髓系细胞触发受体-1与动脉粥样硬化关系的研究进展
陆 娇综述 李承彬审校
髓系细胞触发受体-1与动脉粥样硬化关系的研究进展
陆 娇综述 李承彬*审校
髓系细胞触发受体-1(TREM-1)是新近发现的免疫球蛋白超家族活化跨膜受体,能够触发和扩大炎症反应,在动脉粥样硬化(AS)的炎症机制中起重要作用。本文从TREM-1 的结构特点、表达方式、配体结合、信号通路等论述其与AS发生发展及动脉斑块稳定性的关系和作用,为临床研究AS的靶向治疗提供参考和借鉴。
髓系细胞触发受体-1;炎症反应;动脉粥样硬化
髓系细胞触发受体-1(Triggering Receptor Expressed on Myeloid Cells-1,TREM-1)为TREM家族成员之一,是一种免疫球蛋白超家族活化受体,其在体内的存在方式包括膜结合型(Membrane-bound TREM-1,mTREM-1)和可溶型(Soluble TREM-1,sTREM-1)。动脉粥样硬化(Atherosclerosis,AS)作为冠心病、脑卒中等缺血性疾病的主要病理基础,其特征为脂质、纤维斑块在动脉壁累积,是主要由固有免疫和适应性免疫应答引起的慢性炎症性病变。已有研究[1]表明,mTREM-1介导的信号通路可引起单核细胞大量分泌白细胞介素-1α(Interleukin-1α,IL-1α)、白细胞介素-β(IL-β)等炎性因子,促进动脉粥样化改变,因而mTREM-1与AS的形成与发展有关,有可能成为治疗AS的新靶点。
1 TREM-1的来源和结构
Bouchon等[2]在寻找DNAX激活蛋白12(DNAX-Activating Protein 12,DAP12)相关受体的研究中,通过已知的DAP12相关受体cDNA序列,在文库中筛选发现了新的免疫球蛋白超家族受体,该受体主要表达于单核细胞、巨噬细胞等髓系细胞表面,是一种能够介导炎症反应的受体,因其是TREM家族成员中第一个被发现的,故命名为TREM-1。
mTREM-1分子量约30kDa,由194个氨基酸构成的胞外区、29个氨基酸构成的跨膜区及含有5个氨基酸的胞浆区组成。胞外区又可分为V型免疫球蛋白区(V Type Immune Globulin Domain,Ig-V)
和颈区;Ig-V是mTREM-1配体结合区域,颈区是Ig-V与跨膜区连接的重要部位。mTREM-1跨膜区含有带正电的赖氨酸残基,当mTREM-1与配体结合后,该区可与DAP12跨膜区带负电的天冬氨酸残基结合,从而使mTREM-1的胞外区与DAP12衔接。mTREM-1的胞浆区很短,没有信号转导序列,因此,mTREM-1需与DAP12偶联,通过DAP12胞浆区的免疫受体酪氨酸激活基序(Immunoreceptor Tyrosine-based Activation Motifs,ITAM)将胞外信号传递至胞浆区。
关于sTREM-1的来源目前尚存争议,一种观点认为其来自TREM-1 mRNA的可变剪接体,另一种观点认为其来自mTREM-1经基质金属蛋白酶(Matrix Metallo Proteinases,MMPs)裂解形成的脱落体。有研究[3]发现,被激活的单核细胞在MMPs抑制剂作用下,细胞表面的mTREM-1表达增加,同时伴随sTREM-1含量减少,推测sTREM-1可能是TREM-1的裂解体,这与第二种假说观点一致;该研究同时证实,sTREM-1分子量为 27 kDa,而TREM-1 mRNA可变剪接体分子量最大只有17 kDa,亦即TREM-1 mRNA的可变剪接体不可能成为sTREM-1的来源。虽然sTREM-1的来源与功能尚未阐明,但其分子结构和mTREM-1胞外区相似,具有相同的免疫球蛋白样结构域,是配体的结合位点[4]。
2 TREM-1的表达
mTREM-1表达于中性粒细胞、单核细胞和巨噬细胞等髓系细胞表面,也表达于非髓系细胞中,如平滑肌细胞等[5]。mTREM-1表达受多种因子调节,如维生素 D3和脂多糖能够诱导mTREM-1表达,其机制主要通过转录因子,如激活蛋白-1(Activated Protein-1,AP-1)、核转录因子-κB(Nuclear Transcription Factors-κB,NF-κB)、缺氧诱导因子(Hypoxia Inducing Factors,HIFs)等,与mTREM-1基因启动子区域结合,激活启动子,增强mTREM-1 mRNA表达[6]。此外,真菌、氧化型低密度脂蛋白以及肿瘤坏死因子-α(Tumor Necrosis Factor-α,TNF-α)也能使髓系细胞的mTREM-1表达上调[5, 7, 8]。mTREM-1的表达可触发和扩大炎症的级联反应,对炎性疾病和自身免疫性疾病的发生发展起重要作用[9, 10]。
sTREM-1作为可溶性蛋白受体,主要存在于血浆、尿液、脑脊液等体液中。在炎症性疾病和自身免疫性疾病患者的体液中可以检测到sTREM-1,通常sTREM-1的表达与疾病的严重程度呈正相关[9,11],因此sTREM-1有可能作为疾病诊断和预后的生物学标志物。
3 TREM-1配体
mTREM-1只有与其配体结合,才能引起信号级联反应。EI Mezayen等[12]发现,高迁移率族蛋白1 (High Mobility Group Box1,HMGB1)和热休克蛋白70(Heat Shock Protein70,HSP70)作用于单核细胞,可促进单核细胞分泌炎性因子,封闭mTREM-1可减少单核细胞分泌炎性因子,据此推测HMGB1和HSP70可能是mTREM-1配体。Wong-Baeza等[13]认为, mTREM-1配体也可能存在于病原体,如细菌壁脂多糖,并参与mTREM-1活化。 Read等[14]近年发现,肽聚糖识别蛋白1能激活mTREM-1,增加中性粒细胞和巨噬细胞分泌炎性因子,可能成为mTREM-1配体。总的来看,mTREM-1天然配体尚处探索阶段,有待继续深入研究。由于sTREM-1分子结构与mTREM-1胞外区具有相同的结构域,sTREM-1可与mTREM-1竞争结合配体,对mTREM-1介导的信号转导具有抑制作用[15]。
4 TREM-1的信号通路
由于mTREM-1天然配体还未肯定,故在mTREM-1信号通路和功能研究中,通常采用mTREM-1激动剂。在单核细胞中,激活的mTREM-1跨膜区与衔接蛋白DAP12偶联,触发酪氨酸蛋白激酶Src家族活化,活化的Src激酶促使DAP12的ITAM酪氨酸发生磷酸化,继而激活非受体型脾酪氨酸激酶(Spleen Tyrosine Kinase,SYK),引起下游信号通路的级联反应。下游信号通路涉及调节分子和信号分子的趋化和磷酸化,这些分子主要包括磷脂酰肌醇3激酶(Phosphatidylinositol 3-Kinase,PI3K)、Bruton蛋白激酶(Bruton Protein Kinase,BTK)、磷脂酶C-γ(PhospholipaseC-γ,PLC-γ)和胞外信号调节激酶1/2(Extracellular Signal Regulated Kinase1/2,ERK1/2)[2, 16]。通过这些分子的级联反应引起胞內[Ca2+]快速增高、肌动蛋白细胞骨架重构,促进NF-κB抑制子(Inhibitor of NF-κB,IκB )磷酸化及降解,使NF-κB活性增强和细胞核内NF-κB亚单位P50/P65水平增高,最终引起炎性因子表达。在中性粒细胞中,mTREM-1的激活触发 Src激酶家族、Janus 激酶2(Janus Kinase 2,JAK2)、BTK、丝氨酸/苏氨酸激酶(Serine/Threonine Kinase,AKT)及 ERK1/2磷酸化,最后激活信号转导和转录活化蛋白5/3(Signal Transducer and Activator of Transcription 5/3,STAT5/3)及NF-κB ,增加炎性因子的表达以及活性氧的释放[16, 17]。而树突细胞中的胱天蛋白酶募集域蛋白9(Caspase Recruitment Domain 9,CARD9)和B细胞淋巴瘤10(B-cell Lymphoma 10,Bcl10)复合体对NF-κB也起重要调节作用[18]。而非T细胞激活连接器(Non-T Cell Activation Linker,NTAL)通过与生长因子受体结合蛋白2(Growth Factor Receptor-bound Protein2,Grb2)和鸟嘌呤核苷酸交换因子Sos1结合,可抑制ERK1/2磷酸化,减少炎性因子表达[19]。图1总结了mTREM-1在髓系细胞中的信号转导和调节途径(因sTREM-1缺乏跨膜区及胞浆区,故不能进行信号转导和调节)。
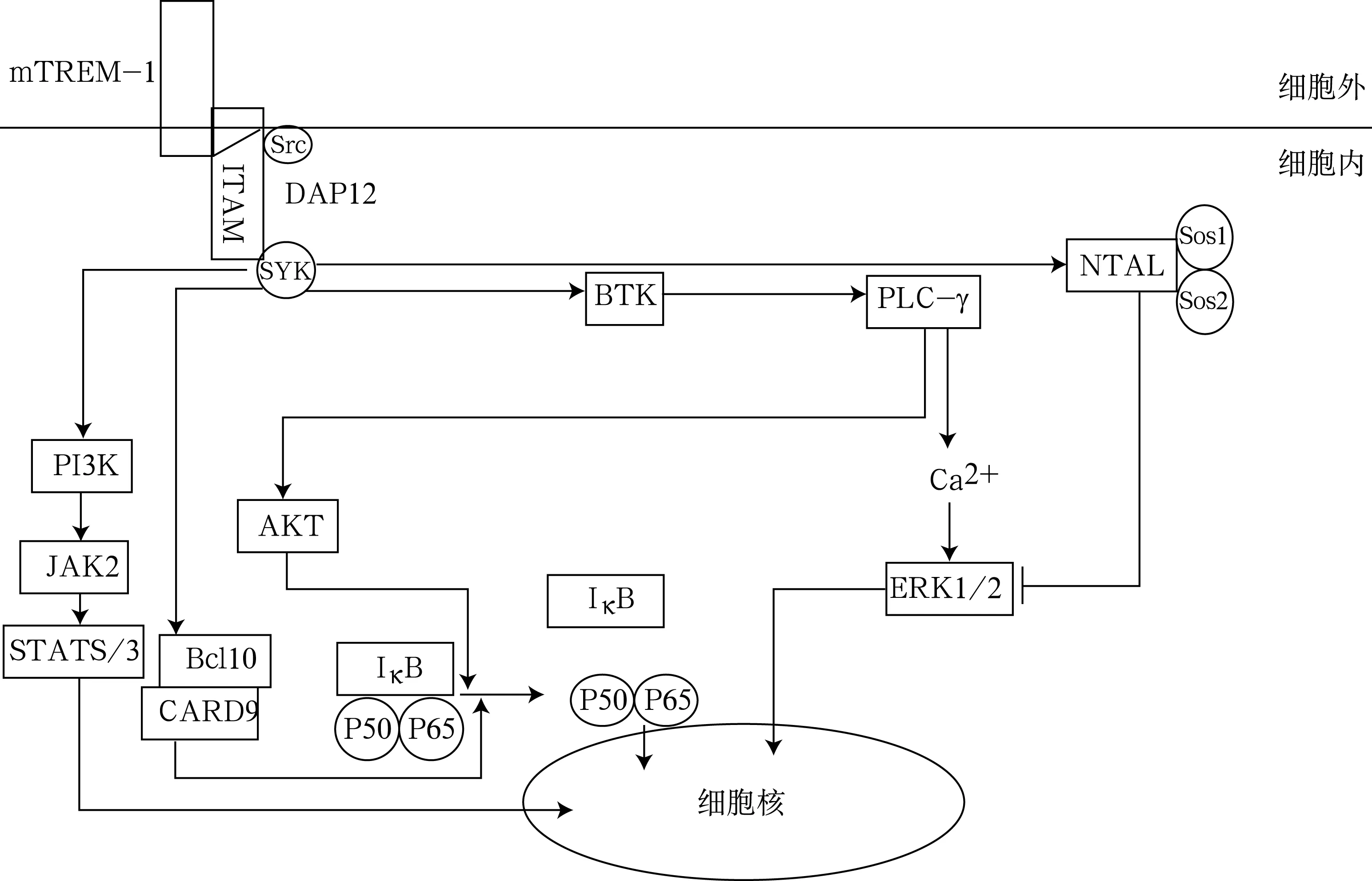
注:mTREM-1:膜结合型髓系细胞受体-1;ITAM:免疫受体酪氨酸激活基序;Src:Src激酶;DAP12:DNAX激活蛋白12;SYK:脾酪氨酸激酶;BTK:Bruton蛋白激酶 ; PLC-γ:磷脂酶C-γ ;NTAL: 非T细胞激活连接器 ;Sos1:鸟嘌呤核苷酸交换因子;Grb2:生长因子受体结合蛋白2;PI3K:磷脂酰肌醇3激酶;JAK2:Janus 激酶2;STAT5/3:信号转导和转录活化蛋白5/3;Bcl10:B细胞淋巴瘤10;CARD9:胱天蛋白酶募集域蛋白9;AKT:丝氨酸/苏氨酸激酶;IκB:核转录因子-κB抑制子;ERK1/2:胞外信号调节激酶1/2
图1 mTREM-1在髓系细胞中的信号转导和调节途径示意图
5 TREM-1对AS的作用
5.1 TREM-1与AS形成
AS的发生包括内皮损伤和基质改变,炎症反应贯穿其中。研究[20, 21]表明,白细胞介素-8(IL-8)能使巨噬细胞积聚于动脉内膜,TNF-α可通过抑制巨噬细胞内脂质代谢,促进泡沫细胞的形成。而mTREM-1能够触发IL-8、TNF-α大量释放,扩大炎症反应,加速AS形成。Zysset等[1]比较高脂食物喂养TREM-1-/- Apoe-/-小鼠和TREM-1+/+ Apoe-/-小鼠动脉硬化病变面积及斑块大小时发现,前者明显小于后者(P<0.001),提示mTREM-1与AS的形成有关;进一步研究发现其机制是血脂异常促进了髓系细胞表面mTREM-1的表达,mTREM-1通过介导有关信号激发骨髓单核细胞分化,引起循环单核细胞增多,进而导致单核细胞-巨噬细胞局部浸润,同时mTREM-1还影响脂类代谢,增加胞內脂质积累,增加泡沫细胞形成。因此,mTREM-1对AS的形成有重要作用。但sTREM-1与AS形成的关联性还不清楚,有待探究。
5.2 TREM-1与动脉斑块稳定性
戴道鹏等[22]通过抽取首次行冠脉造影的稳定型心绞痛患者空腹血,检测血清sTREM-1浓度,结果显示,这些患者中有斑块进展者sTREM-1含量明显高于无斑块进展者,并且sTREM-1水平升高与斑块进展呈正相关,提示血清sTREM-1可以反映斑块进展。Rao 等[5]的研究结果表明,mTREM-1活化能够激活MMP1和MMP9,导致胶原蛋白降解,引起颈动脉斑块不稳定,即mTREM-1可能影响斑块稳定性。继续对患者颈动脉斑块中树突状细胞的mTREM-1观察发现,有症状患者mTREM-1表达量较无症状患者增加,进一步说明mTREM-1水平升高与斑块不稳定有关[23]。
5.3 TREM-1与心肌梗死预后
心肌梗死后引起的免疫应激反应,使机体分泌炎性细胞因子和趋化因子增多,巨噬细胞和单核细胞被募集于梗死区,不但有利于瘢痕形成,持久的炎症还会促进病理性心室重构以及心脏功能失调。通过敲除TREM-1的基因或者使用mTREM-1抑制肽LR12封闭mTREM-1活性,能够抑制心肌的炎症反应,限制白细胞募集,从而改善心脏功能,提高心肌存活率[11]。临床检测结果显示,急性心肌梗死患者血清sTREM-1浓度明显升高,可以作为心肌梗死预后的一个独立预测因子[11]。
6 TREM-1基因多态性与AS
编码TREM-1基因位于人类6号染色体,有rs2234237、rs6910730、rs1817537等1 926个单核苷酸多态位点。截至目前,有关TREM-1基因多态性与AS的研究较少。Golovkin等[24]的研究表明,冠心病的TREM-1基因多态性表现出多重特征:rs2234237位点的AA基因型、 rs6910730位点的GG基因型、rs9471535位点的CC基因型以及rs4711668 位点的TT基因型与冠心病风险呈正相关,而rs1817537 位点的G等位基因、rs2234246位点的T等位基因和 rs3804277位点的T等位基因与冠心病风险呈负相关;rs4711668位点的TT基因型和rs7768162位点的AA基因型与女性冠心病风险的相关性更加显著;携带rs1817537 位点的G等位基因、rs2234246位点的T等位基因和 rs3804277位点的T等位基因女性罹患冠心病风险较低。Kutikhin等[25]也报道rs4711668位点多态性与冠心病的严重程度有关。
7 小结与展望
综上所述,mTREM-1介导的信号通路可促进单核细胞、中性粒细胞等髓系细胞对炎性因子的分泌,在触发和放大炎症反应中发挥重要作用,参与AS形成。然而,mTREM-1的天然配体还未明确,其在AS形成机制中的具体作用有待继续研究。血清sTREM-1与斑块稳定性有关,有可能成为预测动脉斑块稳定性的血清学指标。目前,TREM-1基因多态性与动脉粥样硬化性疾病的相关性备受关注,对其深入研究,有望为临床对相关疾病的诊疗与预后提供新靶标。
◀
本文作者简介:
陆 娇(1992—),女,汉族, 硕士研究生,研究方向为冠心病的机制研究
1 Zysset D,Weber B,Rihs S,et al. TREM-1 links dyslipidemia to inflammation and lipid deposition in atherosclerosis[J]. Nature communications,2016,7(1):13 151.
2 Bouchon A,Dietrich J,Colonna M. Cutting edge: Inflammatory responses can be triggered by TREM-1,a novel receptor expressed on neutrophils and monocytes[J]. The Journal of Immunology,2000,164(10):4 991-4 995.
4 Tammaro A,Derive M,Gibot S,et al . TREM-1 and its potential ligands in non-infectious diseases:from biology to clinical perspectives[J]. Pharmacology & therapeutics,2017,[in press].
5 Rao VH,Rai V,Stoupa S,et al. Tumor necrosis factor-alpha regulates triggering receptor expressed on myeloid cells-1-dependent matrix metalloproteinases in the carotid plaques of symptomatic patients with carotid stenosis[J]. Atherosclerosis,2016,248(1):160-169.
6 Hosoda H,Tamura H,Nagaoka I. Evaluation of the lipopolysaccharide-induced transcription of the human TREM-1 gene in vitamin D3-matured THP-1 macrophage-like cells[J]. International journal of molecular medicine,2015,36(5):1 300-1 310.
7 Li HX,Hong FF,Pan SB,et al. Silencing triggering receptors expressed on myeloid cells-1 impaired the inflammatory response to oxidized low-density lipoprotein in macrophages[J]. Inflammation,2016,39(1):199-208.
8 Zhong J,Huang WL,Deng QC,et al. Inhibition of TREM-1 and dectin-1 alleviates the severity of fungal keratitis by modulating innate immune responses[J]. PLoS One,2016,11(3):e0150 114.
9 Zhang SL,Luo J,Shen BB,et al. Correlation between triggering receptor expressed on myeloid cells-1 and clinical disease activity in Chinese patients with ulcerative colitis[J]. International journal of clinical and experimental medicine,2015,8(2):2 147-2 155.
10 Fan DP,He XJ,Bian YQ,et al. Triptolide modulates TREM-1 signal pathway to inhibit the inflammatory response in rheumatoid arthritis[J]. International Journal of Molecular Sciences,2016,17(4):498.
11 Boufenzer A,Lemarie J,Simon T,et al. TREM-1 mediates inflammatory injury and cardiac remodeling following myocardial infarction[J]. Circulation research,2015,116(11):1 772-1 782.
12 El Mezayen R,El Gazzar M,Seeds MC,et al. Endogenous signals released from necrotic cells augment inflammatory responses to bacterial endotoxin[J].Immunology Letters,2007,111(1):36-44.
13 Wong-Baeza I1,González-Roldán N,Ferat-Osorio E,et al. Triggering receptor expressed on myeloid cells (TREM-1) is regulated post-transcriptionally and its ligand is present in the sera of some septic patients[J]. Clinical and Experimental Immunology,2006,145(3):448-455.
14 Read CB,Kuijper JL,Hjorth SA,et al. Cutting edge:identification of neutrophil PGLYRP1 as a ligand for TREM-1[J]. Journal of Immunology,2015,194(4):1 417-1 421.
15 Baruah S, Keck K,Vrenios M,et al. Identification of a novel splice variant isoform of TREM-1 in human neutrophil granules[J]. Journal of Immunology ,2015,195(12):5 725-5 731.
16 Ormsby T,Schlecker E,Ferdin J,et al. Btk is a positive regulator in the TREM-1/DAP12 signaling pathway[J]. Blood,2011,118(4):936-945.
17 Fortin CF,Lesur O,Fulop T. Effects of TREM-1 activation in human neutrophils:activation of signaling pathways,recruitment into lipid rafts and association with TLR4[J]. International immunology,2007,19(1):41-50.
18 Hara H,Ishihara C,Takeuchi A,et al. The adaptor protein CARD9 is essential for the activation of myeloid cells through ITAM-associated and Toll-like receptors[J]. Nature Immunology,2007,8(6):619-629.
19 Tessarz AS,Weiler S,Zanzinger K,et al. Non-T cell activation linker (NTAL) negatively regulates TREM-1/DAP12-induced inflammatory cytokine production in myeloid cells[J]. The Journal of Immunology,2007,178(4):1 991-1 999.
20 Boisvert WA,Santiago R,Curtiss LK,et al. A leukocyte homologue of the IL-8 receptor CXCR-2 mediates the accumulation of macrophages in atherosclerotic lesions of LDL receptor-deficient mice[J]. The Journal of Clinical Investigation,1998,101(2):353-363.
21 Persson J,Nilsson J,Lindholm MW. Interleukin-1beta and tumour necrosis factor-alpha impede neutral lipid turnover in macrophage-derived foam cells[J]. BMC Immunology,2008,9(1):70.
22 戴道鹏,沈 迎,张瑞岩,等. 血清可溶性髓样细胞触发受体-1水平对冠状动脉粥样斑块进展的预测价值[J]. 国际心血管病杂志,2016,43(5):303-306.
23 Rai V,Rao VH,Shao Z,et al. Dendritic cells expressing triggering receptor expressed on myeloid cells-1 correlate with plaque stability in symptomatic and asymptomatic patients with carotid stenosis[J]. PLoS One,2016,11(5):e0154-802.
24 Golovkin AS,Ponasenko AV,Khutornaya MV,et al. Association of TLR and TREM-1 gene polymorphisms with risk of coronary artery disease in a Russian population[J]. Gene,2014,550(1):101-109.
25 Kutikhin AG,Ponasenko AV,Khutornaya MV,et al. Association of TLR and TREM-1 gene polymorphisms with atherosclerosis severity in a Russian population[J]. Meta Gene,2016,9(1):76-89.
Research Progress of Association between Triggering Receptor Expressed on Myeloid Cells-1 and Atherosclerosis
LU Jiao,LI Cheng-bin*
The Second Clinical Medical College of Yangtze University ,Jingzhou 434020,Hubei Province,China*
The triggering receptor expressed on myeloid cells-1 (TREM-1) that can trigger and expand inflammatory response is a newly discovered activating transmembrane receptor of immunoglobulin superfamily,and it plays a key role in atherosclerosis (AS). To provide a reference or clue for targeted therapy of AS in clinical research,this review discusses effects of TREM-1 on development of AS and artery plaque stability from its structural characteristics,expression,ligand binding,signaling pathways et al.
Triggering receptor expressed on myeloid cells-1;Inflammatory response;Atherosclerosis
长江大学第二临床医学院,荆州 434020;*
,E-mail:jzlcb002@163.com
本文2017-02-01收到,2017-03-04修回
R543.5
A
1005-1740(2017)02-0081-05

