Toxicity effects of water extracts of Holothuria atra Jaeger in mice
Ridzwan Bin Hashim, Nurul Alia Azizan, Zaitunnatakin Zamli, Farah Hanis Zulkipli, Nurzafirah Mazlan, Osama Yousef Althunibat
1Department of Biomedical Science, Faculty of Allied Health Sciences, International Islamic University Malaysia Kuantan Campus, Jalan Sultan Ahmad Shah, Bandar Indera Mahkota 10, 25200 Kuantan, Pahang, Malaysia
2Faculty of Pharmacy, Alzaytoonah University of Jordan, Amman, Jordan
Toxicity effects of water extracts of Holothuria atra Jaeger in mice
Ridzwan Bin Hashim1*, Nurul Alia Azizan1, Zaitunnatakin Zamli1, Farah Hanis Zulkipli1, Nurzafirah Mazlan1, Osama Yousef Althunibat2
1Department of Biomedical Science, Faculty of Allied Health Sciences, International Islamic University Malaysia Kuantan Campus, Jalan Sultan Ahmad Shah, Bandar Indera Mahkota 10, 25200 Kuantan, Pahang, Malaysia
2Faculty of Pharmacy, Alzaytoonah University of Jordan, Amman, Jordan
PEER REVIEW
Peer reviewer
Fedekar Fadel Madkour, Prof. of Marine Ecology, Head of Marine Science Department, Faculty of Science, Port Said University, Egypt.
Tel: 002 0663323608
E-mail: fedekarmadkour@ymail.com
Comments
The present research addressed the toxicity effect of sea cucumber, H. atra, which is consumed as one of the popular food for local consumers in Malaysia in addition to its use in traditional medicine. The findings of this research were needed to raise the awareness of the harmful use of such organisms.
Details on Page 617
Objective:To determine lethal median dose (LD50) and histopathological toxicity of water extract of Holothuria atra (H. atra) in mice.
Holothuria atra, Water extract, Lethal median dose, Animal toxicity
1. Introduction
Holothuria atra (H. atra)Jaeger is a local sea cucumber which can be found abundantly in Malaysian waters. It has been reported to have medicinal values[1-3]. In Sabah, aparting from being consumed as food, its main use is based on local culture and beliefs. Even though there are many people who claim the numerous benefits ofH. atrain traditional medicines and it is safe for human consumption. Support from scientific studies is important to systematically prove the claims. These claims subsequently will convince the public. Therefore, water extract ofH. atrawere investigated for their toxicity effects on mice with a view to ensure whether there are toxic effects ofH. atra.
This study was carried out to determine the lethal median dose (LD50) and histopathological toxicity of the water extract ofH. atra. Toxic effects were assessed on the possible toxic symptoms and histological changes in liver tissues.
2. Materials and methods
2.1. Sample collection and preparation
Samples ofH. atracollected from Pangkor Island, Perak were cleaned with distilled water to remove the visceral organs before they were homogenized for water extraction.
2.2. Water extraction method
About 50 g of the homogenized tissues were dissolved in 300 mL distilled water at room temperature and stirred using a magnetic stirrer for 1 to 2 h. Then, the mixture was filtered by Whatman No.1 filter paper. The filtrate was kept aside. The steps above were repeated by dissolving the remainder residue with 150 mL and 50 mL of distilled water, respectively. All filtrate were pooled and kept in a freezer at -30 °C until freeze drying.
2.3. Experimental animals
Male mice averaging 30 g body weight were obtained from the Institute for Medical Research Kuala Lumpur. They were devided into 8 groups, control (3 mice, normal saline) and 7 test groups (each containing 10 mice, water extraction, 10, 20, 30, 50, 100, 150 and 200 mg/kg respectively, administered viai.p.). The mice were placed in different cages according to the respective group treatments in standard environmental conditions. Food and water were givenad libitium. The mice were monitored for the first 24 h after the administration of the extract and once for every 2 h for the next 24 h and at least twice per day for the rest of the days. The LD50was calculated by Reed-Muench method[4].
2.4. Toxicity study
Water extract ofH. atra, dissolved in normal saline was administered (i.p.); each was administered to groups of 10 male mice. All the treated animals were carefully examined for 14 d for any signs of toxicity (behavioral changes and mortality).
2.5. Histological examination
A small portion of the liver tissue of each mouse was fixed in 10% formalin, processed and embedded in paraffin wax to obtain 5 µm thick slices by using a microtome.
Hematoxylin and eosin stain was applied to the sections. Then they were examined under a light inverted microscope. Staining was needed to observe the color, shape and size of hepatocytes. Hematoxylin stained the nucleus blue. Eosin stained the cytoplasm red.
3. Results
3.1. Median LD50calculation
There were 8 doses ranging from 0 mg/kg to 200 mg/kg were used (Table 1). The mortalities was observed in 10 mice for each dose except for 0 mg/kg (3 mice). The sum of the number of deaths and survivals for each dose gave the denominator to calculate the percent mortality at each level. Since 50% was between 67% and 24%, the LD50would be in between 50 and 30 mg/kg. Figure 1 shows the mortality percentage of mice, which is directly proportional to the dosage administered (singlei.p.dose). It also shows that at 50% of mortality, the LD50ofH. atraextract was at 41 mg/kg. However, there was a sudden increase in the number of mice that died at a dose of 50 mg/kg ofH. atraextract. Thus, since the LD50value ofH. atraextract was relatively low, it could be concluded thatH. atraextract is toxic.
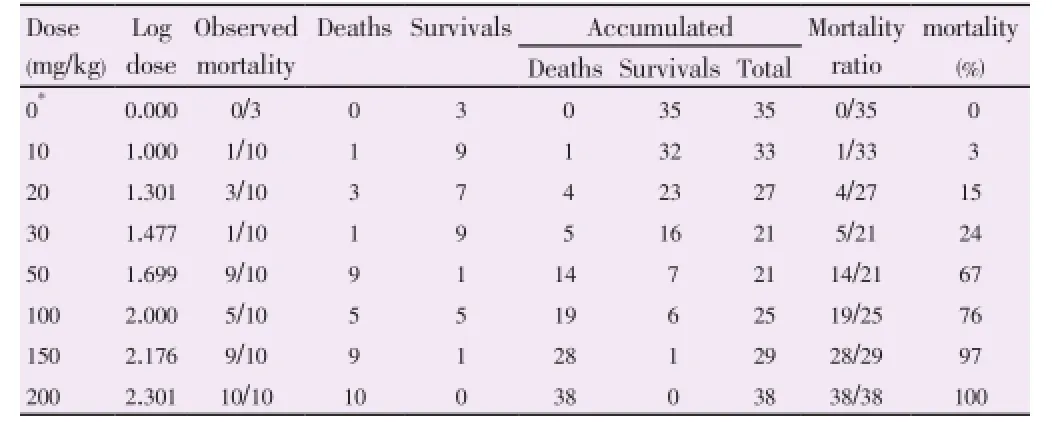
Table 1 Calculation of the LD50by Reed-Muench method.

Figure 1.Dose-mortality curve for H. atra extract in mice.Log (50/30)=0.222 →2Then (1×2): 0.605×0.222=0.134Log LD50=Log 30+0.134=1.611Antilog 1.611=40.872~41 mg/kg.
3.2. Behavioral signs of toxicity
The mortality rate as well as the acute toxicity of theH. atraextract increased progressively as the dose increased from 10 to 200 mg/kg (Table 2). The main observed behavioral signs of toxicity were hypoactivity which was noticed immediately after administration and were more obvious at the higher doses and persisted until death. The acute toxicity (LD50) ofH. atraextract in mice was calculated to be approximately 41 mg/kg.

Table 2 Behavioural signs of toxicity effects of water extract of H. atra administered (i.p.) to mice.
3.3. Histological observation
Histological examination of the liver of mice in control group administrated with normal saline (Figure 2) showed no abnormal changes. On contrary, abnormalities were observed in treated groups with low and high doses (Figure 3 and 4). Abnormalities observed in the liver were hepatocytes arranged disorganized in cords and necrosis of the hepatocytes.

Figure 2.Section of liver from control group (normal saline) indicating polyhedral hepatocytes with defined cell lining arranged in cords and normal round nuclei, with granular cytoplasm, a. Magnification 20×. b. Magnification 40×.

Figure 3.Section of liver from the low doses groups (10, 20 and 30 mg/ kg), indicating the presence of abnormal hepatocytes with a distorted shape and undefined cell lining as well as enlarged nuclei and vacuolation of hepatocytes. Magnification 40×.
4. Discussion
To determine the safety products and drugs for human use, toxicological evaluation is carried out in various experimental animals to predict toxicity and to provide guidelines for selecting a safe dose for humans[5]. The hematological, gastrointestinal and cardiovascular adverse effects in animals and humans is in the highest overall concordance of toxicity[5,6], while certain adverse effects in humans, especially hypersensitivity and idiosyncratic reactions, are poorly correlated with toxicity observed in animals. Furthermore, it is quite difficult to ascertain adverse effects in animals such as headache and abdominal disturbances. In addition, interspecies differences in the pharmacokinetic parameters make it difficult to translate some adverse effects from animals to humans.
Nevertheless, the evaluation of adverse effects of acute toxicity testing dosing in experimental animals may be more relevant in determining the overall toxicity of theH. atraextract. The calculated LD50ofH. atraextract in mice after a single dose was approximately 41 mg/kgi.p.In this study, mortality and symptoms of adverse behavior were noted after the injection of relatively low doses of theH. atraextract in mice which was at 10 mg/kgi.p.The main behavioral sign of toxicity observed was hypoactivity, which was noticed immediately after administration and more obvious at the higher doses and persisted until death. At the higher doses which were 150 mg/kg and 200 mg/kg ofH. atraextracts, the movement was observed to be weak and later convulsed till they died.
Histopathological observation on the hepatic tissues found that the cells underwent necrosis and the organ was hemorrhagic for all the dead mice given the extract, where the severity was directly proportional to the dosage administered. The components of theH. atraextract responsible for the toxic manifestations are not known. The toxicity and the lethality of theH. atraextract may be due to any one or more of the active compounds present in the extract. Since the LD50value ofH. atraextract is relatively low, it can be concluded thatH. atraextract is toxic.

Figure 4.Section of liver from high doses groups (50, 100, 150 and 200 mg/kg) indicating more prominent distortion of polyhedral hepatocytes with undefined cell lining, massive cytoplasm, enlarged nuclei and vacuolated hepatocytes.
The mortality of mice in this present study was directly proportional to the dosage administered. However, there was a sudden increase in the number of mice that died at a dose of 50 mg/kg ofH. atraextract. Nine over ten mice died at dose of 50 mg/kg. Each mouse has different susceptibility to the disease or adverse effects. Thus, at a dose of 50 mg/kg, mice could be more susceptible to injury and die due to the administration ofH. atraextract.
Many exogenous drugs and other chemical substances can cause liver damage by a variety of mechanisms including cellular degeneration and necrosis by interfering directly with various specific biochemical reactions[7]. Depending upon their severity, they may produce cellular degeneration and necrosis. In this study,H. atraextract caused necrosis when it was observed through histological examination of the liver.
In the present study, control liver tissue indicated the presence of normal hepatocytes which are polyhedral in shape with defined cell lining; nuclei are distinctly rounded, with one or two prominent nucleoli. In contrast, the liver section of low and high dose groups indicated the presence of abnormal hepatocytes with a distorted shape and undefined cell lining and massive cytoplasm. Vacuolation of hepatocytes and enlarged nucleus was also observed in the high dose groups. The large vacuole in the cell forces the nuclei to the periphery of the hepatocyte which is usually accompanied by nuclear atrophy. The morphological changes in the liver may be due to the toxic effect of theH. atraextract. Liver is the dominant target site of specific toxins. Liver is the first organ to encounter ingested nutrients, vitamins, metals, drugs and environmental toxicants. Venous blood from the stomach and intestines flows into the portal vein and then through the liver before entering the systemic circulation. Thus, as most drugs and toxic chemicals are metabolized in the liver, these processes may cause liver injuries.
The present results indicate the morphologic evidence of necrosis which is shown by the nuclear and cytoplasmic changes. According to Chandrasoma and Taylor[6], the best evidence of cell necrosis is nuclear change. The chromatin of the cell clumps into coarse stands and the nucleus becomes a shrunken, dense and deeply basophilic mass which is stained dark blue with hematoxylin. This process is called pyknosis. The pyknotic nucleus may then break up into numerous small basophilic particles (karyorrhexis) or undergo lysis as a result of the action of lysosomal deoxyribonuclease (karyolysis). In rapidly occurring necrosis, the nucleus undergoes lysis without a pyknotic stage.
For cytoplasmic changes, its cytoplasm becomes homogenous and deeply acidophilic. It is stained pink with an acidic stain of eosin. This is the first change detectable by light microscope and it is due to denaturation of cytoplasmic proteins and loss of ribosomes. Swelling of mitochondria and disruption of organelle membranes cause cytoplasmic vacoulation. Finally, enzymatic digestion of the cell by enzymes released by the cell’s own lysosomes causes lysis.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
We are thankful to the International Islamic University Malaysia and TechnoFund Grant TF 0409E086 for supporting the work, and to Sist. Shuhamah Abu Dahari for typing the manuscript.
Comments
Background
H. atrais a sea cucumber present abundantly in Malaysia. It is consumed as food and reported to have medicinal values. Scientific evidence is required to prove that whether it is safe for human consumption.
Research frontiers
The present study estimated the LD50and histopathological toxicity of the water extract ofH. atra. Toxicity effects were assessed on the possible toxic symptoms and histological changes in liver tissues.
Related reports
There are many claims of the numerous benefits ofH. atrain traditional medicines and it is assumed to be safe for human consumption but with no sufficient evidences.
Innovations and breakthroughs
The authors calculated the LD50of the water extract ofH. atrato be 41 mg/kg. He also observed that the main behavioral sign of the toxicity was hypoactivity.
Applications
The present study proved thatH. atrais toxic even with low dose and have lethal effect with high dose. The study showed thatH. atrais not safe for human consumption.
Peer review
The present research addressed the toxicity effect of sea cucumber,H. atra, which is consumed as one of the popular food for local consumers in Malaysia in addition to its use in traditional medicine. The findings of this research were needed to raise the awareness of the harmful use of such organisms.
[1] Kamarudin KR, Rehan AM, Lukman AL, Ahmad HF, Anua MH, Nordin NF, et al. Coral reef sea cucumbers in Malaysia. Malays J Sci 2009; 28: 171-186.
[2] Hashim R. [Food resources from coastal areas of Sabah]. Kuala Lumpur, Malaysia: Dewan Bahasa & Pustaka, Ministry of Education, Malaysia; 1993, p. 309. Malay.
[3] Hashim R. Sea cucumbers: a Malaysian heritage. 1st ed. Kuala Lumpur, Malaysia: International Islamic University; 2010, p. 120.
[4] Ahmad Asmadi Y. [The role of vitamin E on paraquat toxicity] [PhD thesis]. Bangi: Universiti Kebangsaan Malaysia; 1999. Malay.
[5] Rhiouani H, El-Hilaly J, Israili ZH, Lyoussi B. Acute and subchronic toxicity of an aqueous extract of the leaves of Herniaria glabra in rodents. J Ethnopharmcol 2008; 118: 378-386.
[6] Zaki MA. Effects of the crude toxin of sea cucumbers Holothuria atra on some hematological and biochemical parameters in rats. Egypt J Nat Toxins 2005; 2: 71-86.
[7] Chandrasoma P, Taylor CR. Concise pathology. 3rd ed. Singapore: McGraw-Hill; 1997.
10.12980/APJTB.4.201414B154
*Corresponding author: Ridzwan B.H, Department of Biomedical Science, Faculty of Allied Health Sciences, International Islamic University Malaysia Kuantan Campus, Jalan Sultan Ahmad Shah, Bandar Indera Mahkota 10, 25200 Kuantan, Pahang, Malaysia.
Email : dr_ridzwan@yahoo.com
Foundation Project: Supported by the International Islamic University Malaysia and TechnoFund Grant TF 0409E086.
Article history:
Received 28 May 2014
Received in revised form 10 Jun, 2nd revised form 19 Jun, 3rd revised form 25 Jun 2014
Accepted 15 Jul 2014
Available online 28 Aug 2014
Methods:The behavioral changes, mortality and histopathology examination on liver were assessed in mice 14 d after the administration (i.p.) of H. atra water extract. Seven doses (10, 20, 30, 50, 100, 150 and 200 mg/kg) of H. atra were used. The control group was treated with normal saline.Results:In the acute study in mice, the water extracts of H. atra caused dose-dependent general behavior adverse affects and mortality. The main behavioral sign of toxicity was hypoactivity, noticed immediately after administration of the extract which was more obvious at the higher doses and persisted until death. Mortality increased with increasing doses, the calculated LD50was 41 mg/kg in mice. The liver toxicity was confirmed by histopathological examination, which indicated the presence of abnormal hepatocytes with a distorted shape and undefined cell lining as well as enlarged nuclei in low doses groups. High doses groups indicated a more prominent distortion of the polyhedral hepatocytes with undefined cell lining, massive cytoplasm, pyknotic, karyorhexis and karyolytic nuclei (necrosis of hepatocytes). Control group showed polyhedral hepatocytes with defined cell lining arranged in cords and normal round nuclei, with granular cytoplasm.
Conclusions:Because of the relatively low LD50value in the acute study in mice, it may be concluded that the H. atra water extract is toxic.
 Asian Pacific Journal of Tropical Biomedicine2014年8期
Asian Pacific Journal of Tropical Biomedicine2014年8期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- An overview of travel-associated central nervous system infectious diseases: risk assessment, general considerations and future directions
- Genetic diversity of the msp-1, msp-2, and glurp genes of Plasmodium falciparum isolates along the Thai-Myanmar borders
- Impact of antibacterial drugs on human serum paraoxonase-1 (hPON1) activity: an in vitro study
- High levels of Zinc-α-2-Glycoprotein among Omani AIDS patients on combined antiretroviral therapy
- Prevention of renal dysfunction by nutraceuticals prepared from oil rich plant foods
- Polyketide and benzopyran compounds of an endophytic fungus isolated from Cinnamomum mollissimum: biological activity and structure
