IgG4相关性自身免疫性胰腺炎影像学表现
徐兵 吕清清 陈静静
[摘要]目的分析IgG4相关性自身免疫性胰腺炎(autoimmune pancreatitis,AIP)的CT和MRI表现,以提高对该病影像学特征的认识,减少影像学误诊。方法回顾性分析47例IgG4相关性AIP患者的临床资料,其中33例行CT平扫及增强扫描,24例行MRI平扫及增强扫描、MRCP检查,10例联合行CT及MRI检查,观察病变胰腺的形态、密度/信号改变及强化特征、胰腺周围及胰管改变、胰腺外表现。结果47例患者中,CT及MRI检查共观察到胰腺弥漫性肿大呈“腊肠状”27例,局灶性肿大20例;CT平扫病变表现为均匀一致的略低密度,增强扫描动脉期病变区呈不均匀斑片状强化,门脉期和延迟期病变区呈现较均匀的延迟渐进性强化;MRI检查病变表现为T1WI-FS呈不均匀稍低信号,T2WI呈稍高信号,增强扫描早期病灶不均匀轻度强化,延迟扫描呈较均匀延迟渐进性强化;CT及MRI检查28例胰腺周围见包膜样边缘,即“胶囊征”,增強扫描呈轻中度延迟强化;MRCP检查示10例患者可见主胰管节段性狭窄,上游胰管局限性轻度扩张;CT及MRI检查共观察到30例患者表现为胆管系统改变,可见胆总管胰腺段管壁增厚、狭窄伴延迟强化,MRCP检查呈“鸟嘴样”改变,上游肝内外胆管不同程度扩张。结论IgG4相关性AIP的CT和MRI表现具有一定的特征性,结合影像学表现及实验室相关检查有助于提高该病的诊断水平。
[关键词]自身免疫性胰腺炎;免疫球蛋白G4相关疾病;体层摄影术,X线计算机;磁共振成像;诊断
[中图分类号]R576.2;R814.42[文献标志码]A
Imaging findings of IgG4-related autoimmune pancreatitisXU Bing, LYU Qingqing, CHEN Jingjing(Department of Radiology, The Affiliated Hospital of Qingdao University, Qingdao 266003, China)
[ABSTRACT]ObjectiveTo analyze the computed tomography (CT) and magnetic resonance imaging (MRI) findings of immunoglobulin G4 (IgG4)-related autoimmune pancreatitis (AIP), to improve the understanding of the imaging features of this disease, and to reduce imaging misdiagnosis. MethodsA retrospective analysis was performed on the clinical data of 47 patients with IgG4-related AIP, among whom 33 underwent plain CT scan and contrast-enhanced CT scan, 24 underwent plain MRI scan, contrast-enhanced MRI scan, and magnetic resonance cholangiopancreatography (MRCP) examination, and 10 underwent combined CT and MRI examination to observe the morphology, changes in density/signal, contrast enhancement characteristics of the diseased pancreas, the peripancreatic and pancreatic duct changes, and the manifestations out of the pancreas. ResultsThe CT and MRI scans showed that among the 47 patients, 27 had a “sausage-like”, diffusely enlarged pancreas and 20 had a focally enlarged pancreas. The plain CT scan showed a uniform and slightly low density; the contrast-enhanced CT scan of the lesion showed an inhomogeneous patchy enhancement in arterial phase, and a uniformly delayed progressive enhancement in portal vein phase and delayed phase. The MRI scan of the lesion showed inhomogeneous and slight hypointensity on T1WI-FS and slight hyperintensity on T2WI, the contrast-enhanced MRI scan showed an inhomogeneous mild enhancement in the early stage and a uniformly delayed progressive enhancement on delayed scan. The CT and MRI scans showed that 28 patients had capsule-like edges around the panc-reas, namely “capsule sign”, with a mild to moderate delayed enhancement on contrast-enhanced images. The MRCP showed that 10 patients had segmental stenosis of the main pancreatic duct and localized mild dilatation of the upstream pancreatic duct. The CT and MRI scans showed that 30 patients had changes in the bile duct system, with wall thickening, stenosis, and delayed enrichment of the pancreatic segment of common bile duct. The MRCP showed “beak-like” changes, and dilatation of upstream intrahepatic and extrahepatic bile ducts to varying degrees. ConclusionThe CT and MRI findings of IgG4-related AIP have certain characteristics. The combination of imaging findings and relevant laboratory tests can help improve the diagnosis of this disease.
[KEY WORDS]Autoimmune pancreatitis; Immunoglobulin g4-related disease; Tomography, X-ray computed; Magnetic resonance imaging; Diagnosis
自身免疫性胰腺炎(autoimmune pancreatitis,AIP)是一种特殊的慢性胰腺炎,目前已经发现其有1型和2型AIP两种类型[1]。1型AIP即IgG4相关性AIP,最常见于亚洲人群,被认为是一种IgG4相关性疾病在胰腺的表现[2];2型AIP在欧洲和美国人群中较多见,其受累器官的组织病理学改变与IgG4相关性疾病不同,而且血清学检查不伴有IgG4的异常。IgG4相关性AIP一般无特异性临床症状,部分急性发作患者常常易被误诊为急性胰腺炎[3-4],造成病情反复发作,甚至部分患者影像学表现易被误诊为胰腺癌[5-6],因此准确诊断具有重要临床意义。本研究回顾性分析总结47例经病理和(或)临床证实的IgG4相关性AIP的CT及MRI影像学特点,旨在进一步提高对该病的诊断水平。
1资料与方法
收集2013年1月—2018年5月我院收治的47例IgG4相关性AIP患者的临床资料,所有纳入患者均符合2011年AIP国际诊断标准[1]。其中33例患者进行了CT平扫及增强扫描,24例患者进行了MRI平扫及增强扫描、MRCP检查,10例患者同时进行了CT及MRI检查。47例IgG4相关性AIP患者中,男40例,女7例;年龄23~84岁,平均57岁;31例表现为腹痛、腹胀,其中6例同时伴有黄疸;12例表现为单纯黄疸;2例表现为食欲不振、乏力;2例体检时发现;4例经手术病理证实;8例经ERCP活檢组织和临床治疗证实;39例血清中IgG4水平不同程度升高,采用激素治疗有效确诊。
对相关影像学资料进行回顾性分析,分析内容包括胰腺形态、密度/信号改变及强化特征、胰腺周围及胰管改变、胰腺外表现。
2结果
2.1胰腺形态学改变
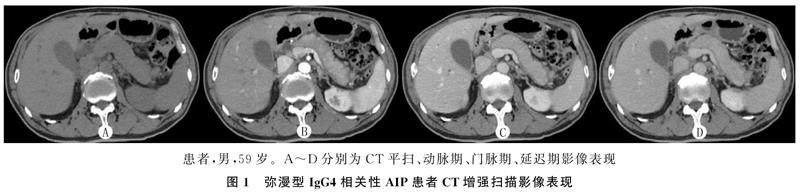
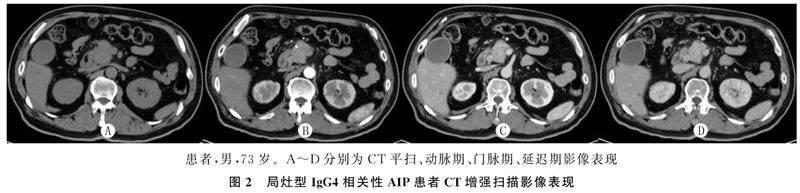
CT及MRI检查共观察到胰腺弥漫性肿大27例,表现为胰腺边缘饱满,正常分叶及羽毛状结构消失,胰尾部短缩,呈“腊肠样”改变(图1);局灶性肿大20例,呈“假肿瘤样”改变(图2),其中肿大部位位于胰头部7例,胰头体部3例,胰体尾部9例,胰尾部1例。
2.2密度/信号改变及强化特征
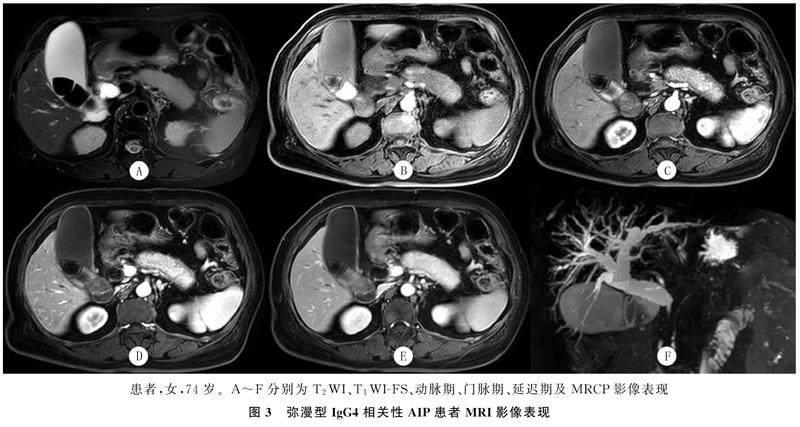
CT平扫检查显示,病变表现为均匀一致的略低密度,增强扫描动脉期病变区的强化有不同程度减低,呈不均匀斑片状强化,门脉期和延迟期病变区呈现较均匀的延迟渐进性强化(图1、2)。MRI检查表现为T1WI-FS呈不均匀稍低信号, T2WI呈稍高信号,增强扫描早期病灶不均匀轻度强化,延迟扫描呈较均匀延迟渐进性强化(图3)。
2.3胰腺周围及胰管改变
CT以及MRI检查28例胰腺周围可见包膜样边缘,即“胶囊征”(图1、3),其中19例见于弥漫性肿大患者,9例见于局灶性肿大患者,增厚包膜CT平扫呈等或稍低密度,T1WI-FS呈等或稍低信号,T2WI呈低信号,CT及MRI增强扫描呈轻中度延迟强化。MRCP检查示10例患者主胰管节段性狭窄,上游胰管可有局限性轻度扩张。
2.4胰腺外表现
CT及MRI检查30例表现为胆管系统改变,其中21例见于弥漫性肿大患者,9例见于局灶性肿大患者,可见胆总管胰腺段管壁增厚、狭窄,MRCP检查呈“鸟嘴样”改变(图3F),增强扫描可见增厚胆总管壁呈延迟强化(图2),上游胆总管及肝内胆管不同程度扩张。2例伴有泪腺改变,1例伴有下颌下腺改变,1例同时表现为肾脏改变,1例伴有脾、胃改变,1例伴有腹膜后纤维化表现。
3讨论
1995年,日本学者YOSHIDA等[7]首先提出了AIP这一概念,认为是一种与自身免疫相关的特殊类型慢性胰腺炎。根据2011年AIP国际诊断标准分为2型[1]。1型AIP为淋巴浆细胞硬化性胰腺炎,表现为显著的导管周围淋巴浆细胞浸润、血清和组织中IgG4阳性浆细胞高水平表达、席纹状纤维化和闭塞性静脉炎,对激素治疗敏感;2型AIP为特发性导管中心性胰腺炎,其典型特征是胰腺小叶和导管大量的中性粒细胞浸润,被称为粒细胞性上皮损害,可导致胰管损伤[8]。依据患者的病理组织学以及血清IgG4水平较高且对激素治疗有效的诊断标准,本研究所纳入的患者均明确为1型AIP,即IgG4相关性AIP。
IgG4相关性AIP好发于老年男性,患者临床表现多样,常以黄疸和腹痛起病多见,其中无痛性梗阻性黄疸是最常见的初期症状[9],发热、厌食、乏力、体质量下降均可出现,一些患者可伴有其他IgG4相关胰腺外的组织器官的炎症(硬化性胆管炎、硬化性涎腺炎、间质性肺炎及腹膜后纤维化等)[10-11]。本组47例患者的年龄分布、性别比例与文献报道结果相近,临床表现则以腹痛为主,其中31例有腹痛、腹胀,18例有黄疸体征,该组中有2例为体检时发现,说明病变较轻的患者,也可无明显临床症状。根据这些临床表现并不能明确诊断,血清IgG4对诊断该病具有一定的特异度和灵敏度[12-13]。本组47例患者中有39例IgG4水平明显升高,但部分患者的IgG4水平在正常范围内。
在AIP国际共识中重点强调了胰腺实质影像学检查在AIP诊断中的主要地位。AIP从形态上分为弥漫型和局灶型[14]。弥漫型最常见,胰腺正常小叶轮廓消失,沿病变胰腺长轴方向不同程度肿胀、增粗,长径显著大于短径,呈典型的“腊肠状”改变,边界清楚;局灶型表现为局灶性肿块,以胰头部常见,易与胰腺癌混淆,病灶边界多清晰。本组47例IgG4相关性AIP,胰腺病变受累范围可呈弥漫性肿大,也可呈局灶性肿大,与文献报道结果一致,但局灶性肿大多数位于胰体尾部,其好发部位有待于大样本研究进一步证实。病变区胰腺的密度均匀,CT平扫时与正常胰腺的密度相近,MRI检查T1WI呈稍低信号,T2WI呈稍高信号,这是由于IgG4相关性AIP很少伴有坏死、囊变、出血、钙化情况,也不伴脂肪变性或脂肪浸润等病理改变[15]。
本組47例IgG4相关性AIP,动态增强CT和MRI影像特点呈现一致性,均表现为延迟渐进性强化,从平扫到延迟期,病变胰腺的密度、信号强度逐渐升高,其增强模式与组织病理学特点有关,病变内增生的纤维组织导致微血管受压、变细,血流速减慢,造影剂通过时间延长,因而呈“缓慢渐进强化”的特点。渐进性强化是IgG4相关性AIP的一个特征性表现[16-17]。本研究中大部分胰腺病变周围可见包膜样边缘,即“胶囊征”,此征象在弥漫型AIP中更常见,其病理上是一种沿胰腺外围增生的慢性炎性纤维组织,CT平扫呈等或稍低密度,T1WI呈等或稍低信号,T2WI呈低信号,增强扫描呈轻中度延迟强化,在显示“包膜样边缘”方面,MRI检查优于CT检查[18-19],“胶囊征”是IgG4相关性AIP的重要征象,有助于明确诊断[20-21]。另外,由于主胰管的浆细胞、淋巴细胞浸润和纤维化,可导致管壁增厚,管腔出现不同程度的狭窄[22]。因此,MRCP显示IgG4相关性AIP胰管呈弥漫性不规则狭窄,很少出现主胰管的显著扩张[23-24],这与胰腺癌不同,本研究也证实了这点。
胆管是最常受到累及的胰腺外结构[25],被称为IgG4相关硬化性胆管炎,多发生于胆总管胰腺段,其主要的潜在病理改变是淋巴细胞、浆细胞浸润及纤维增生引起胆管壁广泛增厚及纤维化。本组47例IgG4相关性AIP,30例表现有胆管系统改变,CT及MRI检查影像学上表现为胆总管远端壁明显增厚、狭窄,增强扫描呈延迟强化,MRCP检查表现为逐渐变细,呈“鸟嘴样”狭窄,近侧胆管有扩张[26-27]。而胰腺癌导致的胆总管狭窄呈浸润性不规则狭窄,远端突然中断[28]。复习相关文献,IgG4相关性AIP可累及多种脏器[29],如泪腺炎、涎腺炎(两者同时存在时称Mikulicz病)、甲状腺炎、甲状腺功能减低、间质性肺疾病、腹膜后纤维化及肾小管间质性肾病等。对于临床怀疑IgG4相关性AIP的患者,试验性激素治疗是必要的[30],CT及MRI检查能直观对比病灶的变化,协助诊断。
总之,IgG4相关性AIP属系统性自身免疫性疾病的一部分,属于慢性增生性炎症,在CT和MRI影像上均具有特征性改变,结合实验室IgG4检查结果,可对大部分病变作出明确诊断,有利于临床制定有效的治疗方案。
作者声明:徐兵、陈静静参与了实验设计;徐兵、吕清清、陈静静参与了论文的写作和修改。所有作者均阅读并同意发表该论文,且均声明不存在利益冲突。
[参考文献]
[1]SHIMOSEGAWA T, CHARI S T, FRULLONI L, et al. International consensus diagnostic criteria for autoimmune pancreatitis: Guidelines of the International Association of Panc-reatology[J]. Pancreas, 2011,40(3):352-358.
[2]OKAZAKI K, UCHIDA K. Current concept of autoimmune pancreatitis and IgG4-related disease[J]. Am J Gastroenterol, 2018,113(10):1412-1416.
[3]CHINTANABOINA J, YANG Z H, MATHEW A. Autoimmune pancreatitis: A diagnostic challenge for the clinician[J]. South Med J, 2015,108(9):579-589.
[4]CHEN X, WANG L H, ZHANG L X, et al. IgG4-related autoimmune pancreatitis mimicking acute pancreatitis: A case report[J]. Chung Kuo I Hsueh Ko Hsueh Tsa Chih, 2017,32(1):65-68.
[5]BEYER G, ELLENRIEDER V, NEESSE A. Autoimmune pancreatitis: Avoiding unnecessary procedures by following international consensus guidelines[J]. Digestion, 2015,92(3):171-172.
[6]BOJKOV M, DT P, DVOKOV J, et al. Immunoglobulin G4, autoimmune pancreatitis and pancreatic cancer[J]. Dig Dis, 2015,33(1):86-90.
[7]YOSHIDA K, TOKI F, TAKEUCHI T, et al. Chronic pancreatitis caused by an autoimmune abnormality. Proposal of the concept of autoimmune pancreatitis[J]. Dig Dis Sci, 1995,40(7):1561-1568.
[8]NEGRELLI R, BONINSEGNA E, AVESANI G, et al. Type 1 and type 2 autoimmune pancreatitis: Distinctive clinical and pathological features, but are there any differences at magnetic resonance? Experience from a referral center[J]. Pancreas, 2018,47(9):1115-1122.
[9]NAGPAL S J S, SHARMA A, CHARI S T. Autoimmune pancreatitis[J]. Am J Gastroenterol, 2018,113(9):1301.
[10]KHANDELWAL A, INOUE D, TAKAHASHI N. Autoimmune pancreatitis: An update[J]. Abdom Radiol (NY), 2020,45(5):1359-1370.
[11]NIKOLIC S, BREHMER K, PANIC N, et al. Cardiovascular and lung involvement in patients with autoimmune pancreatitis[J]. J Clin Med, 2020,9(2):E409.
[12]DAI C, CAO Q, JIANG M, et al. Serum immunoglobulin G4 in discriminating autoimmune pancreatitis from pancreatic cancer: A diagnostic meta-analysis[J]. Pancreas, 2018,47(3):280-284.
[13]LIAN M J, LIU S, WU G Y, et al. Serum IgG4 and IgG for the diagnosis of autoimmune pancreatitis: A systematic review with meta-analysis[J]. Clin Res Hepatol Gastroenterol, 2016,40(1):99-109.
[14]NOTOHARA K, NISHIMORI I, MIZUNO N, et al. Clinicopathological features of type 2 autoimmune pancreatitis in Japan: Results of a multicenter survey[J]. Pancreas, 2015,44(7):1072-1077.
[15]DETLEFSEN S, KLPPEL G. IgG4-related disease: With emphasis on the biopsy diagnosis of autoimmune pancreatitis and sclerosing cholangitis[J]. Virchows Arch, 2018,472(4):545-556.
[16]DONG Y, DONOFRIO M, HOCKE M, et al. Autoimmune pancreatitis: Imaging features[J]. Endosc Ultrasound, 2018,7(3):196-203.
[17]KWON J H, KIM J H, KIM S Y, et al. Differentiating focal autoimmune pancreatitis and pancreatic ductal adenocarcinoma: Contrast-enhanced MRI with special emphasis on the arterial phase[J]. Eur Radiol, 2019,29(11):5763-5771.
[18]LEE S, KIM J H, KIM S Y, et al. Comparison of diagnostic performance between CT and MRI in differentiating non-diffuse-type autoimmune pancreatitis from pancreatic ductal adenocarcinoma[J]. Eur Radiol, 2018,28(12):5267-5274.
[19]王澤锋,王海军,张俊晶,等. 自身免疫性胰腺炎CT及MRI影像学特征与诊断[J]. 中华消化外科杂志, 2017,16(1):95-101.
[20]HAFEZI-NEJAD N, SINGH V K, FUNG C, et al. MR imaging of autoimmune pancreatitis[J]. Magn Reson Imaging Clin N Am, 2018,26(3):463-478.
[21]KAJIHARA Y. Type 1 autoimmune pancreatitis: A capsule-like rim surrounding the enlarged pancreas[J]. QJM, 2019,112(1):51.
[22]SHANKAR A, SRINIVAS S, KALYANASUNDARAM S. Icicle sign: Autoimmune pancreatitis[J]. Abdom Radiol (NY), 2020,45(1):245-246.
[23]PALAZZO M, PALAZZO L, AUBERT A, et al. Irregular narrowing of the main pancreatic duct in association with a wall thickening is a key sign at endoscopic ultrasonography for the diagnosis of autoimmune pancreatitis[J]. Pancreas, 2015,44(2):211-215.
[24]CAZACU I M, CHAVEZ A A L, SAFTOIU A, et al. A diagnostic challenge: Pancreatic cancer or autoimmune pancreatitis? [J]. Curr Health Sci J, 2018,44(2):181-185.
[25]MADHUSUDHAN K S, DAS P, GUNJAN D, et al. IgG4-related sclerosing cholangitis: A clinical and imaging review[J]. AJR Am J Roentgenol, 2019,213(6):1221-1231.
[26]NEGRELLI R, MANFREDI R, PEDRINOLLA B, et al. Pancreatic duct abnormalities in focal autoimmune pancreatitis: MR/MRCP imaging findings[J]. Eur Radiol, 2015,25(2):359-367.
[27]SANDRASEGARAN K, MENIAS C O. Imaging in autoimmune pancreatitis and immunoglobulin G4-related disease of the abdomen[J]. Gastroenterol Clin North Am, 2018,47(3):603-619.
[28]PAN Y, CHEN J Y, YU R S. Accurate imaging diagnosis and evaluation of pancreatic cancer[J]. Zhejiang Da Xue Xue Bao Yi Xue Ban, 2017,46(5):462-467.
[29]LORENZO D, MAIRE F, STEFANESCU C, et al. Features of autoimmune pancreatitis associated with inflammatory bo-wel diseases[J]. Clin Gastroenterol Hepatol, 2018,16(1):59-67.
[30]MADHANI K, FARRELL J J. Management of autoimmune pancreatitis[J]. Gastrointest Endosc Clin N Am, 2018,28(4):493-519.
(本文編辑 耿波 厉建强)

