原发性高血压大鼠血浆外泌体水平及其与微循环变化的相关性研究*
覃伟峰 仉红刚 张秋菊 修瑞娟
原发性高血压大鼠血浆外泌体水平及其与微循环变化的相关性研究*
覃伟峰 仉红刚#张秋菊 修瑞娟
目的:研究13周龄原发性高血压大鼠(SHR)外周血外泌体数量和大小分布与微循环功能的相关性。方法:市购13周龄雄性SHR(实验组)和相同周龄的WKY大鼠(对照组)各7只。采用多功能激光多普勒血流探测仪(LDF)检测两组大鼠耳廓、趾及脑皮质血流量、血细胞聚集度和血流速度,采用血流成像技术(LDPI)观察脑皮质血流分布。取外周血,提取血浆外泌体,采用透射电镜观察外泌体形态,以Apogee流式细胞术检测总外泌体水平和直径<100nm的外泌体水平,分析实验组大鼠外泌体水平与其微循环指标的相关性。结果:LDF结果显示,实验组大鼠脑皮质血流量和血流速度均显著高于对照组(P<0.05或P<0.01),而实验组大鼠趾血流量和血流速度明显低于对照组(P<0.05),两组大鼠的耳廓血流量、血流速度及耳廓、趾和脑皮质的血细胞聚集度差异均无统计学意义(P>0.05);LDPI结果显示,实验组大鼠脑皮质血流灌注量显著高于对照组(P<0.01)。体外实验显示,实验大鼠血浆外泌体大小分布不均,但以直径<100nm者居多;实验组总外泌体水平和直径<100nm外泌体水平均明显高于对照组(P<0.05)。相关性分析显示,实验组大鼠血浆总外泌体和直径<100nm外泌体水平与脑皮质血流量呈显著正相关(P<0.01)。结论:脑微循环功能改变与血浆总外泌体含量,尤其直径<100nm的外泌体水平显著相关,提示外泌体水平的升高可能预警原发性高血压发生和发展过程中的微循环变化,或可作为高血压早期的监测标记物。
原发性高血压;外泌体;微循环;大鼠
原发性高血压由于其高发病率和高致死率已成为危害人类健康的主要疾病之一[1],外泌体在其中扮演的角色受到越来越广泛关注。外泌体是一种由机体多种细胞分泌的直径在30-300nm的囊泡或多囊泡体,研究证实分泌到外周血中的外泌体既能通过携带功能性血管紧张素Ⅱ受体调控血压变化[2],还能通过携带的miRNA调节肺动脉高压[3],故在高血压的发生发展过程中起到重要病理生理作用。本文报道原发性高血压大鼠(SHR)外周血中外泌体大小和数量变化及其与微循环功能的相关性。
1 材料与方法
1.1 动物和分组
SPF级13周龄雄性SHR 7只,体重270g±11g(实验组);同周龄SPF级雄性正常WKY大鼠7只,体重270g±10g(对照组);均购自北京维通利华公司。适应性喂养一周后进行实验。实验中和实验后处理均遵照中国医学科学院北京协和医学院微循环研究所伦理委员会要求。
1.2 主要仪器和试剂
激光多普勒检测仪(LDF)和激光多普勒血流成像仪(LDPI)均为英国Moor公司产品,型号分别为moor VMS-LDF1-HP和moor LDI-HIR;仪器配套数据分析软件分别为moor VMS-PC Version 3.1 for Vascular Monitor System 和moor LDI Image Review Version 5.3。流式细胞仪为英国Apogee Flow System Ltd产品,型号为Apogee A50 Micro,配套分析软件为Histogram Software。电子显微镜为日本电子公司产品,型号:JEM-1400Plus,拍照软件为Radius。外泌体提取试剂盒购自美国101Bio公司(PureExo®Exosome Isolation Kit P101),负染色试剂(1%醋酸铀)由中国医学科学院基础医学院电镜室提供。
1.3 微循环指标观测方法
参照本课题组前期实验方法[4],3%戊巴比妥钠(50mg/kg)腹腔注射麻醉大鼠。室温下,大鼠左侧卧位,分别于右耳耳廓边缘近心端5mm处或右后肢中趾背侧向心端5mm处放置LDF探头,连续测量记录耳廓、趾血流量和血流速度、血细胞聚集度2min;再将大鼠俯卧位,头部备皮,矢状位剪开额顶部皮肤2cm,暴露颅骨前囟及左侧颅骨,标定于前囟左5mm后3mm处放置LDF探头,连续测量记录脑皮质血流量、血流速度和血细胞聚集度2min。最后将脑皮质暴露部位置于LDPI下,检测记录脑血流成像。利用仪器配套数据分析软件直接输出上述检测部位2min内的各项指标数值,选取较平稳时段的均数进行统计学分析。
1.4 血浆外泌体检测
1.4.1 外泌体提取及形态观察:采集外周血,于16℃、1 500g离心10min分离血浆。取500μl血浆,采用外泌体提取试剂盒,按照其操作说明提取外泌体,主要过程包括:首先于3 000g、4℃条件下离心血浆样本15min,以移除细胞碎片;然后加试剂A、B、C于上清液,静置30min;再次离心后出现三层,移除上层和下层,提取中层,外泌体即在该层;最后将外泌体重悬于100μl PBS中。按常规方法进行负染色后用透射电镜观察,不着色小囊泡即为外泌体。
1.4.2 流式细胞术测定外泌体水平:按照流式细胞仪操作手册要求校正仪器,检测PBS溶剂中的微粒浓度作为本底,然后用同样的PBS将外泌体样本稀释10倍后再上Apogee流式细胞仪检测,外泌体水平计算方法为:稀释后的样本中外泌体浓度(个/μl)=稀释后样本中微粒总浓度-PBS中微粒浓度。分别检测总外泌体浓度和通过阈值设门检测直径<100nm的外泌体浓度。
1.5 统计学处理

2 结 果
2.1 两组大鼠耳廓、趾、脑皮质血流量、血流速度和血细胞聚集度比较
与对照组比较,实验组大鼠耳廓血流量无明显差异(t=-0.492,P>0.05);但趾血流量明显降低(U=8.000,P<0.05),脑皮质血流量显著升高(t=3.968,P<0.01),见表1。
与对照组比较,实验组大鼠耳廓血流速度差异无统计学意义(t=0.470,P>0.05),但趾血流速度明显变慢(U=4.000,P<0.01),脑皮质血流速度较快(t=2.514,P<0.05),见表2。
与对照组比较,实验组耳廓、趾和脑皮质的血细胞聚集度差异均无统计学意义(P>0.05),见表3。
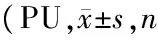
表1 两组大鼠不同部位血流量比较均=7)
注:与对照组比较,1)P<0.05,2)P<0.01
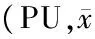
表2 两组大鼠不同部位血流速度比较±s,n均=7)
注:与对照组比较,1)P<0.05,2)P<0.01
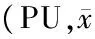
表3 两组大鼠不同部位血细胞聚集度比较±s,n均=7)
2.2 两组大鼠脑皮质血流量比较
LDPI可以监测微循环的血液灌注量,包括毛细血管(营养血管)、微动脉、微静脉和吻合支。结果显示,与对照组比较,实验组大鼠脑皮质血流量升高(988.01±70.19PU vs 883.23+79.03PU),差异有统计学意义(U=7.000,P<0.05),见图1。
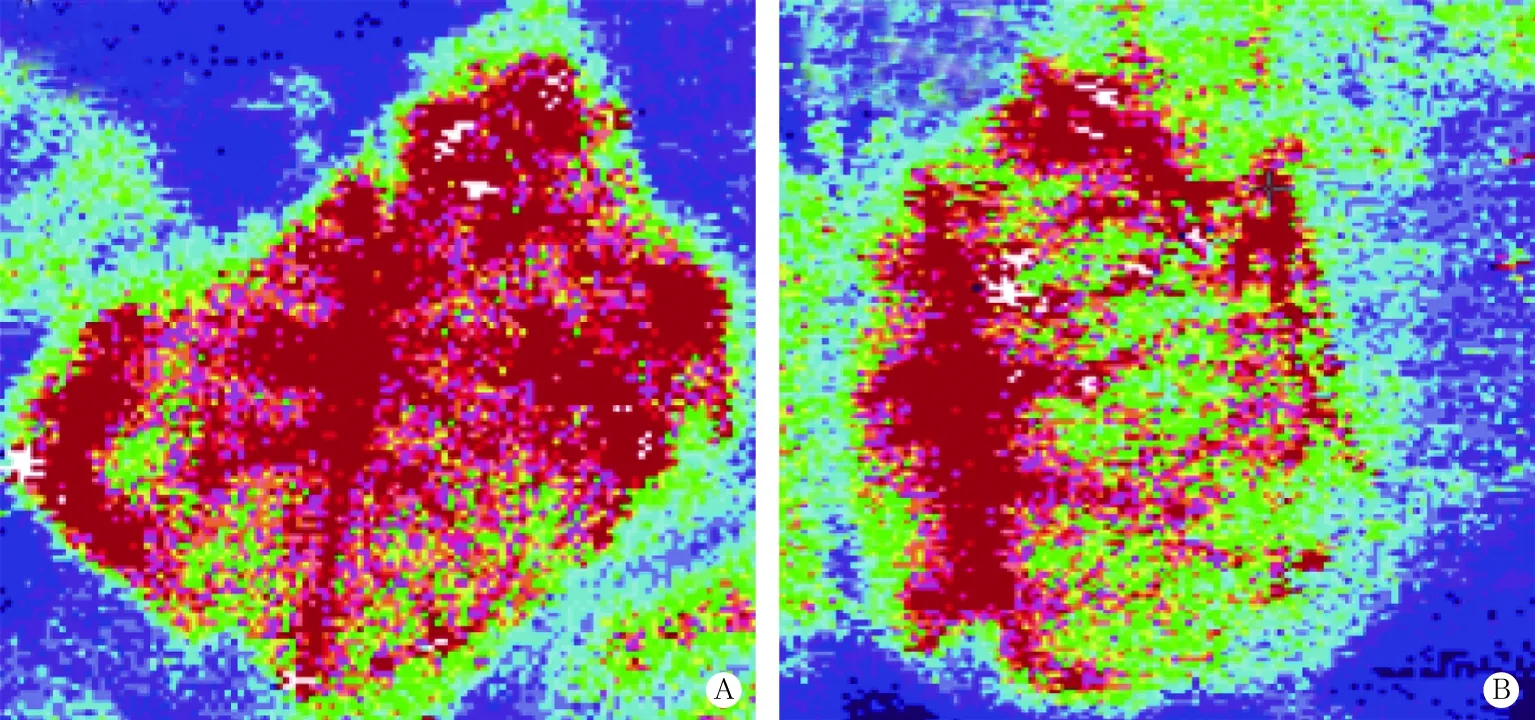
图1 大鼠脑皮质血流成像图(LDPI,A为实验组;B为对照组)
2.3 两组血浆外泌体形态和水平比较
透射电镜见血浆中单个或多个聚集分布的圆形或椭圆形小囊泡状外泌体,直径50-200nm,以直径<100nm者居多,外泌体囊泡内有较多电子致密物(图2)。定量分析显示,实验组血浆总外泌体水平和直径小于100nm的外泌体水平均显著高于对照组,差异均有统计学意义(t=2.313、2.591,P<0.05),见表4。
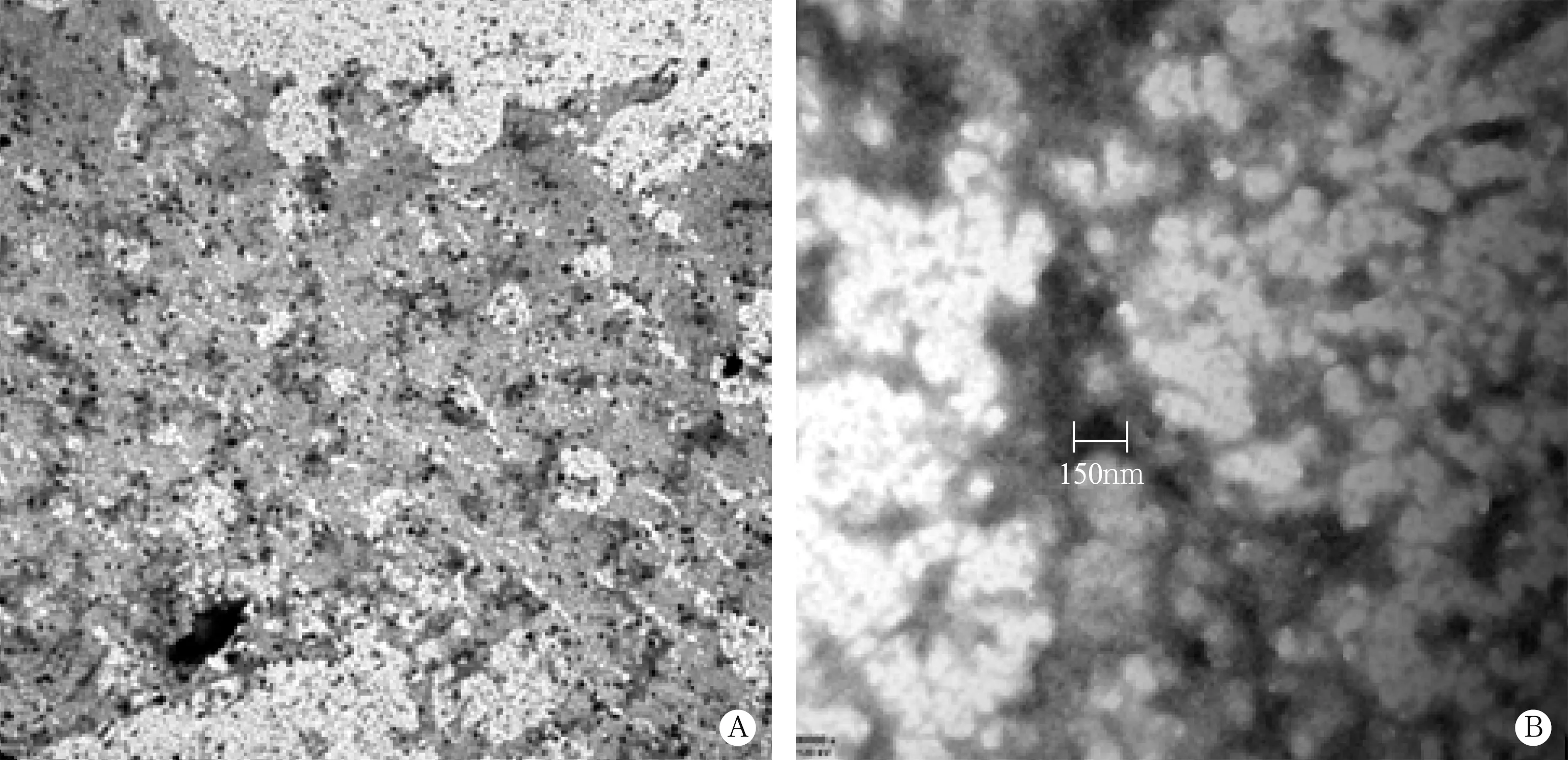
图2 血浆外泌体形态(透射电镜,A×4 000,B×80 000)
表4 两组大鼠血浆总外泌体水平比较(个±s,n均=7)
注:与对照组比较,1)P<0.05
2.4 实验组大鼠血浆外泌体水平与其脑皮质血流量的相关性分析
实验组大鼠血浆总外泌体水平和直径<100nm的外泌体水平均与脑皮质血流量呈直线正相关,后者更显著(r=0.898、0.908,P<0.01),见图3。
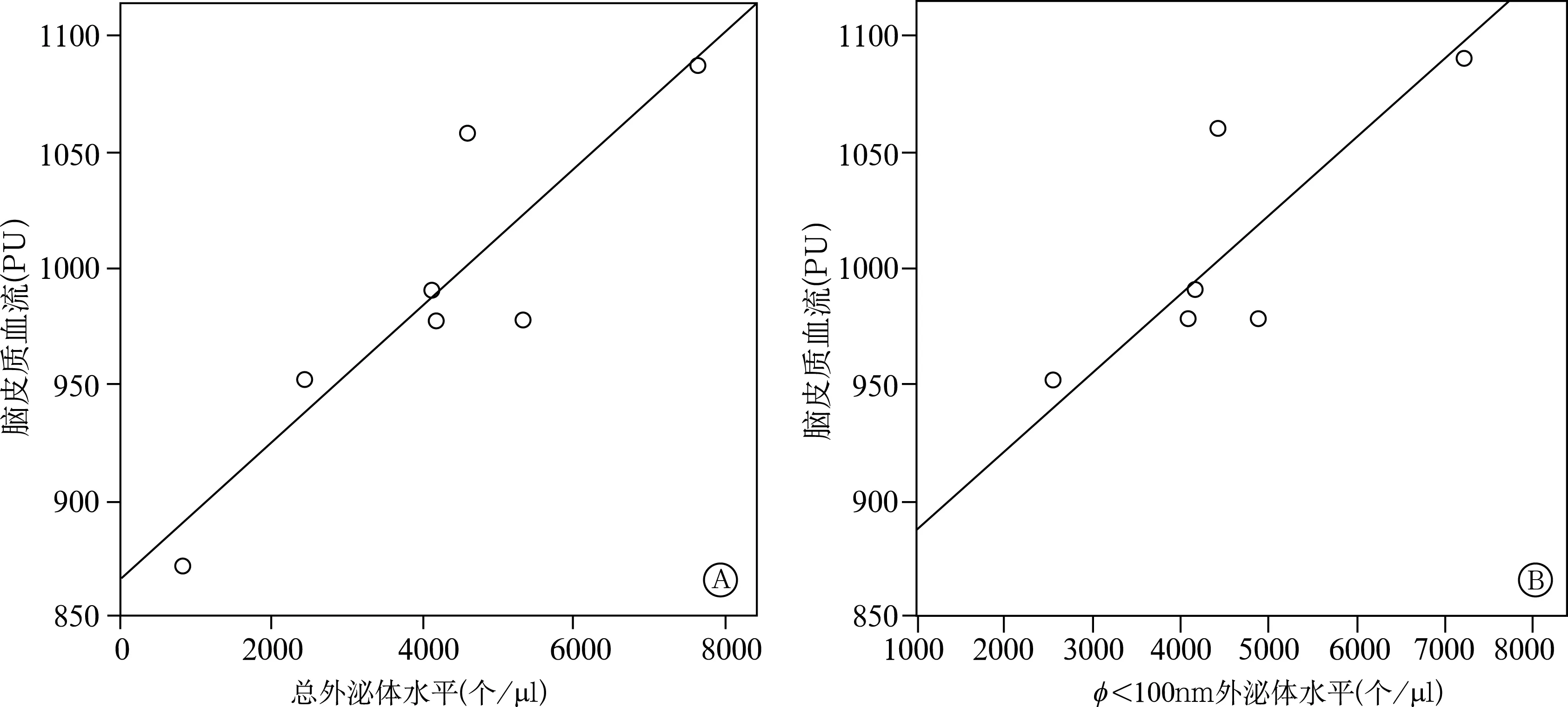
注:A为总外泌体水平与脑皮质血流量相关性曲线;B为直径<100nm外泌体水平与脑皮质血流量的相关性曲线
3 讨 论
本研究结果显示,SHR趾血流量和血流速度均明显低于WKY大鼠,与之前临床相关报道[5,6]一致,进一步表明高血压可致末梢微循环受损。其原因可能由调节皮肤血流的肌源性机制障碍所致[7]。但本研究中13周龄SHR脑皮质血流量却显著高于同周龄WKY大鼠,这可能与高血压病程的病理生理特征密切相关,即与高血压大鼠的周龄密切相关。有研究[8]证实,20周龄以下的SHR处于高血压早期(本文大鼠为13周龄),机体的大、小血管功能均处于可调节阶段,此时大血管管腔开始变窄,而机体能够通过自适应的血管平滑肌肌源性反应保护下游的微血管,使其血流量增加。这种现象也在脑卒中易感型高血压大鼠(SHRSP)中被观察到[9]。随着年龄增长或病程延长,这种调节作用可能降低,从而引起脑血流量减少。
本文采用敏感且高通量外泌体分析技术Apogee流式细胞仪,对SHR和WKY大鼠血浆外泌体的大小分布进行了定量分析,结果显示SHR总外泌体水平显著高于WKY大鼠,并且绝大多数外泌体直径均在100nm以下,这一现象尚未见到相关报道,其形成机制及其在微循环障碍中的作用有待进一步探讨。
本文研究结果还显示,SHR血浆外泌体水平升高与脑皮质血流量呈正相关,即提示外泌体可能参与微循环功能调控。有研究证实,将微小RNA(miR)-143转移到外泌体中能提高微血管密度,而来源于血管内皮细胞的外泌体能携带miR-143至血管平滑肌细胞,从而影响血管功能和血管重塑[10-12],更何况外泌体能稳定地通过血脑屏障,并且携带RNA[13,14],因而对脑皮质血流的影响更显著。
综上所述,血浆外泌体水平,尤其直径<100nm外泌体水平升高可能预警原发性高血压发生和发展过程中的早期微循环变化,或可作为高血压微循环功能障碍的监测标记物。进一步探索其调控分子和机制对高血压及其并发症防治可能提供新的途径和策略。
◀
本文第一作者简介:
覃伟峰(1990-), 男,汉族,硕士研究生,研究方向为高血压微循环功能障碍及其病理生理机制
1 Li H, Zhang X, Wang F, et al. MicroRNA-21 lowers blood pressure in spontaneous hypertensive rats by upregulating mitochondrial translation[J]. Circulation, 2016, 134(10): 734-751.
2 Pironti G, Strachan RT, Abraham D, et al. Circulating exosomes induced by cardiac pressure overload contain functional angiotensin II type 1 receptors[J]. Circulation, 2015, 131(24): 2 120-2 130.
3 Aliotta JM, Pereira M, Wen S, et al. Exosomes induce and reverse monocrotaline-induced pulmonary hypertension in mice[J]. Cardiovasc Res, 2016, 110(3): 319-330.
4 刘晓庆, 仉红刚, 张秋菊, 等.高功率多普勒血流探测仪结合血流成像仪观察氯吡格雷对高血压大鼠微循环的影响[J]. 微循环学杂志, 2015, 25(2): 30-33.
5 Esen F, Caglar S, Ata N, et al. Fractal scaling of laser doppler flowmetry time series in patients with essential hypertension[J]. Microvasc Res, 2011, 82(3): 291-295.
6 Catalano M, Schioppa S, Sampietro G, et al. Skin blood flow during vasoconstrictive and vasodilative stimuli in essential hypertension patients: a laser Doppler flowmetry study[J]. Int J Microcirc Clin Exp, 1997, 17(2): 80-85.
7 Rossi M, Carpi A, Di Maria C, et al. Spectral analysis of laser Doppler skin blood flow oscillations in human essential arterial hypertension[J]. Microvasc Res, 2006, 72(1-2): 34-41.
8 Li Y, Shen Q, Huang S, et al. Cerebral angiography, blood flow and vascular reactivity in progressive hypertension[J]. Neuroimage, 2015, 111:329-337.
9 Henning EC, Warach S, Spatz M. Hypertension-induced vascular remodeling contributes to reduced cerebral perfusion and the development of spontaneous stroke in aged SHRSP rats[J]. J Cereb Blood Flow Metab, 2010, 30(4): 827-836.
10 Su SA, Xie Y, Fu Z, et al. Emerging role of exosome-mediated intercellular communication in vascular remodeling[J]. Oncotarget, 2017, [in press].
11 Deng L, Blanco FJ, Stevens H, et al. MicroRNA-143 activation regulates smooth muscle and endothelial cell crosstalk in pulmonary arterial hypertension[J]. Circ Res, 2017, 117(10):870-883.
12 Aliotta JM, Pereira M, Wen S, et al. Exosomes induce and reverse monocrotaline-induced pulmonary hypertension in mice[J]. Cardiovasc Res, 2016, 110(3): 319-330.
13 Xiong Y, Mahmood A, Chopp M. Emerging potential of exosomes for treatment of traumatic brain injury[J]. Neural Regen Res, 2017, 12(1):19-22.
14 Yang T, Fogarty B, Laforge B. et al. Delivery of small interfering RNA to inhibit vascular endothelial growth factor in zebrafish using natural brain endothelia cell-secreted exosome nanovesicles for the treatment of brain cancer[J]. AAPS J, 2017, 19(2):475-486.
The Concentration of Exosomes from Plasma in Hypertensive Rats and its Correlation to Microcirculation Function
QIN Wei-feng, ZHANG Hong-gang#, ZHANG Qiu-ju, XIU Rui-juan
Institute of Microcirculation, Peking University of Medical College & Chinese Academy of Medical Sciences, Key Laboratory of Microcirculation, Ministry of Health, Beijing 100005,China;#
Objective: To investigate the microcirculation function and its correlation to the concentration and size distribution of circulating exosomes in spontaneous hypertensive rats(SHR). Method: Study group included seven 13-week-old male SHR, control group included seven 13-week-old male Wistar-Kyoto(WKY) rats. The blood flow, blood flow velocity, blood cell aggregation of all animals' cerebral cortices, auricles and toes were detected by the high power laser doppler flowmetry(LDF), and the whole distribution curve of blood flow of their cerebral cortices were measured by the laser doppler perfusion imaging(LDPI). The exosomes were extracted from the plasma, and then detected by transmission electron microscopy. Last, the total concentration of exosomes and the concentration of exosomes with diameter less than 100nm were measured using Apogee flow cytometry. Results: LDF showedthat the blood flow and blood flow velocity of cerebral cortex were both significantly higher in study group than controls(P<0.05 orP<0.01), while the blood flow and blood flow velocity of toes were both significantly lower in study group than in control group(P<0.05 orP<0.01), and the blood cell aggregation of cerebral cortices, auricles and toes showed no differences between the two groups(P>0.05).LDPI showed that the blood flow of cerebral cortex was significantly higher in study group than in control group(P<0.01). Furthermore, the total concentration of exosomes from plasma was significantly higher in study group than control group(P<0.05), and the same as the exosomes with diameter less than 100nm (P<0.05). And most importantly, we found that the total concentration of exosomes and the concentration of exosomes with diameter less than 100nm from plasma were both positively related to the blood flow in cerebral cortex detected by LDPI(P<0.01).Conclusion: The change of brain microcirculation function was significantly related to circulating total concentration of exosomes, especially to the concentration of exosomes with diameter less than 100nm.This indicated that the increase of exosomes might predict microcirculation change in the processes and development of essential hypertension and could be a potential biomarker in the early stage of hypertension.
Essential hypertension; Exosome; Microcirculation; Rat
国家自然科学基金(11274046);协和青年基金(3332016081);中国医学科学院医学与健康创新项目(2016-12M-3006);中国医学科学院中央级公益性科研院所基本科研业务费基金(2016ZX310046)
北京协和医学院,中国医学科学院微循环研究所;卫生部微循环重点实验室,北京 100005;#
,E-mail:zhanghg1966126@imc.pumc.edu.cn
本文2017-01-20收到,2017-02-24修回
R544.1 R331.3+5
A
1005-1740(2017)02-0008-05

