剑叶凤尾蕨化学成分及其细胞毒活性研究
张艳+石玉生+胡文忠+宋丽艳+陈效忠
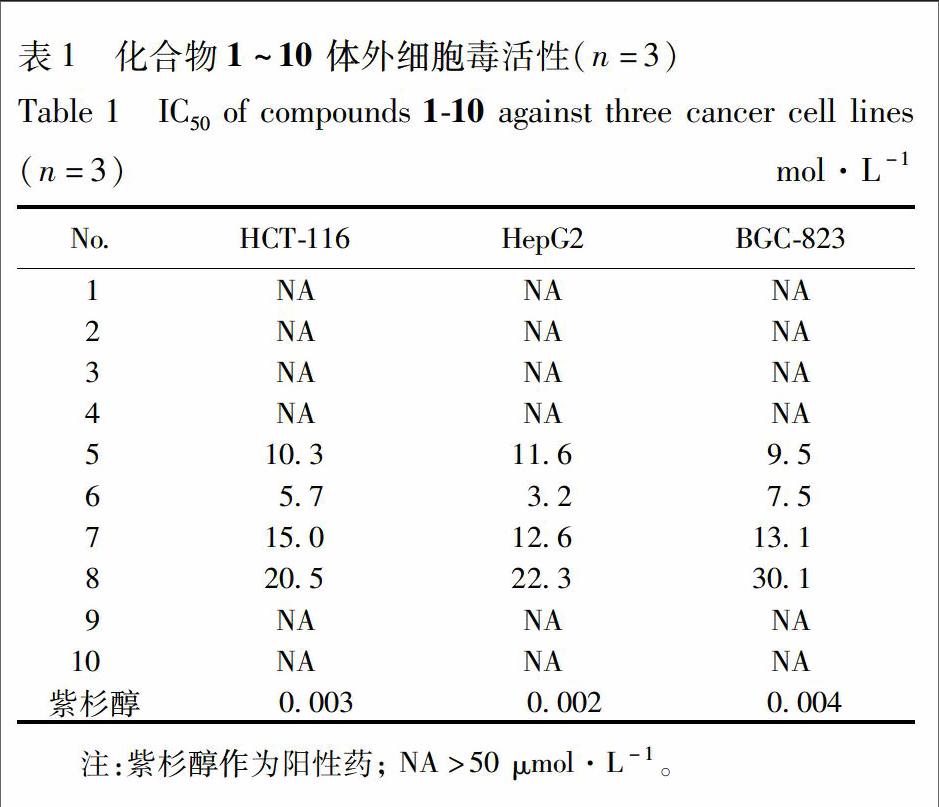
[摘要] 采用95%乙醇提取药材,提取液回收至无醇味后依次过聚酰胺树脂、MCI树脂、中压制备液相色谱和制备型高效液相色谱,从剑叶凤尾蕨全草中分离得到8个二萜类化合物和2个倍半萜类化合物。质谱(ESI-MS)数据和核磁共振(NMR)数据与文献数据对比鉴定分离得到的化合物分别为ent-3β-hydroxy-kaur-16-en-19-al (1),4-epi-kaurenic acid (2),mitrekaurenone (3),7β,16α,17-trihydroxy-ent-kauran-19-oic acid (4),crotonkinin E (5),crotonkinin F (6),pterisolic acid A (7),pterisolic acid C (8),(2R)-pterosin P (9)和dehydropterosin B (10)。化合物1~6首次从凤尾蕨属中分离得到,化合物7~10首次从剑叶凤尾蕨中获得。细胞毒活性测试表明化合物5~8具有一定的抑制人结肠癌细胞HCT-116、 肝癌细胞HepG2和人胃癌细胞BGC-823的活性。
[关键词] 剑叶凤尾蕨;二萜;倍半萜;细胞毒活性
[Abstract] The materials were extracted by 95% ethanol,and the extracting solution was isolated by kinds of chromatographic columns including polyamide,MCI,preparative MPLC,and preparative HPLC. Eight diterpenes and two sesquiterpenes were isolated from the plant. On analysis of ESI-MS and NMR spectroscopic data,the structures were established as ent-3β-hydroxy-kaur-16-en-19-al (1),4-epi-kaurenic acid (2),mitrekaurenone (3),7β,16α,17-trihydroxy-ent-kauran-19-oic acid (4),crotonkinin E (5),crotonkinin F (6),pterisolic acid A (7),pterisolic acid C (8),(2R)-pterosin P (9),and dehydropterosin B (10). Compounds 1-6 were obtained from Pteris for the first time,and compounds 7-10 were obtained from P. ensiformis for the first time. Compounds 5-8 showed moderate activity against HCT-116,HepG2 and BGC-823 cell lines,separately.
[Key words] Pteris multifida;diterpenes; sesquiterpenes;cytotoxic activitiy
doi:10.4268/cjcmm20162420
凤尾蕨科Pteridaceae植物在世界范围内有10个属,400 余种,主要分布于热带和亚热带地区。凤尾蕨属Pteris为凤尾蕨科的一个属,在世界范围内有300余种,我国有66种,该属植物在我国主要分布于西南和华南地区[1]。以往化学成分研究表明对映贝壳杉烷型二萜类和1-氢-茚-1-酮型倍半萜类化合物是凤尾蕨属植物的特征性成分。此外该属植物还含有三萜類、黄酮类、脂肪酸等化学成分[1]。该属植物生物活性广泛,包括抗肿瘤、抗炎、抗菌[2],降血糖[3]等活性。值得关注的是从该属植物半边旗中分离得到的对映贝壳杉烷型二萜ent-11α-hydroxy-15-oxo-kaur-16-en-19-oic-acid(5F)具有抑制多种肿瘤细胞(如结肠癌细胞、胃癌细胞、甲状腺肿瘤细胞、肝癌细胞、咽喉癌细胞)的作用[4],该发现引起国内外学者的广泛关注,5F是极具研究价值的热点分子。药用植物亲缘学表明亲缘关系近的植物含有结构相似的化学成分,结构相似的化学成分其生理活性多相似[5]。根据该原理从凤尾蕨属其他种类植物中寻找具有更强抗肿瘤活性的对映贝壳杉烷型二萜能起到事半功倍的效果。
剑叶凤尾蕨P. ensiformis Burm.为凤尾蕨科凤尾蕨属药用植物,分布于我国华南、西南、华东、台湾等地。全草入药,鲜用或干用均可。具有清热利湿、消肿解毒、凉血止血的功效。现代药理研究表明剑叶凤尾蕨提取物对多种肿瘤细胞具有抑制作用[6-8],然而其抗肿瘤的药效物质一直没有明确。本文对剑叶凤尾蕨化学成分和生物活性进行系统研究,从该植物中分离并鉴定了8个二萜类化合物、2个倍半萜类化合物,并对分离得到的化合物进行了细胞毒活性测试。
1 材料
Bruker Avance DRX-400型核磁共振波谱仪 (布鲁克公司,瑞士),TMS为内标;Agilent 1100 LC/MSD Trap-SL质谱仪(安捷伦公司,美国);Shimadazu LC-6A (SPD-10A) 制备型HPLC,同时配备RID-10A示差折光检测器(岛津公司,日本),Waters XBridge C18 (4.6 mm×250 mm,5 μm) 色谱柱 (沃特世公司,美国);中压制备液相色谱 (拜泰齐公司,瑞典),ODS色谱柱(50 mm×500 mm,50 μm,YMC公司,日本);旋光值在utopol Ⅱ型旋光仪(鲁道夫公司,美国)测定。
药材于2014年7月采集于广东省中山市五桂山,经黑龙江中医药大学佳木斯学院陈效忠副教授鉴定为剑叶凤尾蕨P. ensiformis全草。药材标本保存于黑龙江中医药大学佳木斯学院标本馆(标本号ID-g-20140726)。
2 方法
2.1 提取与分离 95%乙醇提取药材(10 kg) 3次,每次1 h,减压回收提取液至无醇味。提取液过聚酰胺柱色谱,依次用水,30%,50%,95%乙醇溶液洗脱,获得4个洗脱部位Fr.(A~D)。Fr.B (100 g) 过MCI 柱色谱,依次用水,30%,50%,95%乙醇溶液洗脱,获得4个部位 Fr. (B1~B4)。Fr.B-4 (20 g) 过MPLC色谱(甲醇洗脱,10%~100%)获得23个流分。Fr.B-4b(200.5 mg)过制备型HPLC (20%甲醇为流动相)获得化合物 9 (12.0 mg)和10 (10.0 mg)。Fr.B-4e(300.8 mg)过制备型HPLC (20%甲醇为流动相)获得化合物 1 (8.0 mg) 和3 (10.0 mg)。Fr.B-4f(200.8 mg)过制备型HPLC (30%甲醇为流动相)获得化合物 4 (12.0 mg) 和 6 (11.0 mg)。Fr.B-4g(800.8 mg)过制备型HPLC (35%甲醇为流动相)获得化合物 2 (15.0 mg),5 (10.0 mg),7 (15.0 mg)。Fr.B-4h(260.5 mg)过制备型HPLC (40%甲醇为流动相)获得化合物 8 (20.0 mg)。
2.2 MTT法测试细胞毒活性 3种人源肿瘤细胞(结肠癌细胞HCT-116、人肝癌细胞HepG2和人胃癌细胞BGC-823)用于化合物细胞毒活性测试。
方法如下:肿瘤细胞加入96孔细胞培养板,调整细胞悬液浓度达到1×104个/mL,在37 ℃,5%CO2的饱和水汽二氧化碳培养箱中培养3 h。每孔中加入不同浓度的待测样品(样品浓度依次为0.32,1.6,8.0,40.0,200.0 μmol·L-1),继续培养96 h。取出每孔内溶液,离心,吸去培养液,用PBS洗涤1次。每孔加0.1 mL PBS和10 μL MTT染液,继续培养3 h,然后每孔加入100 μL Formanzan溶解液。用酶标仪测定500 nm处的吸收度,最后用Reed-Muench法计算IC50。
3 化合物结构鉴定
化合物1 白色不定型粉末。根据ESI-MS m/z 303 [M+H]+,分子式为C20H30O2。1H-NMR (CDCl3,400 MHz) δ: 1.95 (1H,m H-1a),0.96 (1H,m,H-1b),1.90 (1H,m H-2a),1.88 (1H,m,H-2b),3.19 (1H,m,H-3),1.06 (1H,dd,J=13.0,3.0 Hz,H-5),1.90 (1H,m H-6a),1.60 (1H,m,H-6b),1.63 (1H,m H-7a),1.53 (1H,m,H-7b),1.06 (1H,m,H-9),1.67 (1H,m,H-11a),1.56 (1H,m,H-11b),1.95 (1H,m,H-12a),1.18 (1H,m,H-12b),2.68 (1H,m,H-13),1.60 (1H,m,H-14a),1.50 (1H,m,H-14b),2.10 (1H,m,H-15a),2.07 (1H,m,H-15b),4.83 (1H,m,H-17a),4.77 (1H,m,H-17b),1.29 (3H,s,H-18),9.78 (3H,s,H-19),0.95 (3H,s,H-20);13C-NMR (CDCl3,100 MHz) δ: 38.7 (C-1),28.5 (C-2),77.3 (C-3),52.2 (C-4),56.1 (C-5),20.5 (C-6),41.5 (C-7),43.6 (C-8),54.3 (C-9),39.2 (C-10),18.6 (C-11),39.9 (C-12),43.9 (C-13),32.8 (C-14),48.9 (C-15),155.3 (C-16),103.7 (C-17),19.6 (C-18),208.2 (C-19),16.7 (C-20)。 以上數据与文献[9]对比,故鉴定化合物为ent-3β-hydroxy-kaur-16-en-19-al。
化合物2 无色油状物。ESI-MS m/z 303 [M+H]+,分子式为C20H30O3。1H-NMR (CDCl3,400 MHz) δ: 1.86 (1H,m,H-1a),0.89 (1H,m,H-1b),1.60 (1H,m,H-2a),1.50 (1H,m,H-2b),1.98 (1H,m,H-3a),1.11 (1H,m,H-3b),1.69 (1H,m,H-5),1.20 (2H,m,H-6),1.68 (1H,m,H-7a),1.52 (1H,m,H-7b),1.20 (1H,m,H-9),1.60 (2H,m,H-11),1.63 (1H,m,H-12a),1.48 (1H,m,H-12b),2.68 (1H,m,H-13),1.79 (1H,m,H-14a),1.63 (1H,m,H-14b),2.10 (1H,d,J=16.0 Hz,H-15a),2.09 (1H,d,J=16.0 Hz,H-15b),4.83 (1H,s,H-17a),4.75 (1H,s,H-17b),1.18 (3H,s,H-18),1.09 (3H,s,H-20);13C-NMR (CDCl3,100 MHz) δ: 39.8 (C-1),18.2 (C-2),39.9 (C-3),47.7 (C-4),50.2 (C-5),23.3 (C-6),33.3 (C-7),44.0 (C-8),56.2 (C-9),38.8 (C-10),40.7 (C-11),17.8 (C-12),44.5 (C-13),37.0 (C-14),49.1 (C-15),155.9 (C-16),103.2 (C-17),16.2 (C-18),185.5 (C-19),17.9 (C-20)。 以上数据与文献[10]对比,故鉴定化合物为4-epi-kaurenic acid。
化合物3 无色油状物。ESI-MS m/z 315 [M+H]+,分子式为C20H26O3。1H-NMR (CDCl3,400 MHz) δ: 1.59 (1H,m,H-1a),1.05 (1H,m,H-1b),1.57 (2H,m,H-2),2.23 (1H,m,H-3a),1.46 (1H,m,H-3b),2.27 (1H,d,J=5.0 Hz,H-5),4.89 (1H,d,J=5.0 Hz,H-6),1.67 (1H,m,H-9),2.13 (1H,m,H-11a),1.42 (1H,m,H-11b),1.43 (1H,m,H-12a),1.26 (1H,m,H-12b),2.72 (2H,m,H-13),1.90 (1H,dd,J=13.0,6.0 Hz,H-14a),1.65 (1H,dd,J=13.0,4.0 Hz,H-14b),2.37 (1H,d,J=16.0 Hz,H-15a),2.28 (1H,d,J=16.0 Hz,H-15b),5.08 (1H,s,H-17a),4.93 (1H,s,H-17b),1.33 (3H,s,H-18),0.72 (3H,s,H-20);13C-NMR (CDCl3,100 MHz) δ: 34.6 (C-1),18.3 (C-2),29.2 (C-3),41.8 (C-4),52.9 (C-5),76.8 (C-6),209.3 (C-7),54.0 (C-8),57.5 (C-9),35.6 (C-10),32.0 (C-11),17.2 (C-12),37.6 (C-13),32.3 (C-14),48.4 (C-15),157.0 (C-16),108.5 (C-17),26.8 (C-18),180.2 (C-19),15.9 (C-20)。 以上数据与文献[10]对比,故鉴定化合物为mitrekaurenone。
化合物4 无色油状物。ESI-MS m/z 353 [M+H]+,分子式为C20H32O5。1H-NMR (CDCl3,400 MHz) δ: 1.78 (1H,m H-1a),0.86 (1H,m,H-1b),1.93 (1H,m H-2a),1.41 (1H,m,H-2b),2.08 (1H,d,m,H-3a),1.02 (1H,m,H-3b),1.70 (1H,m,H-5),2.06 (1H,m H-6a),1.90 (1H,m,H-6b),3.56 (1H,br s,H-7),1.37 (1H,m,H-9),1.58 (1H,m,H-11a),1.54 (1H,m,H-11b),1.60 (1H,m,H-12a),1.55 (1H,m,H-12b),2.01 (1H,m,H-13),1.80 (1H,d,J=12.0 Hz,H-14a),1.67 (1H,J=13.0,4.0 Hz,H-14b),1.65 (1H,d,J=14.0 Hz,H-15a),1.52 (1H,d,J=14.0 Hz,H-15b),3.65 (1H,d,J=12.0 Hz,H-17a),3.60 (1H,d,J=12.0 Hz,H-17b),1.09 (3H,s,H-18),0.91 (3H,s,H-20);13C-NMR (CDCl3,100 MHz) δ: 40.0 (C-1),20.1 (C-2),38.6 (C-3),44.1 (C-4),47.5 (C-5),30.0 (C-6),77.6 (C-7),48.7 (C-8),50.7 (C-9),39.8 (C-10),18.6 (C-11),27.0 (C-12),45.6 (C-13),36.1 (C-14),49.3 (C-15),81.8 (C-16),66.0 (C-17),29.1 (C-18),181.7 (C-19),15.8 (C-20)。以上数据与文献[11]对比,故鉴定化合物为7β,16α,17-trihydroxy-ent-kauran-19-oic acid。
化合物5 白色无定型粉末。ESI-MS m/z 403 [M+H]+,分子式为C24H34O5。1H-NMR (CDCl3,400 MHz) δ: 1.93 (1H,m,H-1a),0.87 (1H,m,H-1b),1.64 (2H,m,H-2),1.36 (3H,m,H-3),1.37 (1H,m,H-5),1.64 (1H,m,H-6a),1.11 (1H,m,H-6b),1.93 (1H,m,H-7a),1.36 (1H,m,H-7b),1.47 (1H,m,H-9),5.13 (1H,m,H-11),2.43 (1H,m,H-12a),1.47 (1H,m,H-12b),3.08 (1H,m,H-13),1.95 (2H,m,H-14),5.90 (1H,s,H-17a),5.25 (1H,s,H-17b),3.89 (1H,d,J=12.0 Hz,H-18a),3.66 (1H,d, J=12.0 Hz,H-18b),0.80 (3H,s,H-19),1.10 (3H,s,H-20),1.87 (3H,s,H-22),2.10 (3H,s,H-24);13C-NMR (CDCl3,100 MHz) δ: 39.0 (C-1),18.3 (C-2),35.4 (C-3),36.6 (C-4),48.8 (C-5),17.9 (C-6),33.3 (C-7),50.7 (C-8),59.8 (C-9),38.9 (C-10),68.3 (C-11),36.5 (C-12),36.8 (C-13),38.8 (C-14),209.2 (C-15),150.1 (C-16),113.4 (C-17),72.6 (C-18),17.9 (C-19),18.7 (C-20),170.1 (C-21),21.6 (C-22),171.7 (C-23),21.3 (C-24)。以上數据与文献[12]对比,故鉴定化合物为crotonkinin E。
化合物6 白色无定型粉末。ESI-MS m/z 403 [M+H]+,分子式为C24H34O5。1H-NMR (CDCl3,400 MHz) δ: 1.83 (1H,m,H-1a),0.79 (1H,m,H-1b),1.68 (2H,m,H-2),1.46 (1H,m,H-3a),1.35 (1H,m,H-3b),1.43 (1H,m,H-5),1.86 (1H,m,H-6a),1.43 (1H,m,H-6b),5.17 (1H,dd,J=10.0,4.5 Hz,H-7),1.33 (1H,m,H-9),1.67 (1H,m,H-11a),1.54 (1H,m,H-11b),2.00 (1H,m,H-12a),1.78 (1H,m,H-12b),3.13 (1H,m,H-13),2.18 (1H,m,H-14a),2.09 (1H,m,H-14b),6.01 (1H,s,H-17a),5.30 (1H,s,H-17b),3.90 (1H,d,J=11.0 Hz,H-18a),3.60 (1H,d,J=11.0 Hz,H-18b),0.83 (3H,s,H-19),1.17 (3H,s,H-20),1.90 (3H,s,H-22),2.17 (3H,s,H-24);13C-NMR (CDCl3,100 MHz) δ: 39.1 (C-1),18.2 (C-2),35.9 (C-3),36.7 (C-4),45.9 (C-5),24.8 (C-6),73.5 (C-7),56.7 (C-8),52.3 (C-9),39.8 (C-10),17.8 (C-11),32.6 (C-12),37.6 (C-13),29.8 (C-14),207.9 (C-15),148.9 (C-16),115.6 (C-17),72.3 (C-18),17.9 (C-19),18.5 (C-20),169.7 (C-21),21.4 (C-22),171.6 (C-23),21.5 (C-24)。以上数据与文献[12]对比,故鉴定化合物为crotonkinin F。
化合物7 白色无定型粉末。ESI-MS m/z 347 [M+H]+,分子式为C20H26O5。1H-NMR (CDCl3,400 MHz) δ: 1.87 (1H,m,H-1a),1.09 (1H,m,H-1b),1.76 (1H,m,H-2a),1.34 (1H,m,H-2b),2.08 (1H,m,H-3a),0.87 (1H,m,H-3b),1.26 (1H,d, J=6.0 Hz,H-5),5.10 (1H,m,H-6),2.32 (1H,dd,J=16.0,6.8 Hz,H-7a),1.67 (1H,brd,J=16.0 Hz,H-7b),5.66 (1H,dd,J=4.0 Hz, J=3.0 Hz,H-11),2.50 (1H,dd,J=18.0,3.0 Hz,H-12a),2.29 (1H,m,H-12b),1.79 (1H,d,J=11.0,4.0 Hz,H-14a),1.78 (1H,dd,J=11.0,1.0 Hz,H-14b),6.06 (1H,brs,H-17a),5.67 (1H,brs,H-17b),1.27 (3H,s,H-18),1.00 (3H,s,H-20);13C-NMR (CDCl3,100 MHz) δ: 41.5 (C-1),20.3 (C-2),38.8 (C-3),45.0 (C-4),56.3 (C-5),65.1 (C-6),34.2 (C-7),53.1 (C-8),148.2 (C-9),40.0 (C-10),122.5 (C-11),43.3 (C-12),74.6 (C-13),47.2 (C-14),203.5 (C-15),153.0 (C-16),118.1 (C-17),30.0 (C-18),180.3 (C-19),25.5 (C-20)。以上数据与文献[13]对比,故鉴定化合物为pterisolic acid A。
化合物8 白色无定型粉末。ESI-MS m/z 331 [M+H]+,分子式为C20H26O4。1H-NMR (CDCl3,400 MHz) δ: 1.79 (1H,brd,J=14.0 Hz,H-1a),1.09 (1H,m,H-1b),1.86 (1H,m,H-2a),1.37 (1H,m,H-2b),2.05 (1H,m,H-3a),0.93 (1H,m,H-3b),1.68 (1H,dd, J=11.0,7.0 Hz,H-5),2.16 (1H,m,H-6),1.87 (1H,m,H-7a),1.77 (1H,m,H-7b),5.58 (1H,dd,J=5.0,3.0 Hz,H-11),2.59 (1H,dd,J=16.0,3.0 Hz,H-12a),2.20 (1H,m,H-12b),1.79 (1H,d,J=12.0 Hz,H-14a),1.59 (1H,d,J=12.0 Hz,H-14b),5.87 (1H,brs,H-17a),5.58 (1H,brs,H-17b),1.19 (3H,s,H-18),1.07 (3H,s,H-20); 13C-NMR (CDCl3,100 MHz) δ: 42.0 (C-1),20.8 (C-2),38.5 (C-3),44.9 (C-4),48.0 (C-5),18.6 (C-6),26.0 (C-7),53.0 (C-8),150.0 (C-9),40.3 (C-10),123.5 (C-11),43.6 (C-12),74.2 (C-13),49.0 (C-14),202.9 (C-15),154.8 (C-16),116.7 (C-17),28.5 (C-18),180.3 (C-19),22.7 (C-20)。以上數据与文献[13]对比,故鉴定化合物为pterisolic acid C。
化合物9 白色无定型粉末。[α]25D -20.0 (c 0.15,CH3OH);ESI-MS m/z 235 [M+H]+,分子式为C14H18O3。1H-NMR (CDCl3,400 MHz) δ: 2.70 (1H,m,H-2),3.32 (1H,m,H-3a),2.65 (1H,m,H-3b),7.30 (1H,s,H-4),1.31 (1H,d,J=5.0 Hz,H-11),4.75 (1H,s,H-12),3.10 (2H,t,J=7.0 Hz,H-13),3.92 (2H,t,J=7.0 Hz,H-14),2.63 (1H,s,H-15);13C-NMR (CDCl3,100 MHz) δ: 210.9 (C-1),42.3 (C-2),34.2 (C-3a),125.5 (C-4),146.7 (C-5),135.5 (C-6),138.3 (C-7),134.0 (C-8),153.3 (C-9),16.3 (C-11),59.9 (C-12),30.3 (C-13),61.2 (C-14),13.7 (C-15)。以上数据与文献[14]基本一致,故鉴定化合物为(2R)-pterosin P。
化合物10 白色无定型粉末。ESI-MS m/z 217 [M+H]+,分子式为C14H16O2。1H-NMR (CDCl3,400 MHz) δ: 6.68 (1H,s,H-3a),7.03 (1H,s,H-4),1.89 (1H,s,H-11),2.35 (1H,s,H-12),2.98 (2H,t,J=7.0 Hz,H-13),3.79 (2H,t,J=7.0 Hz,H-14),2.58 (1H,s,H-15);13C-NMR (CDCl3,100 MHz) δ: 200.8 (C-1),126.3 (C-2),141.9 (C-3a),122.3 (C-4),142.9 (C-5),136.7(C-6),138.0 (C-7),135.8 (C-8),144.3 (C-9),10.9 (C-11),21.3 (C-12),32.5 (C-13),62.1 (C-14),13.8(C-15)。以上數据与文献[15]对比,故鉴定化合物为dehydropterosin B。
4 结果与讨论
本文对剑叶凤尾蕨化学成分和细胞毒活性做了系统的研究。从该植物中分离并鉴定了10个化合物,其中化合物1~6为首次从凤尾蕨属中分离获得,化合物7~10为首次从剑叶凤尾蕨中分离得到。细胞毒活性测试表明化合物5~8具有中等强度抑制人结肠癌细胞HCT-116,肝癌细胞HepG2,人胃癌细胞BGC-823活性,IC50见表1。本文的研究对剑叶凤尾蕨的开发利用奠定了理论基础。
[参考文献]
[1] 中国科学院中国植物志编辑委员会. 中国植物志. 第3卷. 第1分册[M].北京:科学出版社,1990:10.
[2] 龚先玲,陈志红,梁念慈. 凤尾蕨属植物化学成分及药理活性研究进展 [J]. 中国中药杂志,2007,32 (14):1382.
[3] 姚学军,孟素蕊,王喆. 凤尾草抗糖尿病活性成分的研究 [J]. 中成药,2014,36 (8):1782.
[4] Chen G G. Ent-11α-hydroxy-15-oxo-kaur-16-en-19-oic-acid inhibits growth of human lung cancer A549 cells by arresting cell cycle and triggering apoptosis [J].Chinese J Cancer Res,2012,24 (2):109.
[5] 陈四保,彭勇,陈士林,等. 药用植物亲缘学 [J]. 世界科学技术,2005,7 (6): 97.
[6] 龚先玲,苟占平,梁念慈,等. 凤尾蕨属6种药用植物抗肿瘤有效部位筛选 [J]. 时珍国医国药,2010,21 (7):1599.
[7] 刘勇,姚振生. 江西凤尾蕨属药用植物资源及其利用 [J]. 江西林业科技,1996 (6):29.
[8] 蔡宏亚,苟占平. 凤尾蕨属草药剑叶凤尾蕨的抗肿瘤活性初步研究 [J]. 辽宁中医药大学学报,2012,14 (8):80.
[9] Dutra L M,Bomfim L M,Rocha S L A,et al. Ent-kaurane diterpenes from the stem bark of Annona vepretorum,(Annonaceae) and cytotoxic evaluation [J]. Bioorg Med Chem Lett,2014,24(15):3315.
[10] Li C,Lee D H,Graf T N,et al. Bioactive constituents of the stem bark of Mitrephora glabra [J]. J Nat Prod,2009,72 (72):1949.
[11] Nhiem N X,Hien N T T,Tai B H,et al. New ent-kauranes from the fruits of Annona glabra,and their inhibitory nitric oxide production in LPS-stimulated RAW264.7 macrophages[J]. Bioorg Med Chem Lett,2015,25(2):254.
[12] Kuo P C,Yang M L,Hwang T L,et al. Anti-inflammatory diterpenoids from Croton tonkinensis [J]. J Nat Prod,2013,76 (2):230.
[13] Fei W,Li Y,Ren F,et al. Pterisolic acids A-F,new ent-kaurane diterpenoids from the fern Pteris semipinnata [J]. Chem Pharma Bull,2011,59 (4):484.
[14] Ouyang D W,Xiang N,Xu H Y,et al. Pterosins from Pteris multifida [J]. Planta Med,2010,76 (16):1896.
[15] Tanaka N,Satake T,Takahashi A,et al. Chemical and chemotaxonomical studies of ferns. XXXIX. Chemical studies on the constituents of Pteris bella Tagawa and Pteridium aquilinum subsp. wightianum (Wall) Shich [J]. Chem Pharm Bull,1982,30: 3640.
[责任编辑 丁广治]
——结构和生物活性
——青蒿素

