基于4-(1-咪唑基)苯甲酸配体构筑的一个单一配体和两个混和配体配位聚合物的结构与性质
李田田 郑盛润
(1贵阳中医学院,贵阳 550002)
(2华南师范大学化学与环境学院,广州 510006)
0 Introduction
The construction of coordination polymers(CPs)have been attracted increasing interesting because of their intriguing architectures,as well as their potential applications in separation,adsorption,fluorescence,magnetic,catalysis,and so on[1-7].In order to obtain desirable CPs,the selection and design of proper organic ligands have been given special attention[8-11].Rigid ligands containing both carboxylate and nitrogen donors,such as pyridine carboxylic acid[12-14],imidazole carboxylic acid[15-19],are versatile linkers that applied to synthesize CPs with interesting structures and properties.Such type of ligands have following characters:(1)they are rigid,thus it is easier to control and predict their resulting structures compared with those based on flexibly ligands,and easier to form porous CPs;(2)they contain both carboxylate and N donors,which are suitable for binding almost all kinds of metal ions,and have potential ability for constructing heterometallic and mixed-ligand CPs.Thus,it is significant to construct CPs based on N-containing carboxylate.
In addition,mixed-ligands CPs become an important type of CPs because they can incorporate of more types of organic ligands into a single unique framework which may bring some new functions derived from organic ligands[20-25].The combination of nitrogen donor ligands and aromatic carboxylate has been proven to be an effective and useful strategy for constructing diverse mixed-ligand CPs[26-28].However,the incorporation of a N-containing carboxylate and carboxylate into a unique framework is relatively less explored.
Considering the above mentioned,in the present study,the imidazole carboxylic acid,4-(imidazol-1-yl)-benzoic acid(HIBA)is selected to construct three new CPs,including two homoligand CPs,{[Cu(IBA)2]·DMF}n(1)and{[Cd(IBA)2(H2O)]·3H2O}n(2),and one mixedligand CP,[Cd3(IBA)2(NPA)2]n(3).Although HIBA is similar to isonictin acid (form the type and position of the donor atoms),CPs based on HIBA are still limited[29-33].Furthermore,the mixed-ligand CPs containing HIBA are still relatively rare that only several CPs have been reported by far[29-30].
1 Experimental
1.1 Materials and equipment
The materials and reagents were obtained commercially and used as purchased without further purification.Elemental (C,H,N)analyses were performed on a Perkin-Elmer 240 element analyzer.The FT-IR spectra were recorded from KBr pellets in the 400~4 000 cm-1range on a Nicolet 5DX spectrometer.X-ray powder diffraction measurements were preformed on a Bruker D8 Advance diffractometer at 40 kV,40 mA with a Cu-target tube(Cu Kα,λ=0.154 06 nm)and a graphite monochromator scanning from 5°to 50°.Solid-state fluorescence spectra were measured by using a Hitachi-2500 spectropho-tometer with a 150 W xenon lamp as light source at room temperature.
1.2 Synthesis of coordination polymer 1
A mixture of HIBA(0.2 mmol),Cu(NO3)2·6H2O(0.1 mmol)and DMF(6 mL)was sealed in a 10 mL Teflon-lined stainless steel autoclave.The mixture was heated at 110℃for 3 days under autogenous pressure,and cooling at 5℃·h-1to room temperature.Blue block crystals of 1 were obtained.Yield:55%(based on the Cu).Elemental analysis Calcd.for C23H21CuN5O5(%):C,54.06,H,4.14,N,13.71.Found(%):C,54.80,H,4.00,N,13.22.IR(KBr,cm-1):3 414(m),3 144(m),1 648(s),1 598(s),1 394(s),860(m),781(m),747(w).
1.3 Synthesis of coordination polymer 2
A mixture of HIBA(0.2 mmol),Cd(Ac)2·2H2O(0.1 mmol),and distilled water(6 mL)was sealed in a 10 mL Teflon-lined stainless steel autoclave and NH3·H2O (1 mol·L-1)was used to adjust the pH value at about 6.The mixture was heated at 160℃for 3 days under autogenous pressure and slow cooling at 5℃·h-1to room temperature.Yellow block-shaped crystals of 2 were obtained.The single crystals could be easily separated from the precipitate by hand,washed with distilled water,and dried in air.Yield:63%(based on the Cd).Elemental analysis Calcd.for[Cd(IBA)2(H2O)]·H2O(C20H18CdN4O6,%):C,45.95,H,3.47,N,10.72.Found(%):C,46.03,H,3.50,N,10.75.IR(KBr,cm-1):3 410(s),1 653(w),1 604(w),1 386(s),1 184(w),1 066(w),862(m),784(m),720(w).
1.4 Synthesis of coordination polymer 3
A mixture of HIBA (0.2 mmol),H2NPA(0.2 mmol),Cd(Ac)2·2H2O(0.1 mmol),and distilled water(6 mL)was sealed in a 10 mL Teflon-lined stainless steel autoclave.The mixture was heated at 160℃for 3 days under autogenous pressure and slow cooling at 5℃·h-1to room temperature.Yellow block-shaped crystals of 3 were obtained.The single crystals could be easily separated from the precipitate by hand,washed with distilled water,and dried in air.Yield:48%(based on the Cd).Elemental analysis Calcd.for C18H10Cd1.50N3O8(%):C,38.27,H,1.78,N,7.44.Found(%):C,38.55,H,1.82,N,7.16.IR(KBr,cm-1):3 429(s),3 115(s),2 366(w),1 616(s),1 520(s),1 346(s),1 251(w),1 179(w),1 128(w),1 066(w),970(w),927(w),831(w),778(w),728(w),639(w),500(w).
1.5 X-ray diffraction determination
Single crystal X-ray diffraction data collections for 1~3 were performed on a Bruker Apex Ⅱ CCD diffractometer operating at 50 kV and 30 mA using Mo Kα radiation(λ=0.071 073 nm)at 293 K.Multiscan absorption corrections were applied with the SADABS program[34].The structures were solved using direct methods and refined by full-matrix least-squares on F2using the SHELX-2016[35].All the hydrogen atoms,except hydrogen atoms in water molecules were placed in idealized positions.The hydrogen atoms on coordinated water molecule in compound 2 were located from different density maps and were refined using AFIX 3.Hydrogen atoms on uncoordinated water molecules in compound 2 were not added but include in the formula.Crystal parameters and details of the data collection and refinement are given in Table 1.Selected bond lengths and angles are given in Table S1.
CCDC:1554061,1;1554062,2;1554063,3.
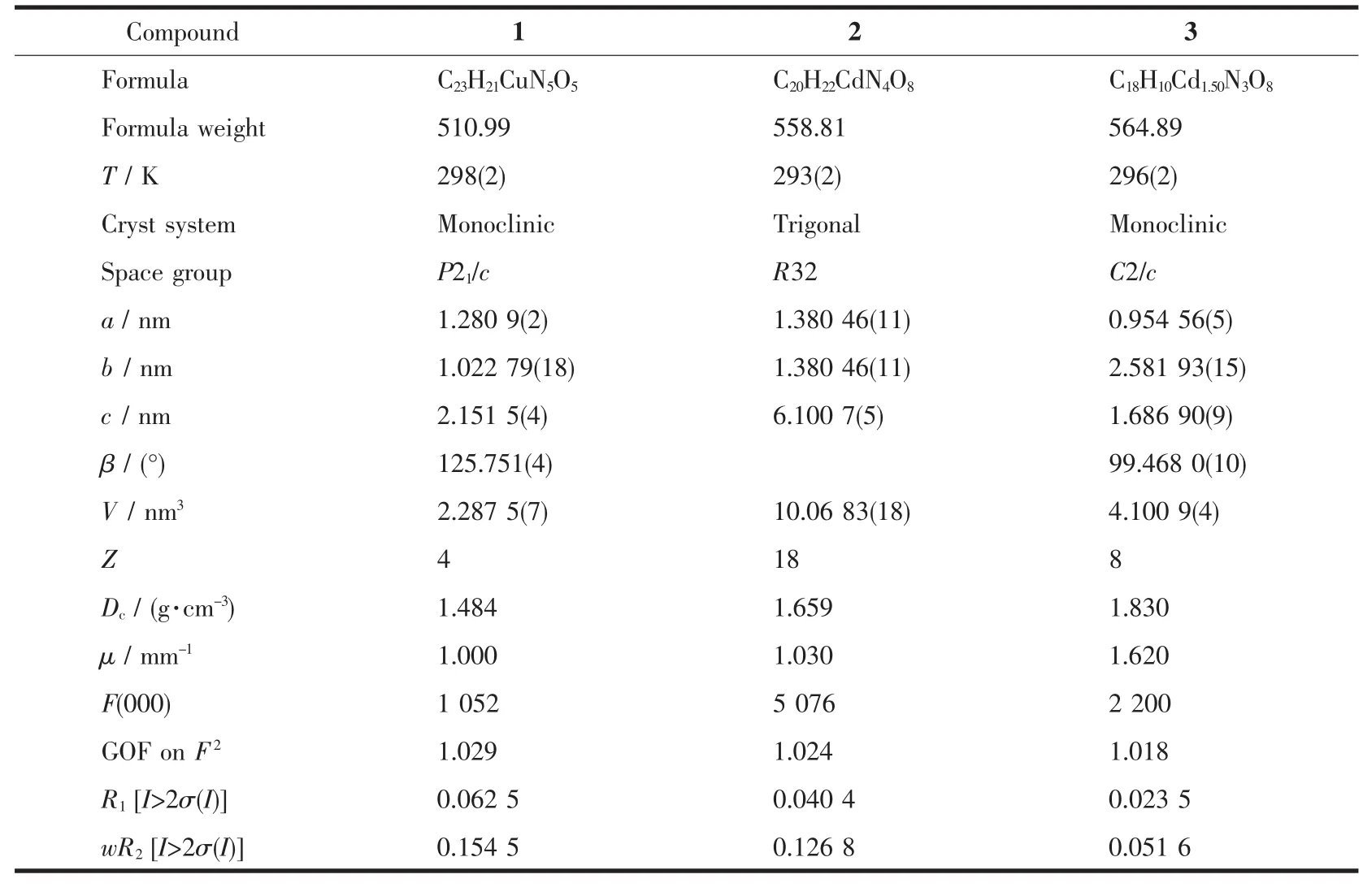
Table 1 Crystallographic data and structure refinement summary for compounds 1~3
2 Results and discussion
2.1 Description of the crystal structures
Compound 1 crystallizes in the monoclinic space group P21/c.There are one Cu(Ⅱ)ion,two IBA-anions,and one DMF molecule in the asymmetric unit.As shown in Fig.1a,Cu1 is six-coordinated by four carboxylate O atoms and two imidazolyl N atoms from four different IBA-anions, giving rise to a distorted octahedral coordination geometry with a CuN2O4coordination mode.The Cu-N bonds range from 0.199 1(5)to 0.198 7(4)nm,whereas Cu-O bonds range from 0.198 7(4)to 0.200 6(4)nm.All of them fall in normal range[36-37].

Fig.1 (a)View of the coordination environment of Cu(Ⅱ)ions in 1;(b)View of the single 3D framework in 1;(c)View of the dia network topology in 1;(d)Four-folded interpentrating dia network in 1
The rigid IBA-anion adopts a μ2-coordination mode that bridges two Cu(Ⅱ)ions via carboxylate and imidazole groups.Thus,the IBA-anion act as rod that connects the Cu(Ⅱ)ions into 3D network,as showed in Fig.1b.For clearly,the Cu(Ⅱ)ion that connects to four IBA-anions can be seen as four-connected nodes,and the 3D net can be described as 4-connected dia network topology[38-39],as showed in Fig.1c.In this single net,the void is large(as it is indicated by Cu…Cu separation of about 1.2 nm),thus four single nets are interpenetrated together to generate a four-fold interpenetrated framework (Fig.1d).There are still small 1D channels along b direction,which are occupied by DMF molecules.
When Cd(Ⅱ) salt was used instead of Cu(Ⅱ) salt in the preparation,compound 2 is obtained.Single X-ray diffraction reveals that compound 2 crystallizes in rhombohedral with space group R32.The asymmetric unit contains one Cd(Ⅱ)ion,two IBA-anions,one coordinated water molecule,and three uncoordinated water molecules.Cd1 ion adopts a distorted pentagonal bipyramid coordination geometry with four carboxylate O atoms from two IBA-anions and one coordinated water O atom on the equatorial plane,as well as two imidazole N atoms on the apex position,as showed in Fig.2a.All the Cd-N and Cd-O bonds are compared with other Cd(Ⅱ)compounds[40-41].The coordination mode of IBA-anion in compound 2 is same as that in compound 1,thus it also act as ditopic linker that connects the Cd(Ⅱ)ions into 3D network(Fig.2b).Similar to that in compound 1,the Cd(Ⅱ)ions surrounded by four IBA-anions can be considered as 4-connected nodes,and the resulting network is also a dia network topology(Fig.2c).The Cd…Cd distance is 0.126 5 nm,indicating that the single network contains large viods.Therefore,three networks are interpenetrated,leading to a three-fold interpenetrated dia framework(Fig.2d).Different from those in compound 1,there are no obvious channels in compound 2.
The differentinterpenetrated frameworks for compounds 1~2 may mainly due to the coordination geometries of metal ions,which can decide the orientation of four IBA-anions around the metal center.The orientation of four IBA-anions can be estimated by the six included angles around the 4-connected node.The angles in compound 1 are 86.37°,86.37°,115.33°,1115.33°,128.04°and 130.84°,whereas in compound 2 are 66.13°,66.16°,116.51°,119.58°,123.07°and 171.88°.This means that the 4-connected node in compound 1 is more nearly to a tetrahedron than that in compound 2,thus it is more suitable for creating body voids.Therefore,itisleading to interpenetrated framework with more number of folds in compound 1.From the reported literatures,the combination of IBA-anions and Cd(Ⅱ)/Cu(Ⅱ) ions often lead to interpenetrated dia framework[42-43],however,the three-fold dia framework of Cu(Ⅱ)compound is firstly observed.
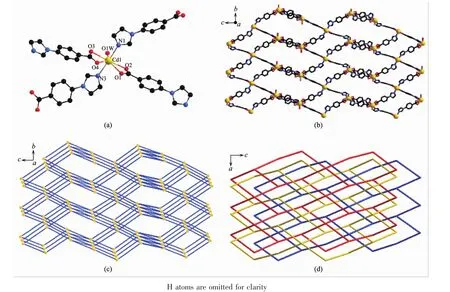
Fig.2 (a)View of the coordination environment of Cd(Ⅱ)ions in 2;(b)View of the single 3D framework in 2;(c)View of the dia network topology in 2;(d)Four-folded interpentrating dia network in 2
When H2NPA was used as a co-ligand,a mixedligand CP (compound 3)is obtained.The asymmetric unit of compound 3 contains two Cd(Ⅱ)ions with 1 and 1/2 site occupancy,respectively,one NPA2-and one IBA-anions.As showed in Fig.3a,Cd1 is sevencoordinated by six O atoms and one N atom from three NPA2-and two IBA-anions,whereas Cd2 is sixcoordinated by six O atoms from four NPA2-and two IBA-anions.Three Cd(Ⅱ)ions are bridged by four carboxylate groups from NPA2-and two carboxylate groups from IBA-anions(with three carboxylate groups between every two Cd(Ⅱ)ions),resulting in a linear trinuclearCd(Ⅱ) building block (Fig.3b)[44-45].The trinuclear building blocks are further interconnected by Cd-O bonds,giving rise to a 1D zigzag secondary building block (SBU)extending along c direction(Fig.3c).The 1D SBUs are linked by NPA2-anions to a 2D layer in ac plane (Fig.3d),which is further connected by IBA-anions into a 3D framework (Fig.3e).Some inorganic anions,such as Cl-,Ac-,and ox2-have been used as a auxiliary ligand to construct CPs based on HIBA.However,very few CPs that use a organic carboxylate acid as auxiliary ligand,as far as we know[29].Obviously,the incorporation of organic carboxylate auxiliary ligand seems more difficult to achieve because the coordination modes and conformations of organic ligands are larger,thus more balance between the building units should be considerated.
2.2 PXRD analyses and luminescent properties
In order to check the purity of compounds 1~3,the as-synthesized crystals of 1~3 were characterized by powder X-ray diffraction(PXRD).As shown in Fig.S1~S3,the observed peaks in the measured patterns are in good agreement with the calculated patterns that generated from single-crystal diffraction data,thus indicating that single phases of 1~3 are formed.The difference in intensity between the simulated and measured patterns may be attributed to a certain degree of preferred orientation of the powder samples during data collection.

Fig.3 (a)View of the coordination environment of Cd(I)ions in 3;View of the triuclear SBU(b)and 1D SBU(c)in 3;(d)View of the 2D framework along b direction in 3;(d)3D framework in 3

Fig.4 Emission spectra of compounds 2(excited at 365 nm)and 3(excited at 335 nm)
The coordination polymers constructed from organic ligands and metal centers with d10electronic configuration have shown competitive prospect for photoactive crystalline materials[46-47].Thus,the solidstate fluorescent properties of 2~3 have been studied at room temperature.As shown in Fig.4,excitation of the microcrystalline samples results in emission bands at 381 nm with a shoulder at 468 nm(λex=365 nm)for 2,and 469 nm(λex=335 nm)for 3.Compared with the fluorescent spectra of free HIBA and H2NPA[48-49],the emission bands at 468 and 469 nm of complexes 2~3 are mainly due to an intraligand emission state of HIBA ligands.The emission bands at 381 nm of compound 2 can be attributed to ligand-to-metal charge transfer(LMCT)between aromatic π systems and the 5s orbitals of Cd(Ⅱ)centers.
3 Conclusions
In conclusion,three new CPs was constructed based on HIBA.Compounds 1 and 2 are frameworks with 4-connected four-fold and three-fold dia network topologies,respectively.Their different interpenetrationsare largely dependenton the coordination geometries of metal centers.Compound 3 is a mixedligand CPs including HIBA ligands,which exhibit 3D framework based on 1D zigzag SBUs and NPA2-and IBA-linkers.This work may be helpful in the construction of homoligand and mixed-ligand CPs based on HIBA.
Supporting information is available at http://www.wjhxxb.cn

