家蚕BmCaspase-8-Like(BmCasp8L)的免疫负调控功能
胡杰,王鑫怡,王菲
家蚕BmCaspase-8-Like(BmCasp8L)的免疫负调控功能
胡杰,王鑫怡,王菲
(西南大学家蚕基因组生物学国家重点实验室,重庆 400716)
【目的】在个体和细胞水平上探讨家蚕()BmCaspase-8-Like(BmCasp8L)的免疫负调控功能,为研究昆虫免疫负调控机制提供参考。【方法】通过RT-PCR技术克隆并进行结构域预测和进化分析;利用荧光定量PCR检测在家蚕各龄期、5龄第3天及预蛹期各组织中的时空表达特征和家蚕感染细菌后的免疫诱导表达特征;合成用于RNAi的dsRNA,通过注射dsRNA在个体中降低的表达水平,并检测对抗菌肽基因表达水平的影响;构建的细胞表达载体,通过质粒或dsRNA的转染在BmE细胞中过表达或降低的表达水平,并利用Western blot或定量PCR予以确认,同时检测抗菌肽基因表达水平的变化及转录因子BmRelish的切割。【结果】BmCasp8L与鳞翅目Caspase-6、哺乳动物Caspase-8同源,其序列与BmDredd、DmDredd N端分别具有61%、42%的相似性,但缺少C端的Caspase结构域。时空表达特征分析表明,在眠蚕期表达量高于起蚕期,在预蛹期、蛹第7天、蛾第1天表达量明显升高;在5龄第3天幼虫体内,在血细胞中的表达量明显高于其他组织,而在预蛹期,主要在丝腺中表达。免疫诱导表达谱显示,5龄第3天家蚕在注射感染黑胸败血芽孢杆菌或粘质沙雷氏菌1 h内,的表达水平上升,此后逐渐恢复正常。对5龄第2天家蚕注射dsCasp8L或dsEGFP,24 h后通过荧光定量PCR检测到的表达量在注射dsCasp8L的家蚕中显著下调,而抗菌肽的表达水平显著上调。在BmE细胞中过表达,抗菌肽的表达水平与对照相比显著降低,且转录因子BmRelish的切割受到抑制。在细胞中转染dsRNA后,的表达水平被有效降低,抗菌肽的表达水平显著上调。【结论】进化分析、表达特征以及细胞和个体上的功能研究表明BmCasp8L是一个具有免疫负调控作用的分子,通过抑制BmRelish的切割,抑制抗菌肽的表达,从而负调控Imd信号通路,降低的表达水平则导致抗菌肽表达水平升高,有助于家蚕抵御微生物病原的侵染。
家蚕;免疫负调控;BmCaspase-8-Like(BmCasp8L);表达特征;抗菌肽;BmRelish
0 引言
【研究意义】昆虫靠进化上保守的先天免疫系统抵御病原微生物的侵染,其免疫方式主要包括抗菌肽表达、吞噬作用、包埋作用和黑化作用,其中抗菌肽的表达主要依赖于两条NF-κB信号通路——Toll和Imd信号通路[1-2]。但NF-κB信号通路的过度激活,往往导致昆虫肠道菌群紊乱、寿命缩短、神经退行性疾病等现象[3-8],因此,免疫负调控分子和负调控机制逐渐被认可为免疫系统的一个重要组成部分。家蚕()作为鳞翅目模式昆虫,对其免疫负调控机制的研究,有助于设计合理的抗病品系,同时有助于深入研究家蚕及其他昆虫先天免疫系统。【前人研究进展】昆虫Imd信号通路主要由革兰氏阴性细菌所激活,革兰氏阴性细菌的胞壁组分DAP型肽聚糖(DAP-PGN)被细胞膜受体蛋白PGRP-LC或胞内受体PGRP-LE所识别,进而招募Imd分子,免疫信号经Imd、FADD、Dredd分子传递,最终激活转录因子Relish[9]。在信号传递过程中,被认为是哺乳动物Caspase-8同源分子的Dredd,既切割Imd又切割Relish:切割后的Imd被E3泛素化连接酶DIAP2催化形成K63-多聚泛素化链从而招募TAK1和IKK使Relish磷酸化;而Relish经Dredd切除其C端的锚蛋白重复序列(ankyrin repeat)后,具有转录活性的N端即进入细胞核启动抗菌肽分子的表达[10-11]。通过突变分析和全基因组RNAi等方法,已鉴定了多种参与Imd信号通路负调控的分子[12-14]。例如PGRP-LB、PGRP-SC1、PGRP-SC2等分泌型PGRPs能通过降解肽聚糖来抑制Imd信号通路的激活[15-18];Pirk通过干扰PGRP-LC、PGRP-LE和Imd的结合从而阻止信号的传递[19-21];IκB蛋白Pickle与Relish相互作用并招募HDAC1蛋白抑制Relish靶基因的启动子活性[22];E3泛素连接酶Dnr1则通过促进Dredd蛋白酶体降解来抑制Imd信号通路[23]。笔者研究团队在研究参与家蚕抗病毒免疫的BmSTING分子时,通过免疫共沉淀和质谱鉴定了一个与BmSTING具有相互作用的蛋白,其氨基酸序列与BmDredd的N端序列相似性高,而缺少其C端酶活结构域[24]。基于其序列特征,将其命名为BmCaspase-8-Like(BmCasp8L)。过表达该分子降低了由BmSTING介导的抗病毒免疫应答水平。该分子是否参与Imd信号通路的调控尚未可知。【本研究切入点】目前,对于Imd信号通路免疫负调控机制的认识主要建立在对果蝇的研究上,而缺乏对其他昆虫免疫负调控分子的鉴定和研究。本研究针对家蚕BmCasp8L,通过研究该分子对转录因子BmRelish的活化及抗菌肽基因表达的影响,解析其负调控机制。【拟解决的关键问题】分析BmCasp8L的序列特征并构建系统进化树,研究的时空表达及细菌免疫诱导表达特征,在此基础上进一步研究其对BmRelish的活化及抗菌肽基因表达的抑制作用。
1 材料与方法
试验于2016年9月至2018年3月在西南大学家蚕基因组生物学国家重点实验室完成。
1.1 供试材料与试剂
供试家蚕品种为大造,由西南大学家蚕基因库提供。试验所用家蚕胚胎细胞系BmE,以及感染家蚕所需黑胸败血芽孢杆菌()和粘质沙雷氏菌(),均由笔者实验室保存。
RNase抑制剂、反转录试剂盒、T7体外转录试剂盒均购自Promega公司;Total RNA Kit购自OMEGA公司;Tubulin抗体(AT819)、HRP标记山羊抗小鼠抗体(A0216)、Myc抗体(AM933)、RIPA细胞裂解液、BCA蛋白浓度测定试剂盒均购自碧云天公司;彩色预染蛋白质分子量标准、Super Signal West Femto化学发光检测试剂均购自Thermo Scientific;DAP型肽聚糖(Peptidoglycan,DAP-PGN)、Flag抗体(F1804-200UG)均购自Sigma公司;细胞转染试剂购自Roche公司;Grace昆虫细胞培养基和胎牛血清均购自Gibco公司;荧光定量PCR相关试剂、pMD-19T载体均购自TaKaRa公司;Myc-BmRelish真核表达载体的构建参考文献[25]。
1.2 BmCasp8L序列分析及系统发育树构建
从家蚕基因组数据库SilkDB(http://silkworm. genomics.org.cn/)中下载BmCasp8L序列BGIBMGA008021(GenBank登录号:XP-012552745.1),通过ClustalX软件进行多个物种同源序列比对,采用MEGA 4.0软件构建系统发生树。
1.3 样品RNA的提取及荧光定量PCR
取各龄期家蚕个体、5龄第3天幼虫及预蛹期个体的各组织,液氮研磨后,用Trizol法提取总RNA,或采用Total RNA Kit提取细胞样品的总RNA,按照反转录试剂盒说明书反转录为cDNA。
荧光定量PCR采用相对定量法,根据目标基因序 列设计特异定量引物,以sw22934作为参比基因,引物序列见表1。扩增程序为95℃ 3 s,60℃ 30 s,共40个循环,在退火/延伸阶段进行荧光信号数据采集,最后对熔解曲线进行分析。
1.4 BmCasp8L dsRNA的合成
根据的mRNA序列,用Primer 5.0软件设计引物BmCasp8L-FL(表1),经PCR扩增得到N端带有FLAG标签的BmCasp8L全长cDNA,连接到pMD19-T载体,经测序验证的阳性克隆用H I和I酶切后,插入pSL1180-A4载体中,用于真核细胞表达。
按照T7体外转录试剂盒的操作说明,用Primer 5.0软件设计用于干涉和(对照)的特异引物dsCasp8L和dsEGFP(表1),并合成相应的dsRNA,用于细胞和个体水平的基因干涉。
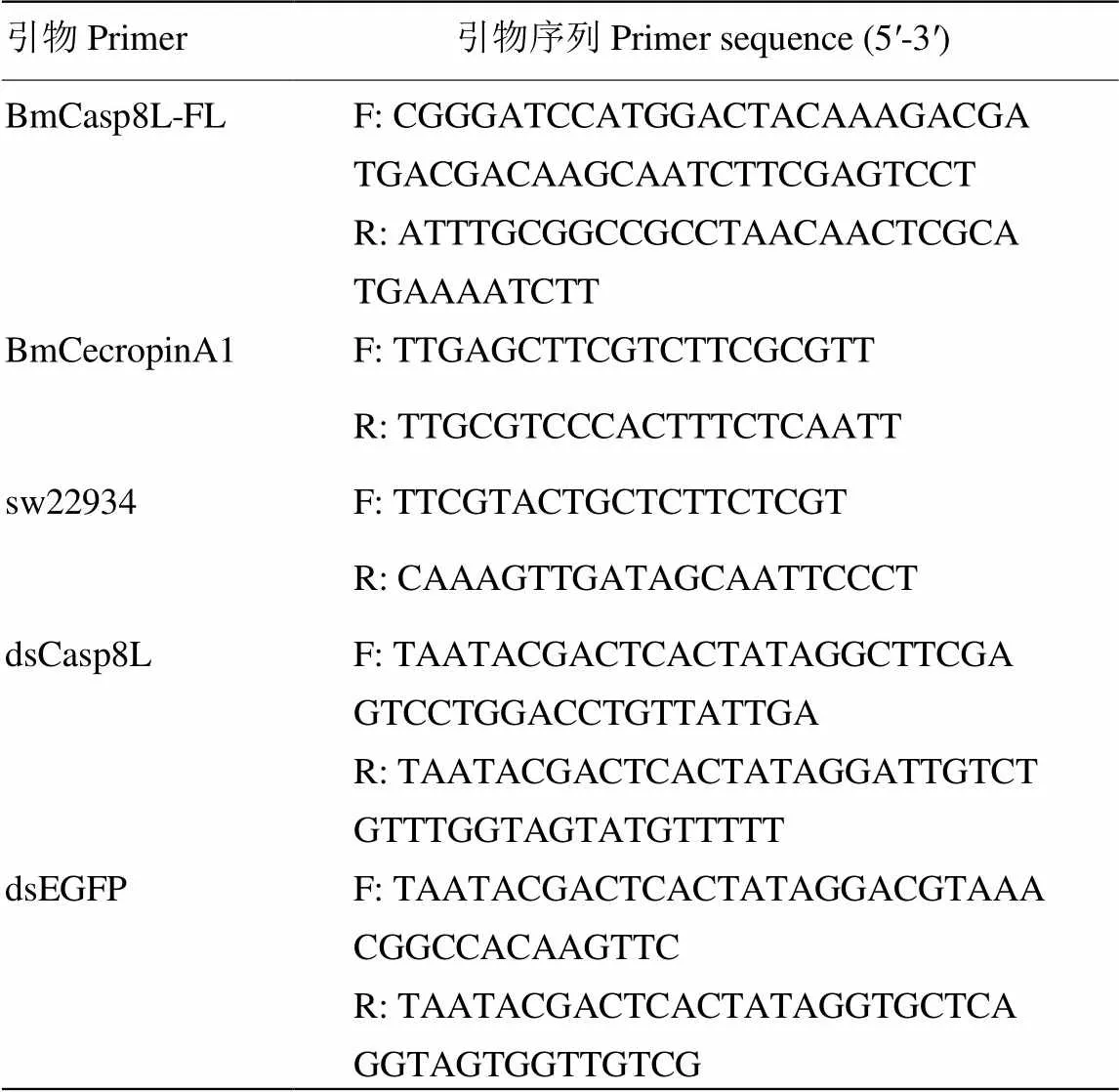
表1 引物序列
1.5 细胞转染
在27℃条件下,用含10%胎牛血清的Grace昆虫细胞培养基培养BmE细胞,血球计数板计数后接种至12孔板中(1×105个细胞/孔),24 h后按照转染试剂操作说明每孔转染1 μg DNA或5 μg dsRNA。转染48 h后加DAP-PGN刺激细胞12 h(10 μg·mL-1),收集细胞用于RNA提取或Western blot检测。
1.6 Western blot检测
用RIPA细胞裂解液裂解细胞后提取总蛋白,然后用BCA蛋白浓度测定试剂盒按照操作说明测定蛋白浓度,等量上样进行12% SDS-PAGE电泳,采用转膜仪将蛋白转移到PVDF膜上,5%脱脂奶粉封闭1 h后,用FLAG抗体、Myc抗体、Tubulin抗体室温孵育1 h,TBST清洗5次后用二抗室温孵育1 h,再用TBST清洗5次,最后用Super Signal West Femto化学发光检测试剂进行显色反应。
1.7 细菌感染试验
将黑胸败血芽孢杆菌和粘质沙雷氏菌于37℃过夜培养并计数后4℃保存备用。选取5龄第2天家蚕幼虫60头,分别用毛细管在腹部倒数第2对气孔处注射dsEGFP、dsCasp8L(2 μg·μL-1,10 μL/头),各注射30头。注射24 h后各取3头经液氮速冻后保存于-80℃用于RNA提取和干涉效率的定量PCR检测,其余幼虫注射粘质沙雷氏菌(1×102个/μL,10 μL/头),并在注射后0、1、3、6、12和18 h各取3头家蚕,经液氮速冻后保存于-80℃用于RNA提取。另取5龄第3天家蚕幼虫60头,以同样的方法直接注射黑胸败血芽孢杆菌或粘氏沙雷氏菌,各注射30头,并在注射后0、0.5、1、3、6和12 h各取3头家蚕,经液氮速冻后保存于-80℃用于RNA提取。
1.8 数据统计与分析
荧光定量PCR依据各样品中目标基因和内参基因的临界循环数(Ct)值,利用2-∆∆Ct计算分析。数据表示为平均值±标准偏差(SD),采用Student’s检验进行显著性差异分析或ANOVA单因素方差分析。<0.05表示差异显著,<0.01表示差异极显著。
2 结果
2.1 BmCasp8L序列分析及系统发生树的构建
BmCasp8L CDS全长858 bp,编码285个氨基酸,位于家蚕第9号染色体nscaf2889上,预测蛋白分子量大小为30 kD。其序列与BmDredd和DmDredd N端分别具有61%、42%的相似性,对该基因进行结构域预测,发现其缺少Dredd分子C端Caspase结构域(图1-A)。进化分析表明BmCasp8L、BmDredd与鳞翅目昆虫Caspase-6均为哺乳动物Caspase-8的同源分子(图1-B)。除家蚕外,在野蚕基因组中也发现了缺少编码Caspase结构域的。但在其他鳞翅目昆虫中,可能由于其基因组数据不完善,尚未鉴定到类似的。果蝇DmDredd具有多种剪接形式,其中Dredd-PF亚型缺乏Caspase酶活结构域。人Caspase-8也有多种剪接异构体,其中Caspase-8L亚型缺少C端酶活结构域。
2.2 BmCasp8L的时空表达及免疫诱导表达
采用荧光定量PCR对5龄第3天各组织内的表达水平进行检测,结果显示在各组织均有表达,在血细胞中表达量明显高于其他组织(图2-A)。对家蚕整个发育时期的表达情况进行定量检测,结果显示的表达水平在眠蚕期显著高于起蚕期;在发育变态期,如预蛹期、蛹第7天及蛾第1天的表达量显著升高(图2-B)。对在预蛹期各组织的表达水平进行荧光定量PCR检测,结果显示在丝腺中表达量最高(图2-C)。
5龄第3天家蚕个体在注射感染黑胸败血芽孢杆菌或粘质沙雷氏菌后的1 h内,的表达水平上升,1 h后的表达水平逐渐恢复正常水平(图2-D),而抗菌肽的表达量在注射粘质沙雷氏菌后持续上升(图2-E)。
2.3 家蚕幼虫干涉BmCasp8L
对5龄第2天家蚕幼虫注射 dsCasp8L(对照为dsEGFP),24 h后再注射感染粘质沙雷氏菌,然后通过荧光定量PCR检测家蚕中抗菌肽的表达量。结果显示,注射dsCasp8L的家蚕与注射dsEGFP的对照家蚕相比,的表达水平下降了53%(图3-A),且在感染粘质沙雷氏菌后,抗菌肽的表达水平明显升高(图3-B)。这一结果说明下调的表达水平导致抗菌肽的表达升高。
2.4 细胞中过表达及干涉BmCasp8L
BmE细胞转染质粒pSL1180-A4-Caspase-8-like后,对BmCasp8L的表达通过Western blot予以了验证(图4-A)。用DAP-PGN刺激过表达BmCasp8L的细胞,其抗菌肽的表达显著低于对照(图4-B)。
将合成的dsRNA转入BmE细胞中干涉的表达,并用荧光定量RCR检测干涉效率,结果显示在转染dsCasp8L的细胞中的表达水平下降了70%(图4-C)。干涉48 h后用DAP-PGN刺激细胞,在干涉的细胞中,抗菌肽的表达水平与对照细胞相比显著上升(图4-D)。
BmE细胞共表达BmCasp8L和BmRelish后,用DAP-PGN刺激细胞,通过Western blot对BmRelish的切割水平进行检测。DAP-PGN的刺激导致BmRelish(BmRelishFL)的活化增强,而过表达BmCasp8L后,BmRelish的活化形式(BmRelishact)明显减弱(图4-E)。这说明BmCasp8L的表达会抑制BmRelish的活化。
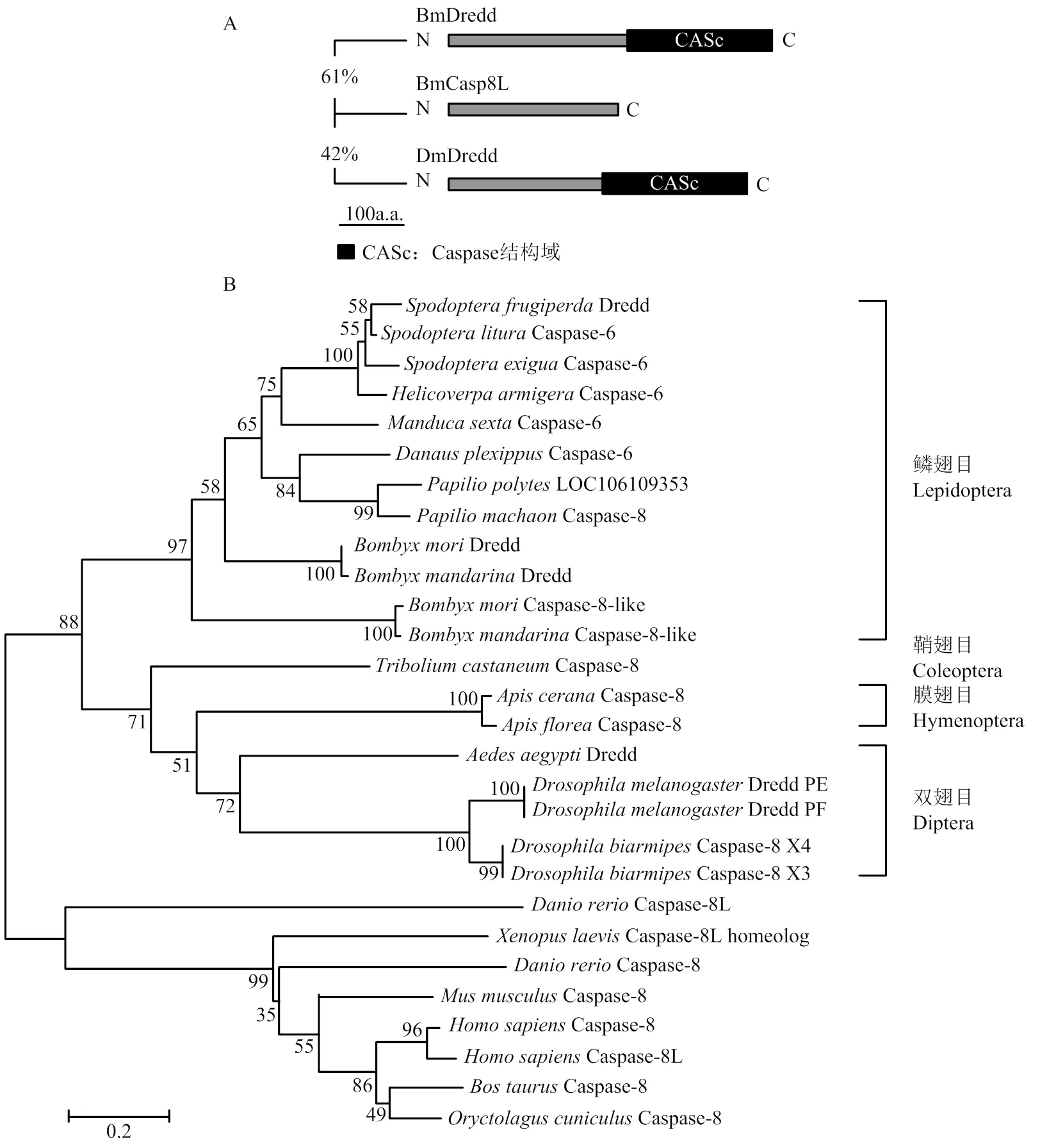
A:BmCasp8L与BmDredd、DmDredd结构域预测Domain prediction of BmCasp8L compared with BmDredd and DmDredd;B:BmCasp8L系统进化树Phylogenetic tree of BmCasp8L
3 讨论
与哺乳动物类似,昆虫的NF-κB信号通路受到严格的调控。Imd信号通路从信号识别、受体复合物的形成到信号传递及转录因子的切割活化等各阶段,均存在不同的负调控分子。对家蚕免疫负调控研究发现,Caspar的同源分子BmFAF,能与转录因子Relish结合并促进其降解,从而抑制抗菌肽的表达[25-26];家蚕肽聚糖识别蛋白PGRP-S5能利用其酰胺酶活性负调控Imd信号通路[27]。本研究在细胞和个体水平上对家蚕BmCasp8L是否影响抗菌肽的表达和BmRelish的活化进行了探索,结果表明其参与Imd信号通路的负调控。
和抗菌肽在家蚕受到细菌感染时,呈现相反的变化规律,暗示其可能对抗菌肽表达具有抑制作用。该抑制作用继而在家蚕个体水平上和细胞水平给予了验证,并且在细胞中过表达BmCasp8L后,BmRelish的活化形式减少,进一步说明BmCasp8L是通过抑制BmRelish的切割来抑制抗菌肽的表达。在预蛹期各组织的表达水平显示其在丝腺中表达量最高。预蛹期为蚕刚吐完丝向蛹期变化的过渡时期,在这个时期高表达可能暗示其参与丝腺细胞的凋亡,但具体作用尚不明确。的时空表达谱还显示,该基因在家蚕变态时期的表达水平显著升高,说明除免疫功能外,BmCasp8L还可能参与家蚕的发育进程。实际上,已有多项研究表明免疫负调控因子在昆虫发育过程中发挥作用。例如,Imd信号通路负调控因子Trabid的缺失会造成果蝇寿命缩短[7]。
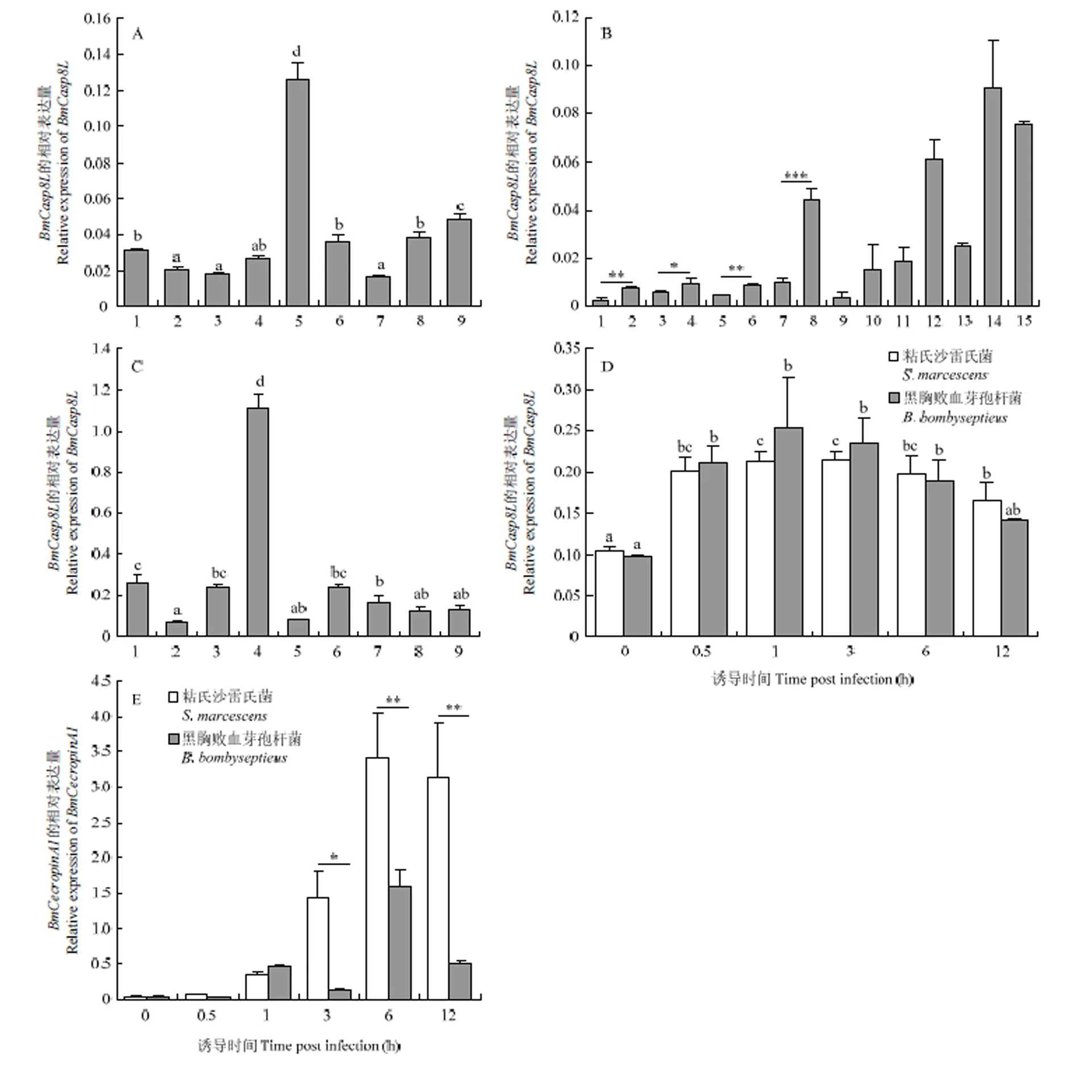
A:BmCasp8L的组织表达谱Expression profile of BmCasp8L in different tissues。1:头部Head;2:表皮Integument;3:精巢Testis;4:卵巢Ovary;5:血细胞Hemocyte;6:中肠Midgut;7:丝腺Silk gland;8:脂肪体Fat body;9:马氏管Malpighian tubule。B:BmCasp8L的时期表达谱Expression profile of BmCasp8L at different development stages。1:蚁蚕Hatch;2:1龄眠蚕1st-instar molting;3:2龄起蚕2nd-instar newly exuviated larva;4:2龄眠蚕2nd-instar molting;5:3龄起蚕3rd-instar newly exuviated larva;6:3龄眠蚕3rd-instar molting;7:4龄起蚕4th-instar newly exuviated larva;8:4龄眠蚕4th-instar molting;9:5龄起蚕5th-instar newly exuviated larva;10:5龄第3天3rd day of 5th-instar;11:5龄第7天7th day of 5th-instar;12:预蛹Prepupa;13:蛹第3天3rd day of pupa;14:蛹第7天7th day of pupa;15:蛾第1天1st day of moth。C:BmCasp8L预蛹期组织表达谱Expression profile of BmCasp8L in different tissues at prepupa stage。1:中肠Midgut;2:头部Head;3:脂肪体Fat body;4:丝腺Silk gland;5:表皮Integument;6:马氏管Malpighian tubule;7:血细胞Hemocyte;8:精巢Testis;9:卵巢Ovary。D:细菌感染家蚕后,BmCasp8L的表达Expression of BmCasp8L after injection of microbes。E:细菌感染家蚕后,抗菌肽BmCecropinA1的表达Expression of BmCecropinA1 after injection of microbes。P<0.05 *, P<0.01 **, P<0.001 ***
当DAP-PGN刺激细胞后,由Imd招募FADD,FADD通过其DED结构域与BmDredd N端DED结构域相互作用,从而形成复合物。而BmCasp8L与BmDredd N端相似性高达61%,因此BmCasp8L可能也具有与FADD结合的能力;通过与FADD的相互作用,干扰了BmDredd与FADD的结合,从而抑制了转录因子Relish的活化并最终影响了抗菌肽的表达。

A:注射不同dsRNA的家蚕中BmCasp8L的表达水平定量检测Quantitative analysis of the expression level of BmCasp8L in B. mori injected with different dsRNA。1:dsCasp8L;2:dsEGFP。B:注射不同dsRNA的家蚕中抗菌肽BmCecropinA1的表达水平定量检测Quantitative analysis of the expression level of BmCecropinA1 inB. mori injected with different dsRNAs。P<0.01 **, P<0.001 ***
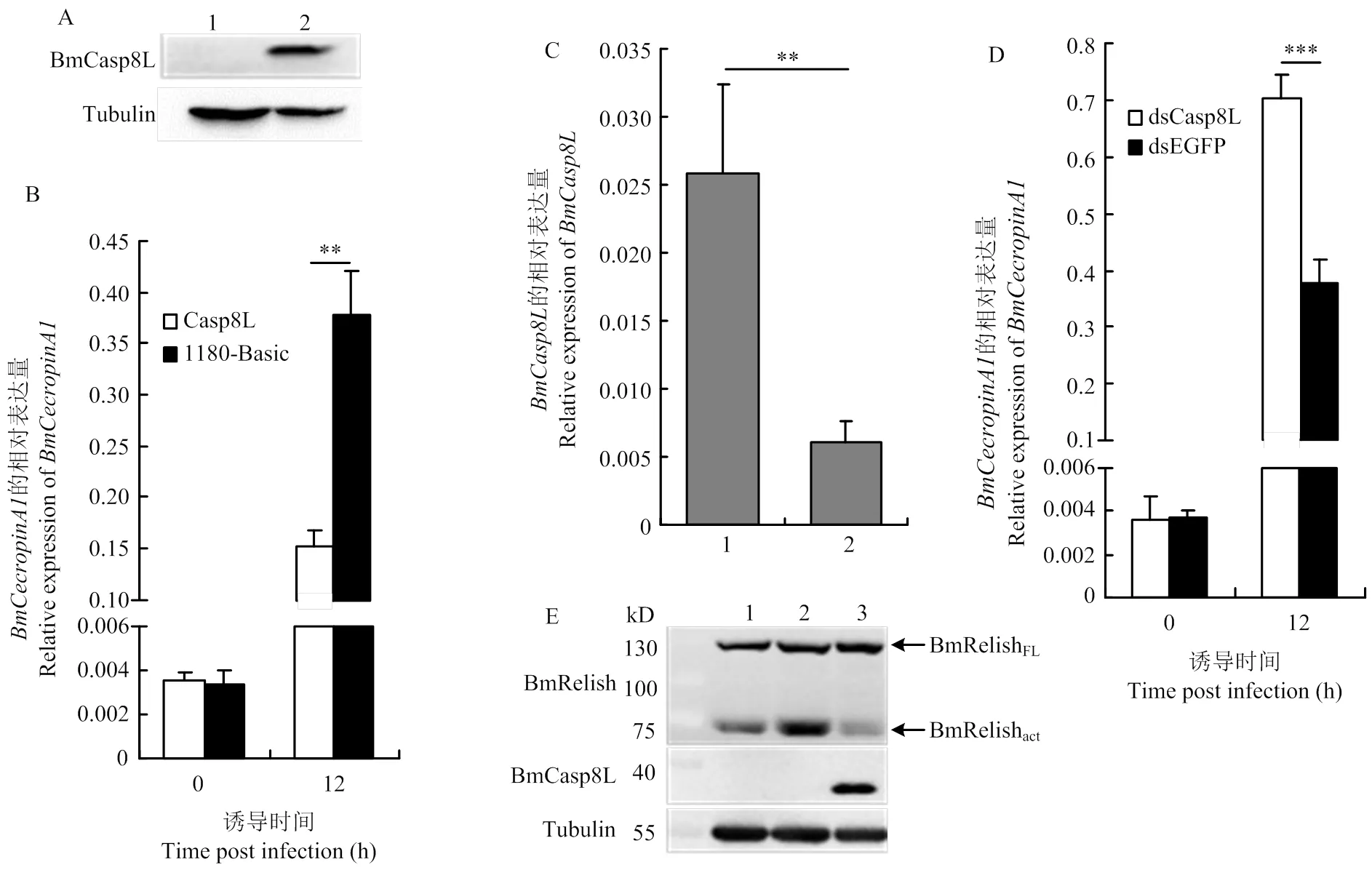
A:BmCasp8L的Western blot检测Western blot detection of BmCasp8L。1:转染pSL1180-A4空质粒的细胞Cells transfected with pSL1180-A4 empty vector;2:转染pSL1180-A4-Caspase-8-like质粒的细胞Cells transfected with pSL1180-A4-Caspase-8-like。B:转染pSL1180-A4-Caspase-8-like并用DAP-PGN刺激后抗菌肽BmCecropinA1的定量检测Quantitative analysis of BmCecropinA1 expression level. Cells transfected with pSL1180-A4-Caspase- 8-like, treated with DAP-PGN。C:BmCasp8L的定量检测Quantitative analysis of BmCasp8L expression level。1:转染dsEGFP的细胞Cells transfected with dsEGFP;2:转染dsCasp8L的细胞Cells transfected with dsCasp8L。D:转染dsCasp8L并用DAP-PGN刺激后抗菌肽BmCecropinA1的定量检测Quantitative analysis of BmCecropinA1 expression level. Cells transfected with dsCasp8L, treated with DAP-PGN。E:BmRelish的Western blot检测Western blot detection of BmRelish。1:转染pSL1180-A4-Relish质粒的细胞Cells transfected with pSL1180-A4-Relish;2:转染pSL1180-A4-Relish质粒并用DAP-PGN刺激的细胞Cells transfected with pSL1180-A4-Relish and treated with DAP-PGN;3:转染pSL1180-A4-Relish及pSL1180-A4-Caspase-8-like质粒并用DAP-PGN刺激的细胞Cells transfected with pSL1180-A4-Relish and pSL1180-A4-Caspase-8-like, treated with DAP-PGN。P<0.01 **, P<0.001 ***
有意义的是,果蝇和人均存在类似的与Caspase N端相似度高但缺乏其酶活结构域的分子。果蝇的Dredd基因编码5个转录本。亚型和亚型(Flybase数据库中现命名为Dredd-PE和Dredd-PD亚型)编码全长的Dredd蛋白,亚型(Dredd-PG亚型)编码的蛋白缺失Dredd的1—54 aa,亚型(Dredd-PF亚型)的mRNA因包含第2个内含子而导致蛋白翻译提前终止,故缺失C端的Caspase结构域。研究表明Dredd-PF亚型在不同组织中均有表达,且Dredd-PF亚型能抑制细胞凋亡。凋亡诱导因子的活性会影响果蝇体内Dredd-PE和Dredd-PF亚型之间的比例——负调控Dredd-PF亚型的积累;且缺失的果蝇在感染后存活率较野生型果蝇低,这些现象说明Dredd-PF亚型参与果蝇的发育和免疫,其高水平表达可能抑制果蝇的免疫应答[28]。人Caspase-8也具有多个剪接异构体,其中Caspase-8L缺少C端的Caspase结构域,研究表明Caspase-8L是Caspase-8a(即全长Caspase-8)的抑制分子,它能与FADD和Caspase-8a结合从而阻止Caspase 8a与FADD结合,因此负调控Fas介导的细胞凋亡[29-30]。并且在自身免疫疾病系统性红斑狼疮患者的外周血细胞中,Caspase-8L mRNA的水平明显低于正常个体,可能与过度免疫反应相关[31]。因此,BmCasp8L是否与Dredd-PF和Caspase-8L类似,在细胞凋亡中发挥负调控作用,也值得深入研究。
4 结论
从家蚕中克隆得到BmCasp8L,序列分析显示该分子与Dredd同源,时空表达和免疫诱导表达检测表明其具有免疫负调控分子特征,细胞及个体中该分子的过表达或干涉试验证明该分子能够抑制转录因子BmRelish的切割,并且抑制抗菌肽的表达从而发挥其免疫负调控功能,为进一步解析该分子在家蚕先天免疫信号通路中的负调控机制打下了基础。
[1] Lemaitre B, Hoffmann J. The host defense of., 2007, 25: 697-743.
[2] Imler J L. Overview ofimmunity: a historical perspective., 2014, 42(1): 3-15.
[3] Dantoft W, DAVIS M M, Lindvall J M, Tang X, Uvell H, Junell A, Beskow A, Engström Y. The Oct1 homolog Nubbin is a repressor of NF-κB-dependent immune gene expression that increases the tolerance to gut microbiota., 2013, 11: 99.
[4] Libert S, Chao Y, Chu X, Pletcher S D. Trade-offs between longevity and pathogen resistance inare mediated by NF-κB signaling., 2006, 5: 533-543.
[5] Kounatidis I, Chtarbanova S, Cao Y, Hayne M, Jayanth D, Ganetzky B, Ligoxygakis P. NF-κB immunity in the brain determines fly lifespan in healthy aging and age-related neurodegeneration., 2017, 19(4): 836-848.
[6] Cao Y,Chtarbanova S, Petersen A J, Ganetzky B. Dnr1 mutations cause neurodegeneration inby activating the innate immune response in the brain., 2013, 110(19): 1752-1760.
[7] Fernando M D, Kounatidis I, Ligoxygakis P. Loss of Trabid, a new negative regulator of theimmune- deficiency pathway at the level of TAK1, reduces life span., 2014, 10(2): e1004117.
[8] Bonnay F, COHEN-BERROS E, Hoffmann M, Kim S Y, Boulianne G L, Hoffmann J A, Matt N, Reichhart J M.gene modulates gut immune tolerance in., 2013, 110(8): 2957-2962.
[9] Myllymaki H, VALANNE S, Ramet M. Theimd signaling pathway., 2014, 192(8): 3455-3462.
[10] Meinander A, RUNCHEL C, Tenev t, Chen l, Kim C H, Ribeiro P S, Broemer M, Leulier F, Zvelebil M, Silverman N, Meier P. Ubiquitylation of the initiator caspase DREDD is required for innate immune signalling., 2012, 31(12): 2770-2783.
[11] 王菲, 李显扬, 化晓婷, 夏庆友. 家蚕抗BmNPV细胞因子的筛选和分析. 中国农业科学, 2018, 51(4): 789-799.
WANG F, LI X Y, HUA X T, XIA Q Y. Screening and analysis of Anti-BmNPV cytokines in silkworm ()., 2018, 51(4): 789-799. (in Chinese)
[12] Foley E, O’farrell P H. Functional dissection of an innate immune response by a genome-wide RNAi screen., 2004, 2(8): e203.
[13] Brennan C A, Anderson K V.: The genetics of innate immune recognition and response., 2004, 22(1): 457-483.
[14] Hoffmann J A. The immune response of., 2003, 426(6962): 33-38.
[15] Bischoff V, Vignal C, Duvic B, Boneca I G, Hoffmann J A, Royet J. Downregulation of theimmune response by peptidoglycan-recognition proteins SC1 and SC2.2006, 2(2): e14.
[16] Zaidman-Remy A, HERVE M, Poidevin M, Pili-Floury S, Kim M S, Blanot D, Oh B H, Ueda R, Mengin-Lecreulx D, Lemaitre B. Theamidase PGRP-LB modulates the immune response to bacterial infection., 2006, 24(4): 463-473.
[17] 陈康康, 吕志强. 昆虫肽聚糖识别蛋白研究进展. 昆虫学报, 2014, 57(8): 969-978.
Chen K K, Lü Z Q. Peptidoglycan recognition proteins (PGRPs) in insects., 2014, 57(8): 969-978. (in Chinese)
[18] Costechareyre D, Capo F, Fabre A, CHaduli D, KELLENBERGER C, ROUSSEL A, CHARROUX B, ROYET J. Tissue-specific regulation ofNF-κB pathway activation by peptidoglycan recognition protein SC., 2016, 8(1): 67-80.
[19] Kleino A, MYLLYMAKI H, Kallio J, Vanha-Aho L M, Oksanen K, Ulvila J, Hultmark D, Valanne S, Ramet M. Pirk is a negative regulator of theImd pathway., 2008, 180(8): 5413-5422.
[20] Aggarwal K, RUS F, Vriesema-Magnuson C, Ertürk- Hasdemir D, Paquette N, Silverman N. Rudra interrupts receptor signaling complexes to negatively regulate the IMD pathway., 2008, 4(8): e1000120.
[21] Lhocine N, RIBEIRO P S, Buchon N, Wepf A, Wilson R. Tenev T, Lemaitre B, Gstaiger M, Meier P, Leulier F. PIMS modulates immune tolerance by negatively regulatinginnate immune signaling., 2008, 4(2): 147-158.
[22] Morris O, LIU X, Domingues C, Runchel C, Chai A, Basith S, Tenev T, Chen H, Choi S, Pennetta G, Buchon N, Meier P. Signal integration by the IkappaB protein pickle shapesinnate host defense., 2016, 20(3): 283-295.
[23] Guntermann S, Primrose D A, Foley E. Dnr1-dependent regulation of theimmune deficiency signaling pathway., 2009, 33(1): 127-134.
[24] Hua X, Li B, Song L, Hu C, Li X, Wang D, Xiong Y, Zhao P, He H, Xia Q, Wang F. Stimulator of interferon genes (STING) provides insect antiviral immunity by promoting Dredd caspase- mediated NF-κB activation., 2018, 293(30): 11878-11890.
[25] Ma X J, Li X Y, Dong S F, Xia Q Y, Wang F. A Fas associated factor negatively regulates anti-bacterial immunity by promoting Relish degradation in., 2015, 63: 144-151.
[26] 马晓娟, 李亚明, 胡翠美, 王菲, 夏庆友. 家蚕免疫负调控分子的功能. 中国农业科学, 2014, 47(15): 3085-3093.
Ma X J, Li Y M, Hu C M, Wang F, Xia Q Y. Functional characterization ofas an immune negative regulatory molecule in silkworm ()., 2014, 47(15): 3085-3093. (in Chinese)
[27] Chen K, Zhou L, Chen F, Peng Y, Lu Z. Peptidoglycan recognition protein-S5 functions as a negative regulator of the antimicrobial peptide pathway in the silkworm,., 2016, 61: 126-135.
[28] Di Fruscio M, STYHLER S, Wikholm E, Boulanger M C, Lasko P, Richard S.interacts genetically with/, andmutants alter the balance ofisoforms., 2003, 100(4): 1814-1819.
[29] Himeji D, HORIUCHI T, Tsukamoto H, Hayashi K, Watanabe T, Harada M. Characterization of caspase-8L:a novel isoform of caspase-8 that behave as an inhibitor of the caspase cascade., 2002, 99(11): 4070-4078.
[30] Miller M a, KARACAY B, Zhu X, O'dorisio M S, Sandler A D. Caspase 8L, a novel inhibitory isoform of caspase 8, is associated with undifferentiated neuroblastoma., 2006, 11(1): 15-24.
[31] Horiuchi T, HIMEJI D, Tsukamoto H, Harashima S I, Hashimura C, Hayashi K. Dominant expression of a novel splice variant of caspase-8 in human peripheral blood lymphocytes., 2000, 272(3): 877-881.
Functional Characterization of BmCaspase-8-Like (BmCasp8L) as an Immune Negative Regulatory Molecule in Silkworm ()
HU Jie, WANG XinYi, WANG Fei
(State Key Laboratory of Silkworm Genome Biology, Southwest University, Chongqing 400716)
【Objective】The objective of this study is to characterize BmCaspase-8-like (BmCasp8L) as an immune negative regulatory molecule in silkworm () cell line as well as inlarvae, and to provide a basis for further studies of negative regulation mechanism in insect immunity.【Method】Domain prediction and phylogenetic analysis were performed after cloning ofby RT-PCR. Then fluorescence quantitative PCR was used to investigate the spatial-temporal expression profile ofat different development stages and in different tissues extracted from the 3rd day of 5th-instar larvae and prepupa, as well as the larvae body after bacterial infection. The dsRNA for RNAi was synthesized to silencein thelarvae, and the effect ofon expression of the anti-microbial peptides was studied. The plasmid for expressingin cells was constructed. After transfection of BmE cells with the expression constructs or dsRNA, Western blot or quantitative PCR was performed to confirm the over-expression or knock-down ofin cells. Meanwhile, the change in expression of the anti-microbial peptides and cleavage of nuclear transcription factor BmRelish were detected.【Result】BmCasp8L is homologous to Lepidoptera Caspase-6 and mammalian Caspase-8. The similarity between BmCasp8L and N-terminal of BmDredd, DmDredd is 61% and 42%, respectively, but it lacks the C-terminal Caspase domain. Spatial-temporal expression profile showed that in molting larvaelevel was higher than in newly exuviated ones, and theexpression level was significantly increased in the prepupa, 7th day of pupa and 1st day of moth stage. theexpression level in the hemocyte was significantly higher than in other tissues in the 3rd day of 5th-instar, but in the prepupa stage, it was mainly expressed in the silk gland. theexpression level in the 3rd day of 5th-instar larvae increased within 1 h post infection ofor, and then gradually returned to normal. Injection of dsCasp8L or dsEGFPinto the 2nd day of 5th-instar larvae,the expression ofwas significantly down-regulated ininjected with dsCasp8L, while the expression of antimicrobial peptidewas up-regulated after 24 h. Over-expression ofin BmE cells led to a remarkable decrease of the anti-microbial peptide. In addition, over-expression ofsuppressed the cleavage of the transcription factor BmRelish. Moreover, the expression level ofcould be efficiently knocked down by dsRNA, at the same time, the expression level ofwas significantly up-regulated. 【Conclusion】Phylogenetic analysis, expression features and functional studies in cells as well as inlarvae all indicated that BmCasp8L acts as an immune negative regulatory molecule by suppressing the cleavage of BmRelish and the expression of anti-microbial peptides, thereby negatively regulating the Imd signaling pathway, and down-regulation ofresulted in an increase of anti-microbial peptides which would potentially increase the resistance oflarvae to bacterial infection.
; immune negative regulation; BmCaspase-8-Like (BmCasp8L); expression feature; anti-microbial peptide; BmRelish
10.3864/j.issn.0578-1752.2018.21.017
2018-06-26;
2018-08-15
国家自然科学基金面上项目(31672495)
胡杰,Tel:023-68250748;E-mail:swuHj2016@163.com。通信作者王菲,Tel:023-68251569;E-mail:fwangswu@gmail.com
(责任编辑 岳梅)

