甲烷中,0℃下加入有机酸(1.7mmol)、EDC·HCl(1.7mmol)和DMAP(0.14mmol),继续搅拌0.5h后升至室温,反应4d后反应液用2mol/L的氢氧化钠溶液调节pH=8。有机相依次用饱和氯化铵溶液、饱和食盐水洗涤,无水硫酸钠干燥。过滤,减压浓缩除去溶剂,柱层析(V(石油醚)/V(乙酸乙酯)=1:1)得中间体。将上述中间体溶解在适量甲醇中,回流10h,减压浓缩除去溶剂,柱层析(V(二氯甲烷)/V(甲醇)=30:1)得目标产物5a~5k。
11,12-环碳酸酯-3-对硝基苯乙酸酯-8-氟-红霉素(5a),黄色固体(0.20g,收率18%)。1H NMR(400MHz, CDCl3) δ(ppm): 8.16(d, J=8.2Hz, 2H), 7.49(d, J=8.2Hz, 2H), 5.80(d, J=11.0Hz, 1H), 4.94(d, J=9.4Hz, 1H), 4.78(s, 1H), 4.56~4.28(m, 1H), 4.23(d, J=7.0Hz, 1H), 3.80(s, 2H), 3.64~3.51(m, 1H), 3.44(s, 1H), 3.36~3.23(m, 1H), 3.18~3.03(m, 2H), 2.90(s, 1H), 2.76(d, J=13.2Hz, 1H), 2.55(s, 6H), 2.32~2.20 (m, 2H), 2.08 (q, J=6.5Hz, 1H), 1.92~1.72 (m, 3H), 1.70 (s, 3H), 1.60 (d, J=23.1Hz, 4H), 1.49 (s, 3H), 1.30 (d, J=5.6Hz, 3H), 1.11 (d, J=6.8Hz, 6H), 0.94 (d, J=7.6Hz, 3H), 0.89 (t, J=7.2Hz, 3H); IR (KBr, cm-1) ν: 3473, 2926, 2787, 2452, 2369, 1803, 1742, 1606, 1523, 1460, 1347, 1240, 1164, 1108, 1038, 974, 855, 722, 633, 567, 485; ESI-HRMS(m/z): Calcd.for C38H55FN2O14, [M+H]+: 783.3716, Found: 783.3732。
11,12-环碳酸酯-3-对硝基苯丙酸酯-8-氟-红霉素(5b),淡黄色固体(0.40g,收率36%)。1H NMR(400MHz, CDCl3) δ(ppm): 8.14(d, J=8.2Hz, 2H), 7.40(d, J=8.3Hz, 2H), 5.82(d, J=10.9Hz, 1H), 4.95(dd, J=9.5, 3.4Hz, 1H), 4.83(s, 1H), 4.23(d, J=7.3Hz, 1H), 4.04(s, 1H), 3.90~3.77(m, 2H), 3.55(s, 1H), 3.46(s, 1H), 3.25~3.16(m, 1H), 3.11(t, J=7.6Hz, 2H), 3.04(s, 1H), 2.85~2.64(m, 4H), 2.44(s, 6H), 2.34~2.20(m, 2H), 2.10(q, J=6.8Hz, 1H), 1.88~1.75(m, 2H), 1.72(s, 3H), 1.67~1.55(m, 4H), 1.49(s, 3H), 1.29(d, J=6.0Hz, 3H), 1.20(d, J=7.3Hz, 3H), 1.12(d, J=5.9Hz, 3H), 0.98~0.85(m, 6H); IR(KBr, cm-1) ν: 3343, 2977, 2788, 2614, 2453, 1804, 1743, 1608, 1522, 1461, 1346, 1243, 1167, 1108, 1039, 855, 772, 634, 568; ESI-HR MS(m/z): Calcd.for C39H57FN2O14, [M+H]+: 797.3872, Found: 797.3892。
11,12-环碳酸酯-3-(2-氟-苯乙酸酯)-8-氟-红霉素(5c),白色固体(0.25g,收率24%)。1H NMR(400MHz, DMSO-d6) δ(ppm): 7.66(d, J=8.0Hz, 2H), 7.50(d, J=7.9Hz, 2H), 5.86(d, J=1.3Hz, 1H), 5.59(dd, J=10.1, 1.8Hz, 1H), 4.88(dd, J=9.8, 3.4Hz, 1H), 4.60(s, 1H), 4.31(d, J=4.2Hz, 1H), 4.10(d, J=7.4Hz, 1H), 3.87~3.81(m, 2H), 3.53~3.42(m, 1H), 3.31(d, J=2.3Hz, 1H), 3.17~3.09(m, 1H), 3.05~2.98(m, 1H), 2.72(dd, J=12.5, 1.9Hz, 1H), 2.47~2.42(m, 1H), 2.21(s, 6H), 2.17~2.07(m, 2H), 1.99(d, J=1.9Hz, 1H), 1.98~1.92(m, 1H), 1.70~1.62(m, 2H), 1.58~1.46(m, 7H), 1.39(s, 3H), 1.14(d, J=6.1Hz, 3H), 1.00(d, J=7.3Hz, 3H), 0.96(d, J=6.9Hz, 3H), 0.88(d, J=7.8Hz, 3H), 0.81(t, J=7.4Hz, 3H); IR(KBr, cm-1) ν: 3449, 2973, 1801, 1739, 1591, 1459, 1384, 1235, 1164, 1101, 1038, 973, 758, 641, 540, 465; ESI-HR MS(m/z): Calcd.for C38H55F2NO12, [M+H]+: 756.3771, Found: 756.3780。
11,12-环碳酸酯-3-(3-氟-苯乙酸酯)-8-氟-红霉素(5d),白色固体(0.20g,收率20%)。1H NMR(400MHz, CDCl3) δ(ppm): 7.25~7.21(m, 1H), 6.97(t, J=8.6Hz, 3H), 5.75(d, J=10.3Hz, 1H), 4.97(dd, J=9.7, 3.5Hz, 1H), 4.82(s, 1H), 4.18(d, J=7.2Hz, 1H), 3.65(s, 2H), 3.58~3.43(m, 2H), 3.39(s, 1H), 3.24~3.17(m, 2H), 3.10(q, J=7.3Hz, 1H), 2.78(d, J=14.2Hz, 1H), 2.72(t, J=11.2Hz, 1H), 2.44(s, 6H), 2.31~2.18(m, 2H), 2.06(q, J=6.9Hz, 1H), 1.84~1.70(m, 3H), 1.67(s, 3H), 1.63~1.55(m, 4H), 1.47(s, 3H), 1.28~1.25(m, 6H), 1.11(d, J=6.9Hz, 6H), 0.95~0.92(m, 3H); IR(KBr, cm-1) ν: 3463, 2977, 2789, 1804, 1741, 1621, 1457, 1384, 1259, 1166, 1105, 1039, 772, 714, 637, 569, 519; ESI-HR MS(m/z): Calcd.for C38H55F2NO12, [M+H]+: 756.3771, Found: 756.3782。
11,12-环碳酸酯-3-(4-氟-苯乙酸酯)-8-氟-红霉素(5e),白色固体(0.45g,收率46%)。1H NMR(400MHz, DMSO-d6) δ(ppm): 7.39~7.28(m, 2H), 7.19~7.11(m, 2H), 5.86(d, J=1.2Hz, 1H), 5.55(dd, J=9.4, 2.1Hz, 1H), 4.89(dd, J=9.8, 3.4Hz, 1H), 4.62(s, 1H), 4.31(d, J=4.1Hz, 1H), 4.09(d, J=7.4Hz, 1H), 3.74(d, J=3.0Hz, 2H), 3.57~3.45(m, 1H), 3.27(d, J=3.0Hz, 1H), 3.14~3.06(m, 1H), 3.06~2.97(m, 1H), 2.75(dd, J=12.6, 1.8Hz, 1H), 2.49~2.43(m, 1H), 2.22(s, 6H), 2.17~2.08(m, 2H), 1.99(d, J=1.9Hz, 1H), 1.95(q, J=6.9Hz, 1H), 1.70~1.62(m, 2H), 1.59~1.46(m, 7H), 1.40(s, 3H), 1.15(d, J=6.0Hz, 3H), 1.00(d, J=7.2Hz, 3H), 0.96(d, J=7.0Hz, 3H), 0.88(d, J=7.7Hz, 3H), 0.83(t, J=7.4Hz, 3H); IR(KBr, cm-1) ν: 3458, 2975, 2788, 2344, 1804, 1742, 1605, 1512, 1460, 1385, 1226, 1165, 1101, 1038, 971, 799, 717, 644, 567, 518; ESI-HR MS(m/z): Calcd.for C38H55F2NO12, [M+H]+: 756.3771, Found: 756.3783。
11,12-环碳酸酯-3-(2-氯-苯乙酸酯)-8-氟-红霉素(5f),白色固体(0.32g,收率30%)。1H NMR(400MHz, CDCl3) δ(ppm): 7.38~7.33(m, 2H), 7.21~7.17(m, 2H), 5.76(dd, J=10.1, 1.4Hz, 1H), 4.99(dd, J=9.7, 3.4Hz, 1H), 4.84(s, 1H), 4.16(d, J=7.3Hz, 1H), 3.86(d, J=3.3Hz, 2H), 3.54~3.46(m, 1H), 3.42(d, J=2.3Hz, 1H), 3.33(s, 1H), 3.13~3.07(m, 2H), 2.93(s, 1H), 2.84(dd, J=13.0, 2.3Hz, 1H), 2.48~2.41(m, 1H), 2.28(s, 6H), 2.23~2.18(m, 2H), 2.06(q, J=6.8Hz, 1H), 1.82~1.71(m, 3H), 1.68(s, 3H), 1.63~1.57(m, 4H), 1.46(s, 3H), 1.25(d, J=7.0Hz, 3H), 1.10(d, J=6.7Hz, 3H), 1.08(d, J=7.3Hz, 3H), 1.00(d, J=7.7Hz, 3H), 0.92(t, J=7.4Hz, 3H); IR(KBr, cm-1) ν: 3465, 2976, 2878, 2788, 1804, 1742, 1649, 1543, 1458, 1384, 1248, 1165, 1106, 1039, 836, 748, 685, 639, 570, 501; ESI-HR MS(m/z): Calcd.for C38H55F2NO12, [M+H]+: 772.3475, Found: 772.3482。
11,12-环碳酸酯-3-(3-氯-苯乙酸酯)-8-氟-红霉素(5g),白色固体(0.28g,收率26%)。1H NMR(400MHz, CDCl3) δ(ppm): 7.30(t, J=2.1Hz, 1H), 7.25~7.16(m, 3H), 5.77(dd, J=10.5, 1.4Hz, 1H), 4.98(dd, J=9.7, 3.5Hz, 1H), 4.83(s, 1H), 4.16(d, J=7.3Hz, 1H), 3.66(s, 2H), 3.57~3.43(m, 2H), 3.40(d, J=2.0Hz, 1H), 3.18~3.06 (m, 2H), 2.93 (s, 1H), 2.82(dd, J=13.0, 2.7Hz, 1H), 2.50~2.40(m, 1H), 2.29(s, 6H), 2.26~2.17(m, 2H), 2.07(q, J=6.8Hz, 1H), 1.83~1.69(m, 3H), 1.68(s, 3H), 1.64~1.59(m, 4H), 1.46(s, 3H), 1.25(d, J=5.4Hz, 3H), 1.14~1.07(m, 6H), 0.95(d, J=7.7Hz, 3H), 0.91(t, J=7.4Hz, 3H); IR(KBr, cm-1) ν: 3467, 2978, 2880, 2789, 1804, 1743, 1638, 1460, 1385, 1242, 1165, 1104, 1039, 973, 806, 762, 637, 568, 500; ESI-HR MS(m/z): Calcd.for C38H55ClFNO12, [M+H]+: 772.3475, Found: 772.3490。
11,12-环碳酸酯-3-(4-氯-苯乙酸酯)-8-氟-红霉素(5h),淡黄色固体(0.38g,收率36 %)。1H NMR(400MHz, DMSO-d6) δ(ppm): 7.39~7.28(m, 4H), 5.85(d, J=1.3Hz, 1H), 5.56(dd, J=9.7, 2.1Hz, 1H), 4.89(dd, J=9.8, 3.4Hz, 1H), 4.61(s, 1H), 4.30(d, J=4.1Hz, 1H), 4.08(d, J=7.4Hz, 1H), 3.71(d, J=4.2Hz, 2H), 3.54~3.42(m, 1H), 3.26(d, J=2.8Hz, 1H), 3.12(qd, J=7.0, 2.2Hz, 1H), 3.06~2.98(m, 1H), 2.74(dd, J=12.6, 1.9Hz, 1H), 2.48~2.41(m, 1H), 2.22(s, 6H), 2.20~2.08(m, 2H), 1.96(q, J=6.7Hz, 1H), 1.70~1.57(m, 3H), 1.57~1.44(m, 7H), 1.40(s, 3H), 1.15(d, J=6.0Hz, 3H), 1.03(d, J=7.3Hz, 3H), 0.96(dd, J=6.9, 1.3Hz, 3H), 0.87(d, J=7.7Hz, 3H), 0.83(t, J=7.3Hz, 3H); IR(KBr, cm-1) ν: 3473, 2875, 2879, 2788, 1804, 1743, 1638, 1459, 1384, 1242, 1165, 1100, 1039, 973, 807, 761, 635, 566, 499; ESI-HR MS(m/z): Calcd.for C38H55ClFNO12, [M+H]+: 772.3475, Found: 772.3487。
11,12-环碳酸酯-3-(4-甲氧基-苯乙酸酯)-8-氟-红霉素(5i),淡黄色固体(0.24g,收率22%)。1H NMR(400MHz, CDCl3) δ(ppm): 7.21(d, J=8.6Hz, 2H), 6.82(d, J=8.6Hz, 2H), 5.75(dd, J=10.4, 1.6Hz, 1H), 4.98(dd, J=9.7, 3.4Hz, 1H), 4.84(s, 1H), 4.15(d, J=7.3Hz, 1H), 3.78(s, 3H), 3.62(s, 2H), 3.56-3.43(m, 1H), 3.39(d, J=2.2Hz, 2H), 3.19~3.05(m, 3H), 2.82(dd, J=13.0, 2.7Hz, 1H), 2.52~2.40(m, 1H), 2.29(s, 6H), 2.26~2.17(m, 2H), 2.07(q, J=6.8Hz, 1H), 1.82~1.68(m, 3H), 1.66(s, 3H), 1.64~1.56(m, 4H), 1.46(s, 3H), 1.24(d, J=6.2Hz, 3H), 1.14(d, J=7.2Hz, 3H), 1.11(d, J=8.2Hz, 3H), 0.95~0.88(m, 6H); IR(KBr, cm-1) ν: 3514, 3285, 2979, 2869, 2663, 1716, 1632, 1461, 1384, 1305, 1167, 1090, 1018, 874, 805, 760, 696, 640, 587, 467; ESI-HR MS(m/z): Calcd.for C39H58FNO13, [M+H]+: 768.3970, Found: 768.3962。
11,12-环碳酸酯-3-(4-三氟甲基-苯乙酸酯)-8-氟-红霉素(5j),黄色固体(0.30g,收率27%)。1H NMR(400MHz, CDCl3) δ(ppm): 7.55(d, J=8.5Hz, 2H), 7.42(d, J=7.8Hz, 2H), 5.79(dd, J=10.8, 1.3Hz, 1H), 4.96(dd, J=9.8, 3.4Hz, 1H), 4.81(s, 1H), 4.16(d, J=7.3Hz, 1H), 3.92(s, 1H), 3.75(s, 2H), 3.54-3.45(m, 1H), 3.42(d, J=1.8Hz, 1H), 3.19~3.12(m, 1H), 3.10(dd, J=10.1, 7.3Hz, 1H), 2.96(s, 1H), 2.81(dd, J=13.0, 2.7Hz, 1H), 2.44(td, J=11.4, 10.1, 3.7Hz, 1H), 2.28(s, 6H), 2.26~2.16(m, 2H), 2.07(q, J=6.1, 5.5Hz, 1H), 1.81~1.71(m, 3H), 1.69(s, 3H), 1.64~1.58(m, 4H), 1.46(s, 3H), 1.25(d, J=7.0Hz, 3H), 1.15~1.05(m, 6H), 0.94(d, J=7.7Hz, 3H), 0.89(t, J=7.4Hz, 3H); IR(KBr, cm-1) ν: 3466, 2978, 2882, 2790, 1804, 1742, 1624, 1460, 1384, 1328, 1244, 1166, 1039, 823, 772, 714, 636, 568, 500; ESI-HR MS(m/z): Calcd.for C39H55F4NO12, [M+H]+: 806.3739, Found: 806.3745。
11,12-环碳酸酯-3-(3-吡啶乙酸酯)-8-氟-红霉素(5k),黄色固体(0.20g,收率22%)。1H NMR(400MHz, CDCl3) δ(ppm): 8.46(s, 1H), 8.00(d, J=6.9Hz, 1H), 7.42(t, J=7.6Hz, 2H), 5.77(d, J=10.5Hz, 1H), 5.03(dd, J=10.5, 7.5Hz, 1H), 4.83~4.76(m, 1H), 4.75(s, 1H), 4.49(d, J=7.6Hz, 1H), 3.67~3.52(m, 4H), 3.10~2.85(m, 2H), 2.74(dd, J=13.1, 3.0Hz, 1H), 2.63(q, J=7.3Hz, 1H), 2.48~2.34(m, 1H), 2.25(s, 6H), 2.24~2.13(m, 2H), 2.04~1.98(m, 1H), 1.96~1.71(m, 7H), 1.69(s, 3H), 1.59(s, 3H), 1.30(d, J=5.9Hz, 3H), 1.12(d, J=7.0Hz, 3H), 0.85(d, J=7.7Hz, 3H), 0.76(t, J=7.4Hz, 3H), 0.36(d, J=7.3Hz, 3H); IR(KBr, cm-1) ν: 3448, 2977, 2785, 1802, 1724, 1669, 1457, 1385, 1272, 1165, 1101, 1035, 975, 804, 772, 713, 636, 569, 509; ESI-HR MS(m/z): Calcd.for C37H55FN2O12, [M+H]+: 739.3817, Found: 739.3817。
1.3 化合物12a~12f的合成(图4)
克拉霉素(10.0g, 13.4mmol),苯甲酸酐(4.5g, 20.0mmol),三乙胺(3.8mL, 26.8mmol)依次加入100mL四氢呋喃中溶解,30℃下搅拌反应20h。将反应液冷却到0~5℃,向反应液中缓慢滴加N,N-二甲基乙二胺(1.0mL, 10.7mmol),继续搅拌30min,TLC监测反应。反应毕,反应液用乙酸乙酯稀释,有机相依次用饱和碳酸氢钠溶液、饱和食盐水洗涤,无水硫酸钠干燥。过滤,减压浓缩,烘干得白色固体6(11.1g,收率97%)。1H NMR(400MHz, CDCl3) δ(ppm): 8.05~7.98(m, 2H), 7.56(t, J=7.4Hz, 1H), 7.43(t, J=7.7Hz, 2H), 5.06~4.97(m, 2H), 4.91(d, J=4.6Hz, 1H), 4.70(d, J=7.4Hz, 1H), 4.02(dd, J=9.1, 6.2Hz, 1H), 3.95(s, 1H), 3.72(d, J=8.8Hz, 1H), 3.69(s, 1H), 3.63(d, J=7.0Hz, 1H), 3.59~3.52(m, 1H), 3.45(s, 3H), 3.17(s, 1H), 3.06(t, J=9.6Hz, 1H), 3.00(s, 3H), 2.93(d, J=6.8Hz, 1H), 2.81~2.67(m, 2H), 2.61~2.52(m, 1H), 2.36(d, J=15.1Hz, 1H), 2.26(s, 6H), 2.21(d, J=10.0Hz, 1H), 1.90~1.83(m, 1H), 1.81~1.70(m, 2H), 1.65~1.57(m, 3H), 1.46~1.35(m, 5H), 1.32(d, J=6.2Hz, 3H), 1.29(s, 3H), 1.26(d, J=6.0Hz, 3H), 1.14(t, J=6.7Hz, 6H), 1.09(d, J=6.8Hz, 3H), 1.02(s, 3H), 0.79(t, J=7.4Hz, 3H), 0.70(d, J=7.5Hz, 3H)。
在180mL乙醇和水[V(乙醇)/V(水)=1:1]的体系中,加入化合物6(12.0g, 14.1mmol),室温搅拌下缓慢滴加浓盐酸(11.7mL, 141.0mmol),在35℃下搅拌反应2h后用氨水调节反应液pH=10~10.5,大量白色固体析出,室温下继续搅拌30min,过滤,去离子水淋洗,真空60℃干燥8h,得白色固体7(9.3g,收率95%)。1H NMR(400MHz, CDCl3) δ(ppm): 8.11~8.01(m, 2H), 7.61~7.52(m, 1H), 7.45(t, J=7.7Hz, 2H), 5.12(dd, J=11.1, 2.3Hz, 1H), 5.04(dd, J=10.5Hz, 7.6Hz, 1H), 4.75(d, J=7.6Hz, 1H), 3.92(s, 1H), 3.77~3.68(m, 2H), 3.61~3.51(m, 1H), 3.49~3.41(m, 1H), 3.22(s, 1H), 2.93(s, 3H), 2.91~2.84(m, 2H), 2.63~2.46(m, 2H), 2.28(s, 6H), 2.03~1.94(m, 1H), 1.94~1.83(m, 1H), 1.82~1.73(m, 1H), 1.70(d, J=6.5Hz, 1H), 1.64~1.62(m, 1H), 1.49~1.36(m, 3H), 1.29(s, 3H), 1.26(d, J=6.1Hz, 3H), 1.22(d, J=6.7Hz, 3H), 1.11~1.04(m, 6H), 1.03(s, 3H), 0.79(t, J=7.4Hz, 3H), 0.69(d, J=7.5Hz, 3H)。

图4 化合物12a~12f的合成Fig.4 Syntheses of compounds 12a~12f
化合物7(5.0g, 7.2mmol)溶解在50mL二氯甲烷和5mL吡啶的混合溶液中,将反应液冷却至0℃,缓慢滴加溶三光气(2.1g, 7.2mmol)的二氯甲烷溶液(15mL)。滴毕,0℃下继续反应5h。反应液倾入饱和食盐水(200mL)中,搅拌15min,二氯甲烷萃取,有机相依次用水、饱和食盐水洗涤,无水硫酸镁干燥。过滤,减压浓缩,柱层析[V(石油醚)/V(丙酮)/V(三乙胺)=10:4:1],得白色固体8(3.9g,收率75%)。1H NMR(400MHz, CDCl3) δ(ppm): 8.11~8.00(m, 2H), 7.56(t, J=7.4Hz, 1H), 7.44(t, J=7.7Hz, 2H), 5.12~4.99(m, 2H), 4.73(d, J=7.6Hz, 1H), 4.68(s, 1H), 3.72(d, J=2.5Hz, 1H), 3.61~3.50(m, 1H), 3.45(dd, J=10.4, 5.9Hz, 1H), 2.96~2.79(m, 5H), 2.65~2.49(m, 2H), 2.27(s, 6H), 1.92~1.74(m, 4H), 1.55~1.40(m, 4H), 1.38(s, 3H), 1.29(s, 3H), 1.27(d, J=6.1Hz, 3H), 1.23(d, J=6.7Hz, 3H), 1.13(d, J=6.7Hz, 3H), 1.05(d, J=7.1Hz, 3H), 0.82(t, J=7.4Hz, 3H), 0.69(d, J=7.5Hz, 3H)。
N-氯代丁二酰亚胺(1.2g, 9.0mmol)溶解在10mL二氯甲烷中,冷却至-5℃,搅拌15min。缓慢滴加二甲硫醚(0.9mL, 12.3mmol)的二氯甲烷溶液(10mL),继续搅拌30min。缓慢滴加化合物8(4.0g, 5.6mmol)的二氯甲烷溶液(50mL),滴毕,继续搅拌反应3 h。三乙胺(1.2mL, 8.6mmol)缓慢滴加到反应液中,继续搅拌30min。升至室温,反应液依次用1%的HCl溶液、饱和食盐水洗涤,无水硫酸镁干燥。过滤,减压浓缩,柱层析[V(石油醚)/V(丙酮)/V(三乙胺)=10: 4: 1],得白色固体9(3.2g,收率80%)。1H NMR(400MHz, CDCl3) δ(ppm): 8.02(d, J=8.3Hz, 2H), 7.59~7.53(m, 1H), 7.44(t, J=7.6Hz, 2H), 5.02(dd, J=10.5, 7.6Hz, 1H), 4.96(dd, J=10.2, 2.1Hz, 1H), 4.59(s, 1H), 4.52(d, J=7.7Hz, 1H), 4.18(d, J=7.9Hz, 1H), 3.67(q, J=6.8Hz, 1H), 3.64~3.56(m, 1H), 2.93~2.82(m, 3H), 2.63(s, 3H), 2.25(s, 6H), 1.90~1.82(m, 1H), 1.82~1.70(m, 3H), 1.64(dd, J=14.5Hz, 2.6Hz, 1H), 1.57~1.48(m, 2H), 1.46(s, 3H), 1.36~1.29(m, 6H), 1.28(d, J=6.0Hz, 3H), 1.16(d, J=6.9Hz, 3H), 1.12(d, J=7.0Hz, 3H), 0.95(d, J=7.5Hz, 3H), 0.85(t, J=7.4Hz, 3H)。
化合物9(3.0g, 4.2mmol)溶解在35mL丙酮中,搅拌条件下滴加1, 8-二氮杂双环[5,4,0]十一碳-7-烯(DBU)(1.6mL, 10.7mmol),加热回流14h。TLC监控反应,反应毕,将丙酮旋干,残余固体用乙酸乙酯溶解,有机相依次用饱和碳酸氢钠溶液、饱和食盐水洗涤,无水硫酸镁干燥。过滤,减压浓缩,柱层析[V(石油醚)/V(乙酸乙酯)=3:2]得白色固体10(2.1g,收率76%)。1H NMR(400MHz, CDCl3) δ(ppm): 8.02(d, J=7.6Hz, 2H), 7.54(t, J=7.4Hz, 1H), 7.42(t, J=7.7Hz, 2H), 6.54(s, 1H), 5.01(dd, J=10.4Hz, 7.7Hz, 1H), 4.94(dd, J=9.8, 2.7Hz, 1H), 4.51(d, J=7.6Hz, 1H), 4.14(d, J=8.4Hz, 1H), 3.66~3.54(m, 2H), 3.22~3.08(m, 1H), 2.96~2.90(m, 1H), 2.84(s, 3H), 2.83~2.78(m, 1H), 2.25(s, 6H), 1.98(s, 3H), 1.95~1.85(m, 2H), 1.83~1.75(m, 2H), 1.60~1.38(m, 3H), 1.35(s, 3H), 1.32~1.26(m, 9H), 1.15(d, J=6.7Hz, 3H), 0.94(d, J=7.3Hz, 3H), 0.88(t, J=7.4Hz, 3H)。
NaH(60%, 0.24g, 6.0mmol)加入到30mL四氢呋喃中,N2保护下,-5℃搅拌15min。快速将化合物10(2.0g, 3.0mmol)添加到反应体系中,-5℃搅拌15min。在N2的保护下,将溶有CDI(1.6g, 9.9mmol)的四氢呋喃溶液(20mL)缓慢的滴加到反应体系中。N2保护下搅拌反应8h。反应毕,将适量的5% KH2PO4溶液和乙酸乙酯加到反应体系中,终止反应,有机相用饱和食盐水洗涤,无水硫酸钠干燥,过滤,减压旋蒸,得化合物11(1.9g,收率82%)。1H NMR (400MHz, CDCl3) δ(ppm): 8.07(s, 1H), 8.03(d, J=7.4Hz, 2H), 7.56(t, J=7.4Hz, 1H), 7.43(t, J=7.6Hz, 2H), 7.35(s, 1H), 7.06(s, 1H), 6.75(s, 1H), 5.64(dd, J=9.8Hz, 3.1Hz, 1H), 5.01(dd, J=10.3Hz, 7.6Hz, 1H), 4.50(d, J=7.6Hz, 1H), 4.13(d, J=8.8Hz, 1H), 3.67~3.60(m, 1H), 3.60~3.52(m, 1H), 3.19~3.08(m, 1H), 2.95~2.88(m, 1H), 2.85~2.79(m, 1H), 2.76(s, 3H), 2.24(s, 6H), 1.88~1.81(m, 2H), 1.80(s, 3H), 1.78~1.77(m, 1H), 1.76(s, 3H), 1.67~1.54(m, 3H), 1.31(s, 3H), 1.30~1.24(m, 6H), 1.22(d, J=6.8Hz, 3H), 0.95(d, J=7.3Hz, 3H), 0.90(t, J=7.4Hz, 3H)。
化合物11(0.5 mmol)和有机胺(1.5mmol)溶解在适量乙腈和水(体积比10:1)的混合溶液中,N2保护下,室温下搅拌反应24h。TLC监测反应,反应毕,向反应液中加适量的水和乙酸乙酯溶液,有机相用饱和食盐水洗涤,无水硫酸钠干燥。过滤,减压浓缩,残余物溶解在适量甲醇中,回流8h。减压浓缩除去溶剂,柱层析[V(二氯甲烷)/V(甲醇)=20:1]得目标产物12a~12f。
3-脱(己吡喃葡萄糖基)-3-O-11-N-(正丙基)-12-O-环氨甲酸酯-6-O-甲基红霉素(12a),白色固体(0.11g,收率33%)。1H NMR(400MHz, CDCl3) δ(ppm): 4.91(d, J=10.3Hz, 1H), 4.22(d, J=7.2Hz, 1H), 4.17(d, J=8.6Hz, 1H), 3.84~3.76(m, 1H), 3.53(s, 1H), 3.51~3.43(m, 3H), 3.16~3.09(m, 1H), 3.09~2.99(m, 2H), 2.59(s, 3H), 2.56~2.49(m, 1H), 2.41(t, J=11.2Hz, 1H), 2.21(s, 6H), 1.93~1.82(m, 1H), 1.75(d, J=13.8Hz, 1H), 1.62(d, J=12.7Hz, 1H), 1.58~1.47(m, 4H), 1.40(s, 3H), 1.32~1.26(m, 5H), 1.26~1.20(m, 4H), 1.19~1.13(m, 5H), 1.10(d, J=6.9Hz, 3H), 0.94(d, J=6.8Hz, 3H), 0.86~0.75(m, 6H); IR(KBr, cm-1) ν: 2973, 2941, 2878, 1753, 1458, 1382, 1163, 1109, 1049, 995; ESI-HR MS(m/z): Calcd.for C34H58N2O10, [M+H]+: 654.4091, found: 654.4076。
3-脱(己吡喃葡萄糖基)-3-O-11-N-(正戊基)-12-O-环氨甲酸酯-6-O-甲基红霉素(12b),白色固体(0.12g,收率34%)。1H NMR(400MHz, CDCl3) δ(ppm): 4.97(d, J=10.1Hz, 1H), 4.33(d, J=6.9Hz, 1H), 4.25(d, J=8.3Hz, 1H), 3.88~3.79(m, 1H), 3.66~3.54(m, 4H), 3.36~3.25(m, 1H), 3.15~3.04(m, 2H), 2.79~2.71(m, 1H), 2.67(s, 3H), 2.63~2.57(m, 1H), 2.45(s, 6H), 2.05~1.90(m, 1H), 1.79(d, J=13.2Hz, 2H), 1.66~1.52(m, 4H), 1.47(s, 3H), 1.43~1.21(m, 18H), 1.17(d, J=6.4Hz, 3H), 1.01(d, J=6.3Hz, 3H), 0.94~0.75(m, 6H); IR(KBr, cm-1) ν: 2972, 2939, 2877, 1752, 1458, 1380, 1165, 1110, 1053, 991; ESI-HR MS(m/z): Calcd.for C36H62N2O10, [M+H]+: 682.4404, found: 682.4402。
3-脱(己吡喃葡萄糖基)-3-O-11-N-(正庚基)-12-O-环氨甲酸酯-6-O-甲基红霉素(12c),白色固体(0.19g,收率51%)。1H NMR(400MHz, CDCl3) δ(ppm): 4.97(d, J=10.0Hz, 1H), 4.31(d, J=6.5Hz, 1H), 4.25(d, J=8.5Hz, 1H), 3.89~3.81(m, 1H), 3.61~3.53(m, 4H), 3.28~3.21(m, 1H), 3.14~3.06(m, 2H), 2.67(s, 3H), 2.63(d, J=9.2Hz, 1H), 2.61~2.56(m, 1H), 2.42(s, 2H), 2.36(s, 6H), 2.05(t, J=12.8Hz, 2H), 1.98~1.93(m, 1H), 1.79(t, J=11.6Hz, 2H), 1.61~1.54(m, 4H), 1.47(s, 3H), 1.38~1.33(m, 6H), 1.31~1.28(m, 6H), 1.27~1.25(m, 6H), 1.16(d, J=7.0Hz, 3H), 1.01(d, J=6.8Hz, 3H), 0.89~0.83(m, 6H); IR(KBr, cm-1) ν: 2971, 2936, 2876, 1753, 1458, 1380, 1166, 1110, 1052, 991; ESI-HR MS(m/z): Calcd.for C38H66N2O10, [M+H]+: 710.4717, found: 710.470。
3-脱(己吡喃葡萄糖基)-3-O-11-N-((3-N-吗啉)正丙基)-12-O-环氨甲酸酯- 6-O-甲基红霉素(12d),白色固体(0.18g,收率47%)。1H NMR(400MHz, CDCl3) δ(ppm): 4.94(d, J=10.3Hz, 1H), 4.31(d, J=7.2Hz, 1H), 4.24(d, J=8.6Hz, 1H), 3.85(q, J=6.8Hz, 1H), 3.72(s, 6H), 3.68~3.60(m, 2H), 3.60~3.50(m, 2H), 3.28~3.20(m, 1H), 3.14~3.04(m, 2H), 2.66(s, 3H), 2.64~2.55(m, 2H), 2.48(s, 4H), 2.45~2.39(m, 2H), 2.37(s, 6H), 2.01~1.90(m, 1H), 1.89~1.70(m, 4H), 1.64~1.52(m, 2H), 1.47(s, 3H), 1.38~1.32(m, 6H), 1.30(d, J=7.5Hz, 3H), 1.26(d, J=5.8Hz, 3H), 1.17(d, J=6.7Hz, 3H), 1.01(d, J=6.8Hz, 3H), 0.86(t, J=7.2Hz, 3H); IR(KBr, cm-1) ν: 2968, 2930, 2855, 1751, 1457, 1378, 1166, 1115, 1076, 991; ESI-HR MS(m/z): Calcd.for C39H65N3O11, [M+H]+: 739.4619, found: 739.4635。
3-脱(己吡喃葡萄糖基)-3-O-11-N-((2-苄基氨基)乙基)-12-O-环氨甲酸酯- 6-O-甲基红霉素(12e),淡黄色固体(0.14g,收率35%)。1H NMR(400MHz, CDCl3) δ(ppm): 7.33~7.30(m, 2H), 7.29~7.25(m, 2H), 7.23~7.17(m, 1H), 5.22(d, J=2.2Hz, 1H), 4.29(d, J=7.3Hz, 1H), 4.24(d, J=8.6Hz, 1H), 3.82~3.81(m, 2H), 3.57(s, 1H), 3.55~3.51(m, 1H), 3.21~3.16(m, 1H), 3.14~3.05(m, 2H), 2.94~2.84(m, 2H), 2.83~2.76(m, 1H), 2.64(s, 3H), 2.63~2.55(m, 1H), 2.49~2.41(m, 1H), 2.28(s, 2H), 2.26(s, 6H), 1.99~1.88(m, 2H), 1.82(dd, J=14.4, 2.1Hz, 1H), 1.71~1.63(m, 2H), 1.57(s, 1H), 1.56~1.50(m, 1H), 1.4(s, 3H), 1.37~1.33(m, 6H), 1.30(d, J=7.5Hz, 3H), 1.24(d, J=6.1Hz, 3H), 1.17(d, J=7.0Hz, 3H), 1.03(d, J=6.9Hz, 3H), 0.75(t, J=7.4Hz, 3H); IR(KBr, cm-1) ν: 2972, 2939, 2877, 1750, 1455, 1379, 1166, 1076, 989; ESI-HR MS(m/z): Calcd.for C40H63N3O10, [M+H]+: 745.4513, found: 745.4505。
3-脱(己吡喃葡萄糖基)-3-O-11-N-(2-((1-萘)氨基)乙基)-12-O-环氨甲酸酯- 6-O-甲基红霉素(12f),红褐色固体(0.08g,收率40%)。1H NMR(400MHz, CDCl3) δ(ppm): 7.91(d, J=7.0Hz, 1H), 7.73(d, J=7.3Hz, 1H), 7.42~7.37(m, 2H), 7.34~7.28(m, 1H), 7.16(d, J=7.9Hz, 1H), 6.63(d, J=7.5Hz, 1H), 5.01(d, J=10.4Hz, 1H), 4.31(d, J=7.0Hz, 1H), 4.26(d, J=8.4Hz, 1H), 4.06(s, 2H), 3.82(d, J=7.0Hz, 1H), 3.67(s, 1H), 3.65~3.49(m, 4H), 3.28~3.20(m, 1H), 3.19~3.11(m, 1H), 3.12~3.02(m, 1H), 2.72(s, 3H), 2.68~2.55(m, 2H), 2.43~2.30(m, 7H), 1.86~1.76(m, 2H), 1.72(d, J=13.4Hz, 1H), 1.61(d, J=12.9Hz, 1H), 1.47(s, 3H), 1.43(s, 1H), 1.37(s, 3H), 1.32(d, J=7.0Hz, 3H), 1.30(d, J=7.5Hz, 3H), 1.25(s, 3H), 1.18(d, J=6.8Hz, 3H), 1.07(d, J=6.6Hz, 3H), 0.53(t, J=7.0Hz, 3H); IR(KBr, cm-1) ν: 2973, 2931, 2874, 1747, 1455, 1379, 1270, 1175, 1108,1055, 989, 712; ESI-HR MS(m/z): Calcd.for C43H63N3O10, [M+H]+: 781.4513, found: 781.4529。
1.4 抗菌活性测试
采用微量稀释法测定化合物的最小抑菌浓度(MIC)。在灭菌的96孔板中加入LB培养液(每孔100μL)。分别设置阳性对照组(红霉素A、克拉霉素、氟红霉素)、空白对照组、溶剂对照组(0.4mL DMSO)。在剩余的孔中从低浓度到高浓度依次加入浓度为50mmol溶于DMSO的待测化合物溶液(重复3孔),使各孔中化合物的浓度分别为0.39、0.78、1.56、3.13、6.25、12.5、25、50、100和200μmol/L。然后在每孔中加入当日在LB培养液新鲜生长、吸光度为0.6~1.0的菌液5μL。微孔板加盖并用胶纸密封,使用多功能酶标仪于37℃孵育18h,并使96孔板在7mm距离上下震荡,每10min静止并在600nm读取吸光度,计算最小抑菌浓度。
2 结果与讨论
2.1 MIC测试结果
采用微量稀释法测定了本文获得的17个化合物对B.subtilis 168、S.aureus USA 300、E.coli DH5a、P.aeruginosa PAO1、A.baumannii ATCC19606的体外抑菌活性,测试结果如表1所示。
氟红霉素衍生物中,5a和5b表现出了较好的抗菌活性。这两个化合物对B.subtilis 168和A.baumannii ATCC19606的活性均为氟红霉素的4倍。克拉霉素衍生物中,12d、12e和12f均表现出了较好的抗菌活性,其中12d的效果最为显著,对E.coli DH5α的抑菌活性是克拉霉素的8倍,对P.aeruginosa PAO1的抑菌活性是克拉霉素的4倍。
2.2 构效关系讨论
(1)氟红霉素衍生物C-3上的不同侧链对化合物的抗菌活性影响较明显。5b的抑菌活性略强于5a,提示在同为对硝基取代苯环的情况下,芳基丙酸取代较芳基乙酸取代可能更有利于提高化合物的抑菌活性。此外,C-3同为卤代芳基乙酸取代时,苯环上卤代的位置对抑菌活性影响不显著,如分别为o-氟苯乙酸、m-氟苯乙酸及p-氟苯乙酸的化合物5c、5d、5e的抑菌活性并无显著差异;(2)克拉霉素衍生物11, 12位氨基甲酸酯环上N取代侧链对化合物的抗菌活性有较大影响。当侧链为脂肪直链时,衍生物有一定的抗菌活性,且脂肪链越长抑菌活性越强。C原子数目n=3时,化合物抑菌活性差,3<n≤7时抑菌活性增强。化合物12d~12f抗菌活性较好,推测侧链长度为4~5个原子、末端为脂肪杂环或芳环时衍生物有较好的体外活性。除S.aureus USA 300外,化合物12d对其余4种受测菌株的抑菌活性均强于化合物12e与12f,推测脂肪链长度为4~5个原子的情况下,长链末端为脂肪杂环取代可能更有利于提高抑菌活性。另外,化合物12e和12f对革兰阳性菌的体外抑制活性均强于革兰阴性菌,推测当侧链末端是芳环时,可能会表现出对革兰阳性菌的选择性。
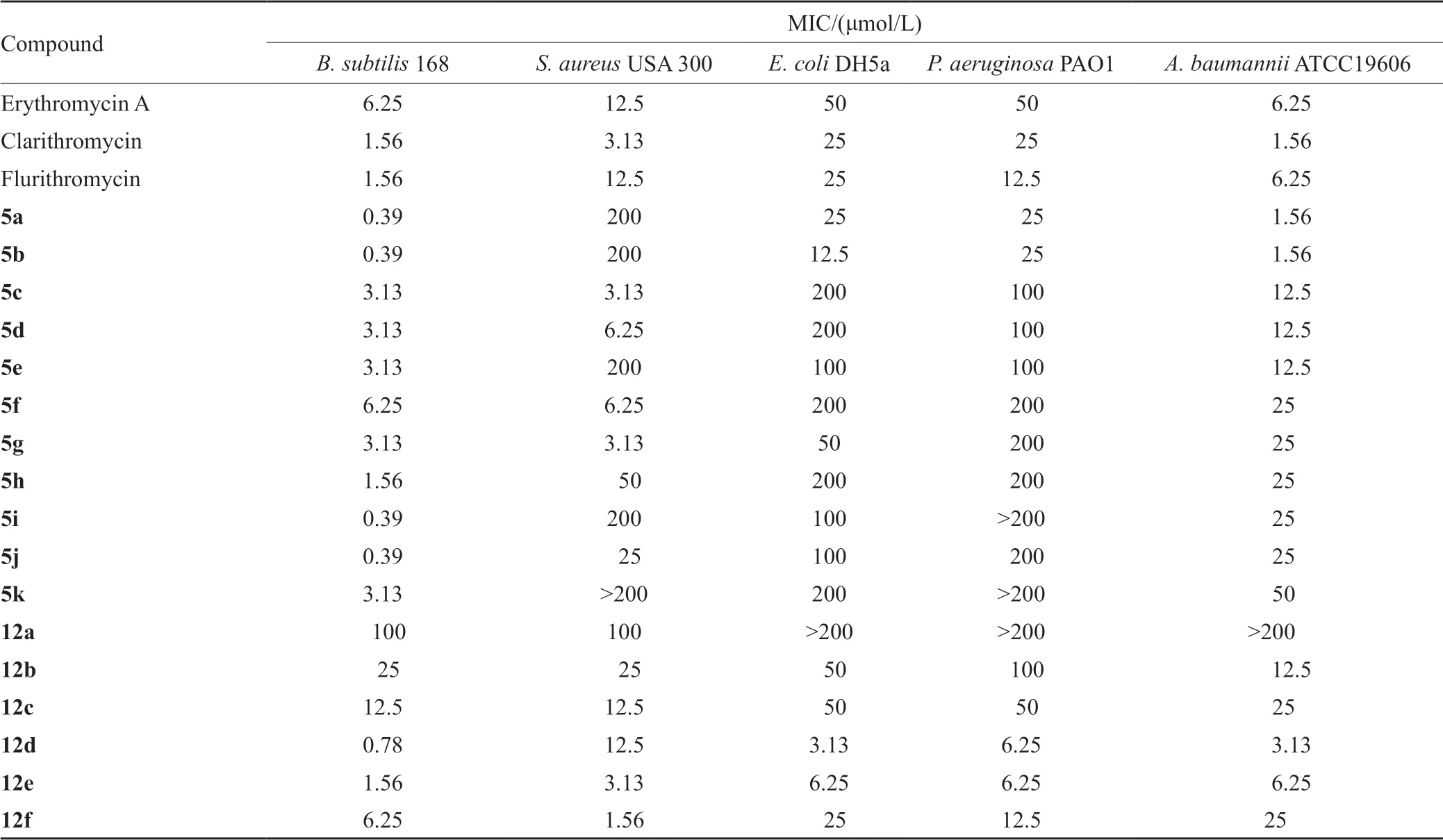
表1 大环内酯类衍生物5a~5k,12a~12f的体外抗菌活性Tab.1 Antimicrobial activities of macrolide derivatives 5a~5k and 12a~12f in vitro
3 结论
本文对氟红霉素和克拉霉素进行结构改造,合成了17个新的大环内酯类衍生物。通过抗菌活性测试,进一步探讨了大环内酯类抗生素的构效关系,为大环内酯类抗生素的后续研究提供了参考。

