Genetic diversity of the msp-1, msp-2, and glurp genes of Plasmodium falciparum isolates along the Thai-Myanmar borders
Kanungnit Congpuong, Rungniran Sukaram, Yuparat Prompan, Aibteesam Dornae
1Bansomdejchaopraya Rajabhat University, Bangkok, Thiland
2Bureau of Vector Borne Disease, Department of Disease Control, Ministry of Public Health, Nonthaburi, Thiland
Genetic diversity of the msp-1, msp-2, and glurp genes of Plasmodium falciparum isolates along the Thai-Myanmar borders
Kanungnit Congpuong1*, Rungniran Sukaram2, Yuparat Prompan1, Aibteesam Dornae1
1Bansomdejchaopraya Rajabhat University, Bangkok, Thiland
2Bureau of Vector Borne Disease, Department of Disease Control, Ministry of Public Health, Nonthaburi, Thiland
PEER REVIEW
Peer reviewer
Maria Dorina Bustos, MD, PhD, Malaria Technical Officer, WHO, Thailand. Tel: (66) 2 591 8198; (66) 2 590 1524, Fax: (66) 2 591 8199; (66) 2 590 1525, E-mail: bustosm@who.int; dorinabustos@ yahoo.com.
Co-reviewers: Dr. Wanna Chaijaroenkul, Pathumthani, Thailand.
Comments
This is an important paper providing baseline information of the genetic diversity of malaria parasite populations with public health implications on future epidemiological studies on the dynamics of parasite transmission, and its impact on malaria control interventions and the drug resistance situation in the border areas.
Details on Page 601
Objective:To study the genetic diversity at the msp-1, msp-2, and glurp genes of Plasmodium falciparum (P. falciparum) isolates from 3 endemic areas in Thailand: Tak, Kanchanaburi and Ranong provinces.
msp-1 gene, msp-2 gene, glurp gene, Plasmodium falciparum, Thai-Myanmar border
1. Introduction
The morbidity and mortality rates due to malaria have been declining gradually in recent years in Thailand, but multidrug resistantPlasmodium falciparum(P. falciparum) remains one of the major health problems in Thailand. Artesunatemefloquine combination has been the first line treatment of uncomplicated falciparum malaria in Thailand since 1995. Declining efficacy of this regimen has been observed in provinces along the Thai-Myanmar borders, especially in Kanchanaburi province during 2009-2011. A successful malaria control and prevention require the coordinated use of several strategies, including understanding the nature and extent of genetic diversity withinP. falciparumwhich is essential in understanding the mechanism underlying its pathology, the acquisition of immunity, the spread of drug resistance and the infection transmission[1]. The merozoite surface protein 1 (msp-1) andmsp-2are abundant surface protein on theblood stage ofP. falciparum. They are thought to play a role in erythrocyte invasion[2]. Four allelic families had been identified in block 2 ofmsp-1gene: K1, MAD20, RO33, and MR and two allelic families inmsp-2gene: FC27 and 3D7[3-6]. Themsp-1,msp-2and glutamate-rich protein (glurp) have been extensively used as markers to investigate the genetic diversity, multiplicity of infection, the level of malaria transmission, immunity against malaria, as well as a discriminatory tool to distinguish new from recrudescent infections of field parasite population[7-15]. This informative tool has been used for distinguishing individual alleles in the therapeutic drug efficacy monitoring program of the Malaria Control Program of Thailand. It was used to investigate the influence of genetic diversity ofP. falciparumin a malaria endemic area along the Thai-Cambodia border[16]. However, data were not available from endemic areas along the Thai-Myamar border[17]. The aim of this study was to assess the genetic diversity and allele frequencies ofmsp-1,msp-2andglurpgenes ofP. falciparumisolates from malaria patients in 3 provinces along the Thai-Myanmar border namely; Tak, Kanchanaburi and Ranong.
2. Materials and methods
2.1. Study sites and samples
A total of 144P. falciparuminfected blood were collected from uncomplicated falciparum malaria adult patients enrolled into the therapeutic efficacy monitoring of artemisinin derivatives antimalarial drugs during January, 2012 to June, 2013 in malaria endemic areas along the western border of Thailand with Myanmar. Ethical approval was obtained from the Research Ethics Committee of the Department of Disease Control, Ministry of Public Health, Thailand. Pre-treatment blood samples collected on Whatmann 3MM filter paper for DNA extraction was obtained from patients in 3 areas, 1) Maesot district of Tak province (57 cases), 2) Saiyok district of Kanchanaburi (55 cases), and 3) Kraburi district of Ranong (32 cases) (Figure 1).

Figure 1.Map of Thai-Myanmar border showing the studied areas; Maesot district of Tak province, Saiyok district of Kanchanaburi province, and Kraburi district of Ranong province.
2.2. Genotyping of P. falciparum msp-1, msp-2 and glurp genes
Parasite DNA for PCR was extracted from dried blood spot using the QiaAmp DNA mini kit (Qiagen, Hilden, Germany) according to manufacturer’s instructions. Polymorphic regions fromP. falciparummsp-1,msp-2, andglurpgenes were used as genetic markers for the genotyping of parasite populations. Formsp-1andmsp-2, the presence of unique sequences was used to divide the variants into distinct allelic families. The polymorphic repetitive regions Block 2 ofmsp-1; Block 3 ofmsp-2and RII repeat region ofglurpwere amplified by nested PCR using the primers and methods as recommended genotyping protocol[18]. In brief, the primary PCR, primer pairs corresponding to conserved sequences spanning the polymorphic regions of each gene were included in separate reactions. The product generated in the primary PCR was used as a template in six separate nested PCR, using in each case a specific primer pair in order to determine the presence of allelic variants of the K1, MAD20 and RO33 families ofmsp-1block2; the 3D7 and FC27 families ofmsp-2repeats, and the RII blocks ofglurp. Each polymorphic domain was amplified from 5 µL of DNA solution in a 20 µL reaction mixture containing 0.12 µmol/L of each primer, 2 mmol/L MgCl2, 125 µmol/L of each deoxyribonucleoside triphosphate, 0.4 U Amplitaq Gold® 360 Master Mix (Invitrogen) and PCR buffer.
The thermocycling conditions in the thermocycler (Thermo Scientific Hybaid Px2 Thermal Cycler, Fisher Scientific) formsp-1,msp-2andglurpprimary PCR andglurpnested PCR were as follows: 5 min at 95 °C, followed by 30 cycles for 1 min at 94 °C, 2 min at 58 °C and 2 min at 72 °C and final extension of 10 min at 72 °C. For themsp-1andmsp-2nested PCR, conditions were as followed: 5 min at 95 °C, followed by 30 cycles for 1 min at 94 °C, 2 min at 61 °C and 2 min at 72 °C and final extension of 5 min at 72 °C. The amplified products were either stored at 4 °C or analyzed immediately by electrophoresis on a 2% molecular grade agarose gel and visualized by UV transilluminator after gel SYBR® safe staining. The sizes of the PCR products were estimated using Imagelab software version 3.0 (Biorad) with the size computed automatically by the software based on the 100 base pairs DNA ladder calibrator (Real Biotech Corporation). Standard 3D7, Dd2 and RO33 clones were used as positive controls for 3D7 and K1; FC27 and MAD20; and RO33 alleles, respectively.
The detection of a single PCR fragment for each locus was classified as an infection with one parasite genotype (monoclonal infection) for that locus. Isolates with more than one genotype were considered as polyclonal infection[10]. Alleles in each family were considered the same if fragment size were within 20 bp interval formsp-1andmsp-2genes[11], and 50 bp interval forglurpgene. In each isolate, number of genotypes and allelic type (or family) of each gene were described.
2.3. Statistical analysis
Themsp-1andmsp-2allele frequencies were calculated as the proportion of alleles found for the allelic family out of the alleles detected in isolates. The proportions of alleles observed at each genetic locus within each group were compared using theChi-square test statistics. Multiplicity of infection (MOI) was defined as the number of parasite genotypes per infection.MOI was calculated for each gene (msp-1,msp-2, andglurp) independently. Estimation of the overall MOI of the isolates was also calculated by combining the three markers, namely by using the highest number of bands detected in one marker. The maximum number of bands detected whatever the locus was considered as the MOI of that infection. Mean MOI was calculated by dividing the total number of fragments detected inmsp-1,msp-2orglurpby the number of samples positive for the same marker. The median MOI was compared among isolates from Tak, Kanchanaburi and Ranong using the non-parametric Kruskal Wallis H test. Statistical significance was defined as aP-value≤0.05. All statistical analyses were performed using SPSS statistical software, version 17.0.
3. Results
The study population comprisedP. falciparumisolated collected from 144 uncomplicated falciparum malaria patients before treatment; 57, 55, and 32 samples were from Tak, Kanchanaburi and Ranong provinces, respectively.
3.1. Distribution of block 2 of msp-1 gene
Allelic families ofmsp-1were not evenly distributed among the isolates from Tak, Kanchanaburi, and Ranong provinces (P<0.000 1). Forty-one (74.5%)P. falciparumisolates from Kanchanburi province were positive for the K1 allele, in contrast to 23 (40.4%) of Tak and 14 (43.8%) of Ranong isolates (Table 1). Isolates from Kanchanaburi also carried the highest proportion of RO33 allele (45.5%), followed by isolates from Tak (12.3%) and Ranong (9.4%) but carried significantly the lowest proportion (21.8%) of MAD20 allele (P=0.000 1).
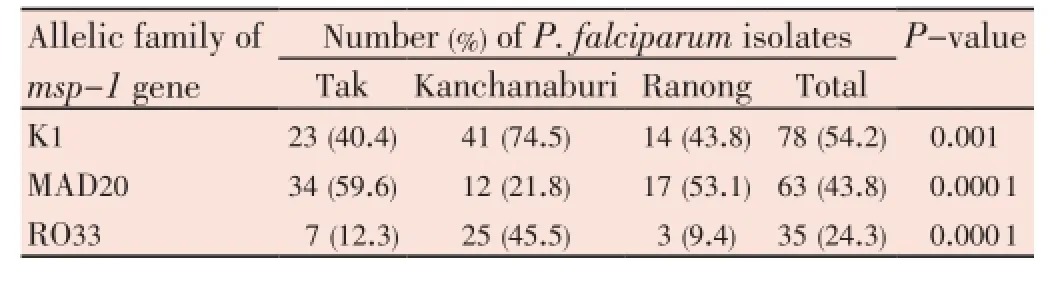
Table 1 Distribution of merozoite surface protein-1 (msp-1) allelic family of Plasmodium falciparum.
3.2. Distribution of block 3 ofmsp-2gene
FC27 and 3D7 had similar distribution pattern in the samples from Tak, Kanchanaburi, and Ranong provinces (Table 2). Forty-two (73.7%)P. falciparumisolates from Tak province were positive for the 3D7 allele and 34 (59.6%) for FC27. Kanchanaburi isolates had slightly lower proportion of FC27 allele (45.5%) but higher 3D7 allelle (80.0%). Ranong isolates had quite similar proportion of FC27 (59.4%) to Tak isolates but had higher proportion of 3D7 (93.8%). However, there was no significant difference (P>0.05).
3.3. Genetic diversity of infection
Of the 144P. falciparumisolates genotyped, 139 (96.5%) comprised multiple genotypes (Table 3). A high degree of genetic diversity was detected at themsp-1andmsp-2genes whereas less diversity was observed at theglurpgene. MOI calculated for each gene showed that the mean MOI formsp-2was the highest (2.93), followed bymsp-1(2.15), andglurp(1.26). The difference in MOI ofmsp-1among the isolates from Tak, Kanchanaburi and Ranong was statistically significant (P=0.015). The mean MOI ofmsp-1was the highest in Kanchanaburi isolates (2.47), followed by Ranong (2.19) and Tak (1.89). Proportion of isolates having MOI≥3 was the highest in Ranong isolates (25.0%), followed by Kanchanaburi (23.7%) and Tak (15.8%).
Similar difference among the locations was also observed in median MOI formsp-2gene (P=0.013). Isolates from Ranong had the highest mean MOI of 3.34, followed by Tak (2.95) and Kanchanaburi (2.62). The highest proportion of isolates having MOI≥3 was also found in Ranong isolates (81.2%), followed by Tak (61.4%) and Kanchanaburi (49.2%).P. falciparumisolates in this study had less complexity if using onlyglurpas a marker gene. Most isolates (84.7%) had only one allele. Isolates from Ranong had no diversity at theglurplocus; all isolates carried only one genotype. Estimation of the overall MOI of the isolates showed that the mean MOI was the highest in Ranong isolates (3.47), followed by Kanchanaburi isolates (3.15) and Tak isolates (3.14) but the difference was not statistically significant (P=0.164).
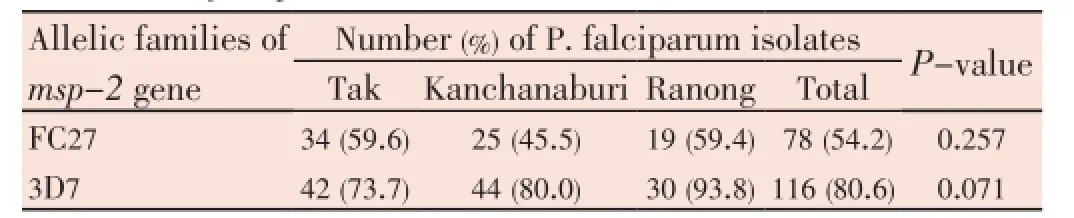
Table 2 Distribution of merozoite surface protein-2 (msp-2) allelic family of Plasmodium falciparum.

Table 3 Multiplicity of infection (MOI) of msp-1, msp-2 and glurp genes.
4. Discussion
In this study we have examined the allelic diversity ofP. falciparumand attempted to understand whether the polymorphism and frequency of alleles ofmsp-1,msp-2andglurpvaries with locations of the parasite isolates.P.falciparumisolates from 3 locations along the western Thailand bordered to Myanmar,i.e.Tak, Kanchanaburi, and Ranong provinces were studied.P. falciparumisolates in these areas had very high complexity of infection; 95% of the isolates had multiple genotype infection (75.7%, 88.3%, and 15.3% formsp-1,msp-2, andglurp, respectively). The highest number of alleles per isolate was 3, 5, and 5 for themsp-1,msp-2, andglurp, respectively. Overall mean MOI was 3.21. K1 and MAD20 were the predominant allelic families at themsp-1gene, whereas alleles belonging to 3D7 were more frequent at themsp-2gene. Lower polyclonal infectedP. falciparumisolates was found in the western Cambodia[16]; 31% of the isolates had polyclonal infection (17%, 26%, and 8% formsp-1,msp-2, andglurp, respectively). Genotypic similarity ofP. falciparumisolates in the eastern provinces of Thailand bordered to Cambodia such as Chanthaburi and Trat provinces is interesting to further study and compare with the isolates from those obtained from the western border of Thailand demonstrated in this study.
In the Thai-Mynmar border, population structure ofP. falciparumisolates from Tak and Ranong provinces were similar as revealed by the presence of similar proportions of MAD20 and K1 alleles atmsp-1loci, 3D7 and FC27 alleles atmsp-2loci as well as comparable mean multiplicity of infections. In contrast, isolates from Kanchanaburi had different structure; the most prevalent alleles were K1 and RO33. The difference is probably due to the local factors as previously described[19], such as vector population, human host immunity, local transmission, as well as drug susceptibility pattern of the parasites in the areas. A recent efficacy study of artesunate-mefloquine combination in these 3 provinces showed that the cure rate declined rapidly in Kanchanaburi province from 92.7% in 2009 to 80% in 2011. High proportion of RO33 allele found in isolates from Kanchanaburi (45.5%) may relate with the reduction in treatment outcome. It is interesting to further monitoring the association of RO33 allelic family with the treatment outcome and drug resistant malaria.
As shown in the map, Kanchanaburi situates between Tak and Ranong. It was surprised to see the similar allelic patterns ofmsp-1andmsp-2genes ofP. falciparumisolates from Tak and Ranong, instead of Tak and Kanchanaburi or Kanchanburi and Ranong where there are shared border. In order to explain the 2 different allelic patterns, Tak and Ranong in one group and Kanchanaburi in another group, infection history of the patients were reviewed from the malaria case investigation forms recorded by the malaria clinics in the areas. It was found that most Kanchanaburi isolates were obtained from indigenous malaria cases contracted the disease in the villages or nearby forest in Bongti Subdistrict of Saiyok District. The parasite genotypes found in Kanchanaburi isolates were thus the genotypes circulated in the area. In contrast, most patients in Tak and Ranong were non-permanent Myanmese immigrants crossing border to seek employment or health facilities in Thai side.
Although Tak and Ranong had similar allelic patterns ofmsp-1andmsp-2genes, Ranong had higher diversity as measured by MOI. This may be resulted from higher malaria transmission in the area. High diversity of parasite isolates may also affect the treatment outcome. Data from artesunatemefloquine combination efficacy monitoring in 2009 of these 3 provinces showed that the cure rate in Ranong (87%) was lower than that in Tak (90.4%) and Kanchanaburi (92.7%). In addition, efficacy study of artemether-lumefantrine in 2012 in Tak and Ranong also showed lower cure rate in Ranong (88.4%) compared to 93.8% in Tak.
There was a limitation in the analysis of PCR products by gel electrophoresis. Varies in measuring of PCR product by gel electrophoresis was common. There was no standardized criterion to define distinct genotype. In the present study, lower number of distinct alleles was demonstrated in isolates from Thai-Myanmar border compared to the isolates from the western Cambodia near the eastern border of Thailand[16]. However, it was not able to conclude that the parasite from the Thai-Myanmar border had lower diversity because different setting criterion was used to define different alleles. A minimum of ten base pairs size difference was required to define an additional genotype in the study in western Cambodia[16], while 20 base pairs size difference was used in this study.
The present study shows thatP. falciparumisolates from Tak and Ranong provinces had similar allelic pattern ofmsp-1andmsp-2and diversity but different from Kanchanaburi isolates. These allelic variant profiles are valuable baseline data for future epidemiological study of malaria transmission and for continued monitoring of polymorphisms associated with antimalarial drug resistance in these areas.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
We acknowledge the collaboration of the Bureau of Vector Borne Disease (BVBD), Department of Disease Control, Ministry of Public Health, Thailand and the USAID. In addition, we thank the laboratory staffs of the Reference Laboratory of the BVBD and data collectors in the malaria clinics situated along the Thai-Myanmar border. This study was supported by a grant from Research Institute, Bansomdejchaopraya Rajabhat University, Grant no. 2555.
Comments
Background
This study aims to assess the genetic diversity and allele frequencies ofmsp-1,msp-2andglurpgenes ofP. falciparumisolates from malaria patients in 3 provinces along the Thai-Myanmar border. These markers are used to investigate the genetic diversity, multiplicity of infection, and to distinguish new from recrudescent infections in the therapeutic efficacy monitoring of the Malaria Control Program of Thailand.
Research frontiers
Such genetic diversity ofP. falciparumhas been observed in a malaria endemic area along the Thai-Cambodia border,but this is the first study along the Thai-Myanmar border. It would shed light on how divergent parasite populations affect the transmission dynamics in this part of the country.
Related reports
The genotyping ofP. falciparummsp-1,msp-2andglurpgenes follows the standard protocols for DNA extraction, PCR and sequencing for allelic variants. The multiplicity of infections and frequency of alleles can vary with different locations of the parasite isolates.
Innovations and breakthroughs
This is the first study on the genetic diversity ofP. falciparum, comparing families of allelic variants from 3 different malaria endemic provinces along the western border of Thailand. It showed a high multiple genotype of infection and a significantly different distribution of allelic families ofmsp-1from the 3 locations.
Applications
The allelic variant profiles are valuable baseline information for future epidemiological studies of malaria parasite populations and for continued monitoring of polymorphisms associated with antimalarial drug resistance in these border areas with a worsening drug resistance situation.
Peer review
This is an important paper providing baseline information of the genetic diversity of malaria parasite populations with public health implications on future epidemiological studies on the dynamics of parasite transmission, and its impact on malaria control interventions and the drug resistance situation in the border areas. It also depicts a healthy collaborative partnership between the academe and the Ministry of Public Health of Thailand, with the application of basic science research in public health issues.
[1] Kiwanuka GN. Genetic diversity in Plasmodium falciparum merozoite surface protein-1 and 2 coding genes and its implications in malaria epidemiology: a review of published studies from 1997-2007. J Vector Borne Dis 2009; 46: 1-12.
[2] Boyle MJ, Langer C, Chan JA, Hodder AN, Coppel RL, Anders RF, et al. Sequential processing of merozoite surface proteins during and after erythrocyte invasion by Plasmodium falciparum. Infect Immun 2014; 82: 924-936.
[3] Salem MSOA, Ndiaye M, Abdallahi MO, Lekweiry KM, Bogreau H, Konaté L, et al. Polymorphism of the merozoite surface protein-1 block 2 region in Plasmodium falciparum isolates from Mauritania. Malar J 2014; 13: 26.
[4] Snounou G, Zhu X, Siripoon N, Jarra W, Thaithong S, Brown KN, et al. Biased distribution of msp-1 and msp-2 allelic variants in Plasmodium falciparum populations in Thailand. Trans R Soc Trop Med Hyg 1999; 93: 369-374.
[5] Atroosh WM, Al-Mekhlafi HM, Mahdy MA, Saif-Ali R, Al-Mekhlafi AM, Surin J. Genetic diversity of Plasmodium falciparum isolates from Pahang, Malaysia based on msp-1 and msp-2 genes. Parasit Vectors 2011; 4: 233.
[6] Amodu OK, Hartl DL, Roy SW. Pattern of polymorphism in genomic regions flanking three highly polymorphic surface antigens in Plasmodium falciparum. Mol Biochem Parasitol 2008; 159: 1-6.
[7] Cattamanchi A, Kyabayinze D, Hubbard A, Rosenthal PJ, Dorsey G. Distinguishing recrudescence from reinfection in a longitudinal antimalarial drug efficacy study: comparison of results based on genotyping of msp-1, msp-2, and glurp. Am J Trop Med Hyg 2003; 68: 133-139.
[8] Mwingira F, Nkwengulila G, Schoepflin S, Sumari D, Beck HP, Snounou G, et al. Plasmodium falciparum msp-1, msp-2 and glurp allele frequency and diversity in sub-Saharan Africa. Malar J 2011; 10: 79.
[9] Al-abd NM, Mahdy MAK, Al-Mekhlafi AMQ, Snounou G, Abdul-Majid NB, Al-Mekhlafi HM, et al. The suitability of P. falciparum merozoite surface proteins 1 and 2 as genetic markers for in vivo drug trials in Yemen. PLoS One 2013; doi: 10.1371/journal. pone.0067853.
[10] Kiwuwa MS, Ribacke U, Moll K, Byarugaba J, Lundblom K, Färnert A, et al. Genetic diversity of Plasmodium falciparum infections in mild and severe malaria of children from Kampala, Uganda. Parasitol Res 2013; 112: 1691-1700.
[11] Mayengue PI, Ndounga M, Malonga FV, Bitemo M, Ntoumi F. Genetic polymorphism of merozoite surface protein-1 and merozoite surface protein-2 in Plasmodium falciparum isolates from Brazzaville, Republic of Congo. Malar J 2011; 10: 276.
[12] Pratt-Riccio LR, Perce-da-Silva Dde S, Lima-Junior Jda C, Theisen M, Santos F, Daniel-Ribeiro CT, et al. Genetic polymorphisms in the glutamate-rich protein of Plasmodium falciparum field isolates from a malaria-endemic area of Brazil. Mem Inst Oswaldo Cruz 2013; 108: 523-528.
[13] Hamid MM, Mohammed SB, El Hassan IM. Genetic diversity of Plasmodium falciparum field isolates in central Sudan inferred by PCR genotyping of merozoite surface protein 1 and 2. N Am J Med Sci 2013; 5: 95-101.
[14] Akter J, Thriemer K, Khan WA, Sullivan DJ Jr, Noedl H, Haque R. Genotyping of Plasmodium falciparum using antigenic polymorphic markers and to study anti-malarial drug resistance markers in malaria endemic areas of Bangladesh. Malar J 2012; 11: 386.
[15] Amodu OK, Oyedeji SI, Ntoumi F, Orimadegun AE, Gbadegesin RA, Olumese PE, et al. Complexity of the msp2 locus and the severity of childhood malaria in south-western Nigeria. Ann Trop Med Parasitol 2008; 102: 95-102.
[16] Gosi P, Lanteri CA, Tyner SD, Se Y, Lon C, Spring M, et al. Evaluation of parasite subpopulations and genetic diversity of the msp1, msp2 and glurp gene during and following artesunate monotherapy treatment of Plasmodium falciparum malaria in Western Cambodia. Malar J 2013; 12: 403.
[17] Yuan L, Zhao H, Wu L, Li X, Parker D, Xu S, et al. Plasmodium falciparum populations from northeastern Myanmar display high levels of genetic diversity at multiple antigenic loci. Acta Trop 2013; 125: 53-59.
[18] Felger I, Snounou G. Recommended genotyping procedure (RGPs) to identify parasite populations. Genava: WHO; 2008. [Online] Available from: http://www.who.int/malaria/publications/atoz/ rgptext_sti.pdf [Accessed on 23rd Febuary, 2013]
[19] Joshi H, Valecha N, Verma A, Kaul A, Mallick PK, Shalini S, et al. Genetic structure of Plasmodium falciparum field isolates in eastern and north-eastern india. Malar J 2007; 6: 60.
10.12980/APJTB.4.2014APJTB-2014-0156
*Corresponding author: Dr. Kanungnit Congpuong, Bansomdejchaopraya Rajabhat University, 1061 Soi Isaraparp 15, Isaraparp Street, Hiranrujee Subdistrict, Dhonburi District, Bangkok, 10600, Thailand.
Tel: 66 084 376 7400
E-mail: k.congpuong@gmail.com
Foundation Project: Supported by a grant from Research Institute, Bansomdejchaopraya Rajabhat University (Grant no. 2555).
Article history:
Received 4 May 2014
Received in revised form 12 May, 2nd revised form 20 May, 3rd revised form 27 May 2014
Accepted 27 Jun 2014
Available online 28 Jul 2014
Methods:A total of 144 P. falciparum isolates collected prior to treatment during January, 2012 to June, 2013 were genotyped. DNA was extracted; allele frequency and diversity of msp-1, msp-2, and glurp genes were investigated by nested polymerase chain reaction.
Results:P. falciparum isolates in this study had high rate of multiple genotypes infection (96.5%) with an overall mean multiplicity of infection of 3.21. The distribution of allelic families of msp-1 was significantly different among isolates from Tak, Kanchanaburi, and Ranong but not for the msp-2. K1 and MAD20 were the predominant allelic families at the msp-1 gene, whereas alleles belonging to 3D7 were more frequent at the msp-2 gene. The glurp gene had the least diverse alleles. Population structure of P. falciparum isolates from Tak and Ranong was quite similar as revealed by the presence of similar proportions of MAD20 and K1 alleles at msp-1 loci, 3D7 and FC27 alleles at msp-2 loci as well as comparable mean MOI. Isolates from Kanchanaburi had different structures; the most prevalent alleles were K1 and RO33.
Conclusions:The present study shows that P. falciparum isolates from Tak and Ranong provinces had similar allelic pattern of msp-1 and msp-2 and diversity but different from Kanchanaburi isolates. These allelic variant profiles are valuable baseline data for future epidemiological study of malaria transmission and for continued monitoring of polymorphisms associated with antimalarial drug resistance in these areas.
 Asian Pacific Journal of Tropical Biomedicine2014年8期
Asian Pacific Journal of Tropical Biomedicine2014年8期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- An overview of travel-associated central nervous system infectious diseases: risk assessment, general considerations and future directions
- Impact of antibacterial drugs on human serum paraoxonase-1 (hPON1) activity: an in vitro study
- High levels of Zinc-α-2-Glycoprotein among Omani AIDS patients on combined antiretroviral therapy
- Toxicity effects of water extracts of Holothuria atra Jaeger in mice
- Prevention of renal dysfunction by nutraceuticals prepared from oil rich plant foods
- Polyketide and benzopyran compounds of an endophytic fungus isolated from Cinnamomum mollissimum: biological activity and structure
