西双版纳粗榧内生真菌菌株CM112中分离到的两个新bisabolane型倍半萜
叶峰 刘梦玉洁 曹玉风 鲁春华,*
(1 化学生物学教育部重点实验室,山东大学药学院,济南 250012;2 山东中医药大学附属医院,济南 250011)
1 Introduction
Many phenolic bisabolane-type sesquiterpenoids have been isolated from different material, such as fungi Verticillium tenerum[1], Aspergillus sp.[2-3], Aspergillus sydowii[4], Scopulariopsis sp.[5], coral Pseudopterogorgia rigida[6], and sponges[7-8].These compounds showed structural diverse and potent biological activities including antibacterial activity[3,9], the acetylchol inesterase inhibitory activity[10], and cytotoxic activity[11].
In the antibacterial activity screening, the strain Aspergillus sp.CM112 isolated from Cephalotaxus mannii Hook.f.[12]was selected as a positive strain.The culture extract of CM112 was subjected to MPLC, followed by Sephadex LH-20 and semi-preparative HPLC, two new (1 and 2) with three known compounds (3~5) were purified and identified as bisabolane-type sesquiterpenoids (Fig.1).In this study, we described the fermentation, isolation, and structure elucidation of the isolated compounds from strain CM112.Their antibacterial activity against Staphylococcus aureus ATCC25923, Pseudomonas aeruginosa PA01 and Salmonella enterica serovar Typhimurium UK-1χ8956 were also evaluated.
2 Material and Methods
2.1 General experimentalequipment
HR-ESI-MS was recorded on a LTQ Orbitrap Velos Pro HR-MS instrument (Thermo Fisher).NMR spectra were obtained on a Bruker Avance DRX-400 NMR spectrometer.Optical rotations were measured on an Anton Paar MCP200 automatic polarimeter.
2.2 Microorganism material
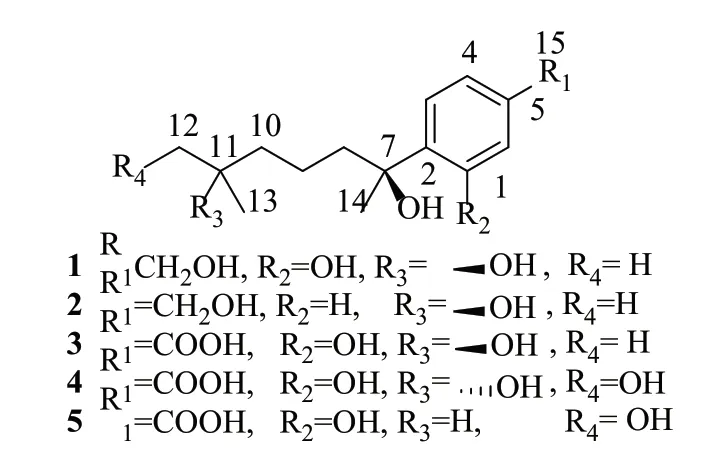
图1 化合物1~5的结构式Fig.1 Structures of compounds 1~5
The fungal stain CM112 was isolated from the stems of Cephalotaxus mannii Hook.f.collected in Xishuangbanna, Yunnan, China, in 2010.The strain CM112 and C.mannii were deposited in School of Pharmaceutical Sciences, Shandong University, Jinan.The strain CM112 grows well on potato dextrose agar (PDA) and ISP3 agar medium.And it was identified as Aspergillus sp.according to its ITS sequence of rDNA (ITS1-5.8S-ITS2).
2.3 Fermentation and extraction
Aspergillus sp.CM112 was cultivated on PDA media in petri dishes at 28℃ for ten days to obtain seed cultures.The fermentation was conducted on ISP3 agar medium (oatmeal 30g, saline standard solution 1mL, dH2O 1L, agar 15g/L, pH7.2, 50 petri dishes per litre medium) for 10 days at 28℃ with a total volume of 10 litres.The culture media with the mycelia were collected, chopped, diced and extracted with EtOAc-MeOH (4:1, V/V) at room temperature.The filtration was collected and concentrated under vacuum at 40℃ to remove organic solution.And the aqueous solution was extracted with ethyl acetate (EtOAc).The EtOAc extract was further patitioned with petroleum ether (PE) and MeOH.The MeOH soluble part was concentrated under vacuum at 40℃ to obtain MeOH extract (7.5g).
2.4 Isolation and identification of compounds 1~5
The MeOH extract (7.5g) was subjected to column chromatography over RP-18 (140g) eluted with 30%, 50%, 70% and 100% MeOH, respectively.In accordance with TLC results, 10 fractions Fr.A~J were obtained.Fr.C (330mg) was subjected to Sephadex LH-20 (80g) eluted with MeOH to yield Fr.C1-C7.Fr.C4 (32mg) was purified by preparative HPLC (ZORBAX Eclipse XDB-C18(9.4mm×250mm, 4mL/min, UV 320nm) eluted with 15% acetonitrile to afford 4 (6.4mg).Fr.D (300mg) was chromatographed over Sephadex LH-20 (80g) eluted with MeOH to obtain Fr.D1-D8.Fr.D2 (40mg) was purified by preparative HPLC (ZORBAX Eclipse XDB-C18, 9.4mm×250mm, 4mL/min, UV 320nm) eluted with 20% acetonitrile to afford 2 (3.3mg) and 1 (19.0mg).Fr.E+F (576.8mg) was chromatographed over Sephadex LH-20 (100g) eluted with MeOH to obtain Fr.E1-E6.Fr.E3 (95mg) was purified by preparative HPLC (ZORBAX Eclipse XDB-C18, 9.4mm×250mm, 4mL/min, UV 320nm) eluted with 20% acetonitrile to afford 3 (25mg) and 5 (4.5mg).
2.5 Antibacterial assay
The antibacterial activity of compounds 1~5 against S.aureus ATCC25923, P.aeruginosa PA01 and S.entericas erovar Typhimurium UK-1c8956 were measured with a paper disc diffusion assay[13].Test compounds were first dissolved in methanol and then absorbed onto individual paper disks (6 diameter) at 30μg/disc and dried up, and finally placed on the surface of the agar.The assay plates were incubated at 37℃ for 24h.Kanamycin was used as positive control (4μg/disc) and the diameters of the inhibition zones including the 6mm disc diameter were measured after 24h of incubation at 37℃.
3 Results and Discussion
3.1 Structure elucidation
Compound 1 was obtained as colorless powder.The molecular formula of 1 was determined to be C15H24O4based on the analysis of the high resolution ESI-MS m/z 291.1568[M+Na]+(calcd for C15H24O4Na+, 291.1567).The1H,13C NMR and HMQC spectra data (Tab.1) of 1 showed 15 carbons including five quaternary carbons (including one oxygenated aromatic carbon at δC157.0 (C-1), two oxygenated sp3carbons at δC78.0 (C-7) and 71.4 (C-11), three tertiary aromatic carbons, four methylenes (one oxygenated at δC64.8 (C-15)), and three singlet methyl groups.A tri-substituted benzene ring was indicated by the1H NMR spectral data at δH7.11 (d, J=7.9Hz, H-3), 6.79 (dd, J=8.0, 1.6Hz, H-4), and 6.75 (d, J=1.6Hz, H-6).The hydroxymethyl group attached at C-5 was confirmed by the HMBC correlation of H-4 and H-6 to C-15, and H-15 to C-4, C-6 and C-5 (Tab.1).The hydroxyl attached at C-1 was confirmed by the low field shifted of C-1 (δC157.0).The detailed analysis of the1H and13C NMR spectra revealed that 1 is a phenolic bisabolane-type sesquiterpenoid, which is similar to sydonol[14].The planar structure of 1 was confirmed by2D NMR (1H-1H COSY, HSQC and HMBC) spectra.The absolute configurations of C-7 and C-11 in 1 were assigned as S, identical to that of (S)-(+)-sydonolMeOH))[14]and 12-acetoxysydonic acid0.11, MeOH))[15]on the basis of the optical rotation data of 1(c 0.1, MeOH)).Therefore, 1 was elucidated as a derivative of sydonol and named (7S,11S)-(+)-11-hydroxyl-sydonol.

表1 化合物1的核磁数据Tab.1 The NMR data for 1
Compound 2 was isolated as colorless powder with(c 0.1, MeOH).The molecular formula of 2 was determined to be C15H24O3on the basis of analysis of the HR-ESI-MS m/z 253.1799 [M+H]+(calcd for C15H25O3
+, 253.1798).The NMR spectra are similar to those of 1, except that the ABX spin system was replaced by a A2B2spin system in 2 just like in aspergiterpenoid A[3], which indicated the presence of a 1,4-disubstitued benzene ring (δH7.31 (d, J=8.3Hz, 2H) and 7.43 (d, J=8.3Hz, 2H).The1H,13C NMR and HMQC spectra (Tab.2) of 2 suggested the presence of three singlet methyl groups [H3-12 (δH1.08), H3-13 (δH1.10), H3-14 (δH1.51)], four methylenes [one oxygenated δC65.5 (C-15)], and two oxygenated quaternary carbons [δC75.30 (C-7) and 71.4 (C-11)], which confirmed the same substituent group of 2,6-dimethylheptane-2,6-diol at C-2 as that of in 1.The optical rotation value for 2 was+1.4 (c 0.1, MeOH), which is identical to that of 1 and contrary to that of aspergiterpenoid A(c 3.21, CHCl3))[3].Therefore, compound 2 was elucidated as (7S, 11S)-(+)-11-hydroxyl aspergiterpenoid A.
The known compounds were elucidated by comparison their NMR data (Tab.3) and value with those reported as (7S,11S)-(+)-11-hydroxysydonic acid (3)[9,16]with(c 0.1, MeOH), (7S,11S)-(+)-11,12-dihydroxysydonic acid (4)[17]with0.1, MeOH)), (7S,11S)-(+)-12-hydroxysydonic acid (5)[5]with(c 0.1, MeOH), respectively.
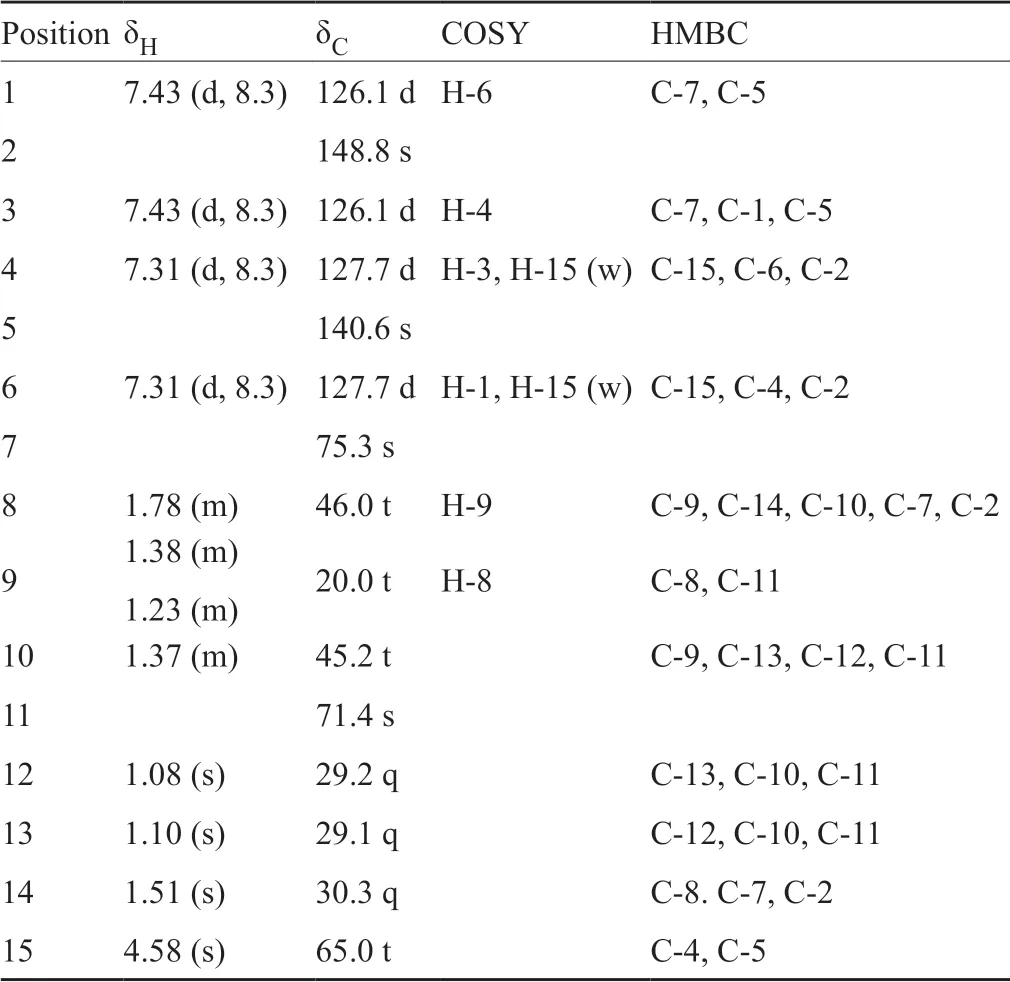
表2 化合物2的核磁数据Tab.2 The NMR data for 2
3.2 Antibacterial activity
Compounds 1~5 were evaluated for their antibacterial activity by paper disc diffusion assay against S.aureus ATCC25923, P.aeruginosa PA01 and S.enterica ser ovar Typhimurium UK-1χ8956.Only compounds 2 and 3 exhibited selective antibacterial activities against S.aureus ATCC25923 with the inhibition zones of 9mm and 12mm at 30μg/disc, respectively.Kanamycin was used as positive control.The antimicrobial activity observed by the agar diffusion test was further confirmed by the micro-dilution assays.The lowest MIC values for 2 and 3 against S.aureus ATCC25923 were 256 and 128μg/L, respectively.
In all, five bisabolane-type sesquiterpenoids (1~5) were purified and identified from A.sp.CM112, an endophytic fungus of C.mannii.1 and 2 are new compounds, and 2 and 3 are responsible for the antibacterial activity against S.aureus ATCC25923.
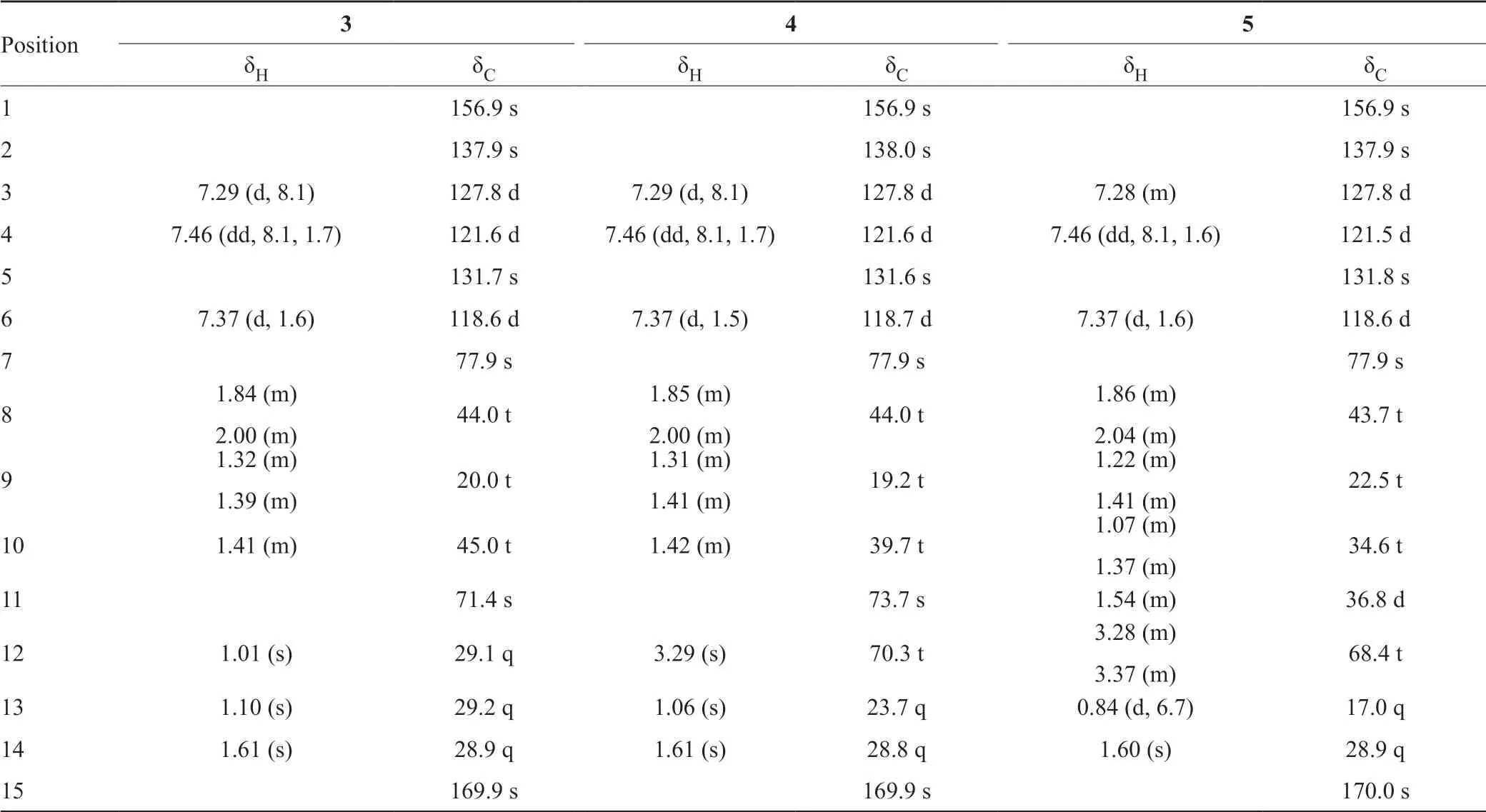
表3 化合物3~5的1H 和13CNMR核磁数据Tab.3 The 1H and 13C NMR data for 3~5
——结构和生物活性

