Glucose-6-phosphate dehydrogenase (G6PD) deficiency is associated with asymptomatic malaria in a rural community in Burkina Faso
Abdoul Karim Ouattara, Cyrille Bisseye,2, Bapio Valery Jean Télesphore Elvira Bazie, Birama Diarra, Tegwindé Rebeca Compaore, Florencia Djigma, Virginio Pietra, Remy Moret, Jacques Simpore*
1Centre for Biomolecular Research Pietro Annigoni (CERBA) LABIOGENE UFR/SVT, University of Ouagadougou BP 364 Ouagadougou, Burkina Faso
2Laboratory of Molecular and Cellular Biology (LABMC), University of Science and Technology of Masuku (USTM), BP 943 Franceville, Gabon
Glucose-6-phosphate dehydrogenase (G6PD) deficiency is associated with asymptomatic malaria in a rural community in Burkina Faso
Abdoul Karim Ouattara1, Cyrille Bisseye1,2, Bapio Valery Jean Télesphore Elvira Bazie1, Birama Diarra1, Tegwindé Rebeca Compaore1, Florencia Djigma1, Virginio Pietra1, Remy Moret1, Jacques Simpore1*
1Centre for Biomolecular Research Pietro Annigoni (CERBA) LABIOGENE UFR/SVT, University of Ouagadougou BP 364 Ouagadougou, Burkina Faso
2Laboratory of Molecular and Cellular Biology (LABMC), University of Science and Technology of Masuku (USTM), BP 943 Franceville, Gabon
PEER REVIEW
Peer reviewer
Yuki Eshita, Ph.D., Associate Professor, Department of Infectious Disease Control, Faculty of Medicine, Oita University, 1-1 Idaigaoka, Hasama-machi, Yufu-shi, Oita 879-5593, Japan.
Tel: +81-97-586-5701
Fax: +81-97-586-5701
E-mail: yeshita@oita-u.ac.jp
Comments
This is a good study in which the authors showed that the G6PD A-variant associated with protection against a symptomatic malaria in Burkina Faso was probably the most common deficient variant. The results suggested that G6PD deficiency seemed to prevent the normal development of P. falciparum in the body.
Details on Page 657
Objective:To investigate 4 combinations of mutations responsible for glucose-6-phosphate dehydrogenase (G6PD) deficiency in a rural community of Burkina Faso, a malaria endemic country.
Polymerase chain reaction, Mutations, Glucose-6-phosphate dehydrogenase deficiency, Asymptomatic malaria, Burkina Faso
1. Introduction
The glucose-6-phosphate dehydrogenase (G6PD) deficiency is the most common disease-producing enzymopathy in humans, affecting 400 million people worldwide[1,2]. Its prevalence is highest in malaria endemic areas, because of the selective advantage conferred to carriers against malaria[3,4]. Approximately 140 mutations or combinations of mutations responsible for this deficiency have been described[5-7]. In sub-Saharan Africa, 3 variants occur with polymorphic frequencies above 1%: wild type G6PD B, a non-deficient variant G6PD A and the deficient variant G6PD A-[8,9]. The variant G6PD A results from a point mutation A376G in exon 5 whereas the deficient variant G6PD A- has an A376G mutation and an additional one G202A in exon 4. Other deficient variants associate the mutation A376G and the following mutations: A542T (exon 6), G680T (exon 7) and T968C (exon 9) in the G6PD gene.
In previous studies carried out in Burkina Faso, the G6PD deficiency has been explored mainly by measuring the enzymatic activities[10]. Furthermore, the genotyping of the G6PD variants have been focused on single 202A/376G G6PD A- variant considered as the most common in Africa[9,11]. However, studies in West Africa have shown the presence of point mutations other than 202A/376G associated with the G6PD deficiency with relatively high frequencies. In fact, it has been shown that the 376G/542T G6PD Santamaria, 376G/968C G6PD Betica Selma and T968C alleles were more frequent in Serer[11] and the general population in the Gambia[12]. In this study four combinations of mutations responsible for the G6PD deficiency were investigated in a rural community in Burkina Faso.
2. Materials and methods
2.1. Settings and type of study
Unrelated participants were recruited in Koubri (a rural community located at 25 km south of Ouagadougou, Burkina Faso), where malaria transmission is perennial because of dams associated with agricultural activities.
2.2. Study population
Two hundred individuals aged from 1 to 79 years were included. The participants were in their great majority from the Mossi ethnic group. They were already involved in another project entitled “Study of fine specific immune responses againstPlasmodium falciparum(P. falciparum) peptides candidate vaccines”.
2.3. Blood collection
Venous blood (5 mL of blood per adult and about 3 mL of blood per child) was collected on ethylene diamine tetraacetic acid impregnated tubes. After centrifugation at 15 000 r/min for 5 min, plasma was separated from cell pellet. The pellet was stored at -20 °C for DNA extraction.
2.4. DNA extraction and genotyping of G6PD-deficient variants
All DNA samples were extracted using a standard saltingout procedure[13] or the QIAamp DNA Mini Kit from QIAGEN (QIAGEN, Hilden, Germany). DNA purities were estimated spectrophotometrically, and the final concentrations were determined using Biodrop µLITE (Isogen Life Science N.V./S.A, Temse, Belgium).
The mutations A376G, G202A and A542T were genotyped with 20 ng of DNA by the TaqMan assays (ABI, Applera International Inc, Foster City, CA, USA) in a reaction volume of 25 µL, using the ABI 7500 FAST real-time polymerase chain reaction (PCR) systems. Fluorescence curves were analyzed with the 7500 FAST Sequence Detection Software version v.2.1 (Applied Biosystems) for allelic discrimination. The G680T and T968C mutations were genotyped by PCR followed by restriction fragment length polymorphism (RFLP) (PCR/RFLP) as previously described by Beutleret al[14].
2.5. Statistical analysis
Data were analyzed using the Statistical Package for Social Sciences 17.0 and EpiInfo version 6 software. The chi square test was used for comparisons. The difference was considered significant forP<0.05.
2.6. Ethical considerations
This study was approved by the Ministry of Health and the CERBA/Saint Camille Ethics Committee. Informed consent was obtained from adults and parents or guardians of children under 5 years before blood collection.
3. Results
3.1. Prevalence of G6PD deficiency
The study population consisted of 58% and 42% of women and men, respectively. Nearly three quarters of the subjects (74.5%) were carriers of the G6PD normal alleles; G6PD-heterozygous women represented 16.0% (32/200), while 9.5% (19/200) were 202A/376G G6PD A-. None of the G6PD-deficient variants 376G/542T, 376G/680T and 376G/968C were detected in this study (Table 1). The G6PD deficiency prevalence was significantly higher in men compared to women (14.3%vs6.0%,P=0.049).
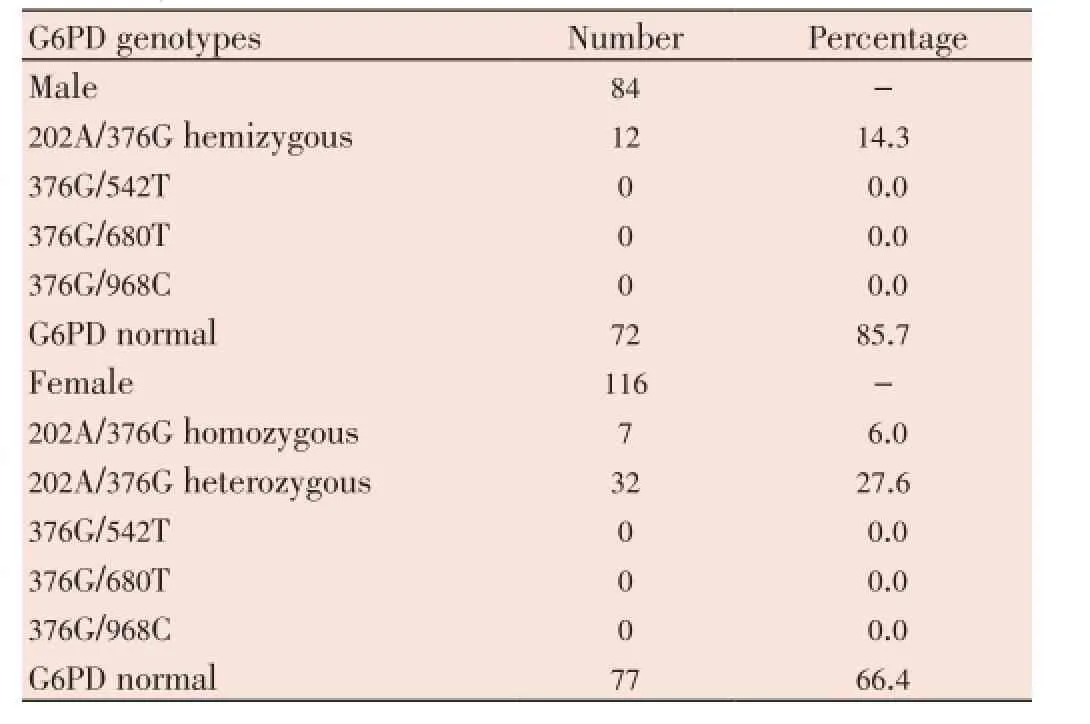
Table 1 Prevalence of four combinations of mutations responsible for G6PD deficiency.
3.2. Asymptomatic malaria and G6PD
教师是教学活动的设计者与引导者,其自身的观念与行为对教学活动的开展起到决定性的作用.有一部分教师已经认识到化学与生活实际相结合的重要性.然而,不同职称、不同年龄层的教师对这一教学理念的接受程度是不一样的.教师对生活化的教学方法接受程度越高,那么他的教学设计就会有更多的生活化体现,这样的课堂会更加轻松,学生更容易接受,对知识点的掌握也会更加牢固.少部分教师依旧拘泥于传统的教学方法,以课本为绝对的主体,那么学生只能是学习有限的理论知识,化学的应用性、趣味性得不到体现,学生学习起来也缺乏乐趣,单纯是当成一门课程来完成.
The presence of theP. falciparumparasite was investigated in 15 out of the 19 G6PD-deficient individuals. The absence of parasitaemia was observed in 53.3% of the G6PD-deficient and 57.0% of the non-deficient persons, respectively. Among the G6PD-heterozygous women,P. falciparumasymptomatic malaria was found in 48.1% (13/27) of them.
No statistically significant difference was found by comparing the prevalence ofP. falciparuminfection between deficient and non-deficient persons on one hand, and between non-deficient and heterozygous individuals on the other hand. However, the geometric mean of parasites in infected individuals from the 3 groups were significantly higher in the non-deficient compared to the deficient persons (1104vs204 parasites/µL,P<0.001) and also in the non-deficient compared with the individuals who are heterozygous (1104vs628 parasites/µL,P<0.001).
4. Discussion
In this study, we investigated four combinations of mutations (202A/376G; 376G/542T; 376G/680T; 376G/968T) responsible for G6PD deficiency in individuals living in a malaria endemic area, such as Burkina Faso. The G6PD deficiency prevalence was 9.5% in our study population. This prevalence is comparable to that of 9.0% reported by Carteret al[8], in six African countries, but lower than the prevalence of 19.6% and 16.3% respectively reported by Modianoet al[15], and Simporeet al[10], in Burkina Faso. These variations could be explained not only by the small size of our population sample, but also by the diagnostic methods used for the detection of the G6PD (realtime PCR, PCR/RFLP versus measurement of enzyme activity) deficiency. Furthermore, these differences could also be due to the difficulty of distinguishing deficient and non-deficient by genotyping heterozygous women[16].
Indeed, in a previous study it was shown that among 81 G6PD heterozygous women, 53% had a normal enzyme activity, while 33% had an intermediate activity and 14% had a biochemical deficiency[17].
Four deficient genotypes were sought in this study, and only the genotype 202A/376G G6PD A- was found. These results are similar to those obtained by Carteret al[8], in six African countries including Burkina Faso, and confirms the results shown by previous studies that the 202A/376G G6PD A- is the most common deficient variant in sub-Saharan Africa[9,18]. The prevalence of G6PD deficiency was significantly higher among men (14.3%) compared to women (6.0%). These results are comparable to that found by Simporeet al. who observed 20.5% of deficiency among men and 12.3% among women[10]. The prevalence of parasitaemia in infected individuals was significantly higher among those who are not deficient with respect to those who are deficient (P<0.001). In addition, heterozygous females had a significantly high prevalence of parasitaemia (P<0.001).
Our results are comparable to those of a study in Gabon which has shown that there is an association between the G6PD deficiency and the protection against asymptomatic malaria[19]. Indeed, G6PD deficiency seems to prevent the normal development ofP. falciparumin the body.
The protection mechanism of the G6PD deficiency against malaria remains hypothetical and various mechanisms are developed[20]. This study shows that the variant 202A/376G G6PD A- is associated with the protection against asymptomatic malaria in Burkina Faso. However, other genotyping studies are needed to confirm the absence of other deficient variants, and to determine more accurately the 202A/376G mutation frequency in the general population and specific ethnic groups.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
We are grateful to all the participants of this study. We would like to thank the Italian Episcopal Conference (CEI) and the West African Economic and Monetary Union (WAEMU) (through the Programme d’appui et de développement des centres d’excellence régionaux (PACER II) for their financial support. We also like to thank the all staff of CERBA/LABIOGENE for their help.
Comments
The G6PD deficiency is the most common diseaseproducing enzymopathy in humans. Its prevalence is highest in malaria endemic areas, because of the selective advantage conferred to carriers against malaria. Approximately 140 mutations or combinations of mutations responsible for this deficiency have been described.
Research frontiers
In this study, four combinations of mutations responsible for the G6PD deficiency were investigated in a rural community in Burkina Faso. This study showed that the 202A/376G G6PD A- variant associated with protection against a symptomatic malaria in Burkina Faso is probably the most common deficient variant.
Related reports
Approximately, 140 mutations or combinations of mutationsresponsible for this deficiency have been described. The G6PD deficiency has been explored by the enzymatic activities in Burkina Faso. The genotyping of the G6PD variants have been reported on single 202A/376G G6PD A-variant in Africa, and also on 376G/542T, 376G/968C and T968C in the Gambia.
Innovations and breakthroughs
Four deficient genotypes (202A/376G, 376G/542T, 376G/680T and 376G/968T), responsible for G6PD deficiency were searched in Burkina Faso, but only the genotype 202A/376G G6PD A- was found. Results showed that there was an association between the G6PD deficiency and the protection against asymptomatic malaria.
Applications
The authors investigated mutations which are responsible for G6PD deficiency in individuals living in a malaria endemic area. It may be significant to know the genotyping of the G6PD variants. Accumulation of the additional information may lead to the solution of the protection mechanism of the G6PD deficiency against malaria.
Peer review
This is a good study in which the authors showed that the G6PD A- variant associated with protection against a symptomatic malaria in Burkina Faso was probably the most common deficient variant. The results suggested that G6PD deficiency seemed to prevent the normal development ofP. falciparumin the body.
[1] Nkhoma ET, Poole C, Vannappagari V, Hall SA, Beutler E. The global prevalence of glucose-6-phosphate dehydrogenase deficiency: a systematic review and meta-analysis. Blood Cells Mol Dis 2009; 42(3): 267-278.
[2] Isaac I, Mainasara A, Erhabor O, Omojuyigbe S, Dallatu M, Bilbis L, et al. Glucose-6-phosphate dehydrogenase deficiency among children attending the Emergency Paediatric Unit of Usmanu Danfodiyo University Teaching Hospital, Sokoto, Nigeria. Int J Gen Med 2013; 6: 557-562.
[3] Beutler E. Glucose-6-phosphate dehydrogenase deficiency: a historical perspective. Blood 2008; 111(1): 16-24.
[4] Allahverdiyev AM, Bagirova M, Koc RC, Ates SC, Baydar SY, Yaman S, et al. Glucose-6-phosphate dehydrogenase deficiency and malaria: a method to detect primaquine-induced hemolysis in vitro. In: Canuto RA, editor. Dehydrogenases. Rijeka, Croatia: InTech; 2012.
[5] Cappellini MD, Fiorelli G. Glucose-6-phosphate dehydrogenase deficiency. Lancet 2008; 371(9606): 64-74.
[6] Zhao X, Li Z, Zhang X. G6PD-MutDB: a mutation and phenotype database of glucose-6-phosphate (G6PD) deficiency. J Bioinform Comput Biol 2010; 8(Suppl 1): 101-109.
[7] Manganelli G, Masullo U, Passarelli S, Filosa S. Glucose-6-phosphate dehydrogenase deficiency: disadvantages and possible benefits. Cardiovasc Hematol Disord Drug Targets 2013; 13(1): 73-82.
[8] Carter N, Pamba A, Duparc S, Waitumbi JN. Frequency of glucose-6-phosphate dehydrogenase deficiency in malaria patients from six African countries enrolled in two randomized anti-malarial clinical trials. Malar J 2011; 10: 241.
[9] Van Malderen C, Van Geertruyden JP, Machevo S, González R, Bassat Q, Talisuna A, et al. Glucose-6-phosphate dehydrogenase deficiency, chlorproguanil-dapsone with artesunate and post-treatment haemolysis in African children treated for uncomplicated malaria. Malar J 2012; 11: 139.
[10] Simpore J, Ilboudo D, Damintoti K, Sawadogo L, Maria E, Binet S, et al. Glucose-6-phosphate dehydrogenase deficiency and sickle cell disease in Burkina Faso. Pak J Biol Sci 2007; 10(3): 409-414.
[11] Howes RE, Dewi M, Piel FB, Monteiro WM, Battle KE, Messina JP, et al. Spatial distribution of G6PD deficiency variants across malaria-endemic regions. Malar J 2013; 12: 418.
[12] Clark TG, Fry AE, Auburn S, Campino S, Diakite M, Green A, et al. Allelic heterogeneity of G6PD deficiency in West Africa and severe malaria susceptibility. Eur J Hum Genet 2009; 17(8): 1080-1085.
[13] Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 1988; 16(3): 1215.
[14] Beutler E, Kuhl W, Vives-Corrons JL, Prchal JT. Molecular heterogeneity of glucose-6-phosphate dehydrogenase A-. Blood 1989; 74(7): 2550-2555.
[15] Modiano D, Luoni G, Sirima BS, Lanfrancotti A, Petrarca V, Cruciani F, et al. The lower susceptibility to Plasmodium falciparum malaria of Fulani of Burkina Faso (west Africa) is associated with low frequencies of classic malaria-resistance genes. Trans R Soc Trop Med Hyg 2001; 95(2): 149-152.
[16] Peters AL, Van Noorden CJ. Glucose-6-phosphate dehydrogenase deficiency and malaria: cytochemical detection of heterozygous G6PD deficiency in women. J Histochem Cytochem 2009; 57(11): 1003-1011.
[17] Kaplan M, Hammerman C. Neonatal screening for glucose-6-phosphate dehydrogenase deficiency: biochemical versus genetic technologies. Semin Perinatol 2011; 35(3): 155-161.
[18] Williams O, Gbadero D, Edowhorhu G, Brearley A, Slusher T, Lund TC. Glucose-6-phosphate dehydrogenase deficiency in Nigerian children. PLoS One 2013; 8(7): e68800.
[19] Mombo LE, Ntoumi F, Bisseye C, Ossari S, Lu CY, Nagel RL, et al. Human genetic polymorphisms and asymptomatic Plasmodium falciparum malaria in Gabonese schoolchildren. Am J Trop Med Hyg 2003; 68(2): 186-190.
[20] Howes RE, Battle KE, Satyagraha AW, Baird JK, Hay SI. G6PD deficiency: global distribution, genetic variants and primaquine therapy. Adv Parasitol 2013; 81: 133-201.
10.12980/APJTB.4.2014APJTB-2014-0100
*Corresponding author: Prof. Jacques SIMPORE. Biomolecular Research Center Pietro Annigoni (CERBA) LABIOGENE UFR/SVT, University of Ouagadougou BP 364 Ouagadougou, Burkina Faso. Burkina Faso, West Africa.
Tel: +226 50361232/+226 70230792
E-mail: jacques.simpore@yahoo.fr
Foundation Project: Supported by West African Economic and Monetary Union (WAEMU) through the Programme d’appui et de développement des centres d’excellence régionaux. Grant No. PACER II.
Article history:
Received 28 Jun 2014
Received in revised form 5 Jul, 2nd revised form 13 Jul, 3rd revised form 20 Jul 2014
Accepted 21 Aug 2014
Available online 28 Aug 2014
Methods:Two hundred individuals in a rural community were genotyped for the mutations A376G, G202A, A542T, G680T and T968C using TaqMan single nucleotide polymorphism assays and polymerase chain reaction followed by restriction fragment length polymorphism.
Results:The prevalence of the G6PD deficiency was 9.5% in the study population. It was significantly higher in men compared to women (14.3% vs 6.0%, P=0.049). The 202A/376G G6PD A-was the only deficient variant detected. Plasmodium falciparum asymptomatic parasitaemia was significantly higher among the G6PD-non-deficient persons compared to the G6PD-deficient (P<0.001). The asymptomatic parasitaemia was also significantly higher among G6PD nondeficient compared to G6PD-heterozygous females (P<0.001).
Conclusions:This study showed that the G6PD A- variant associated with protection against asymptomatic malaria in Burkina Faso is probably the most common deficient variant.
 Asian Pacific Journal of Tropical Biomedicine2014年8期
Asian Pacific Journal of Tropical Biomedicine2014年8期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- An overview of travel-associated central nervous system infectious diseases: risk assessment, general considerations and future directions
- Genetic diversity of the msp-1, msp-2, and glurp genes of Plasmodium falciparum isolates along the Thai-Myanmar borders
- Impact of antibacterial drugs on human serum paraoxonase-1 (hPON1) activity: an in vitro study
- High levels of Zinc-α-2-Glycoprotein among Omani AIDS patients on combined antiretroviral therapy
- Toxicity effects of water extracts of Holothuria atra Jaeger in mice
- Prevention of renal dysfunction by nutraceuticals prepared from oil rich plant foods
