发光稀土⁃硫杂杯[4]芳烃配合物的研究进展
李子萍,毕研峰
发光稀土⁃硫杂杯[4]芳烃配合物的研究进展
李子萍,毕研峰
(辽宁石油化工大学 石油化工学院,辽宁 抚顺 113001)
硫杂杯芳烃作为配体具有识别能力强、可“衍生化”、稳定性较好等优点。稀土离子与硫杂杯[4]芳烃配体易形成多功能金属簇配合物。稀土⁃硫杂杯[4]芳烃因具有独特的催化、磁学和光学等性质而受到越来越多的关注。硫杂杯[4]芳烃与稀土离子可通过酚羟基和桥连硫的配位发生有效的“天线效应”,使稀土离子敏化发光。综述了有关稀土⁃硫杂杯[4]芳烃配合物在光学性质方面的研究现状,论述了其结构、光学性质及应用。
硫杂杯[4]芳烃; 稀土离子; 配位簇; 发光性质
金属簇化合物是原子簇化合物的一种,一般分为两类:一是含两个或两个以上金属原子且存在金属⁃金属键的化合物;二是没有金属⁃金属键存在的多核金属配合物。金属簇化合物因具有大的化学活性比表面积、量子尺寸效应等基本特性,在光学、磁学、电学和生物学等方面表现出了新颖的性能及广泛的应用前景,已经成为众多科研工作者们关注和研究的焦点[1⁃3]。由于稀土离子(LnⅢ)的特殊电子层结构,稀土簇在光、电、磁等方面大都显示出了优异的性能,尤其是在发光性能方面具有以下优势:发射光谱的种类多样丰富;f⁃f跃迁光谱呈尖锐的线状,发光的色纯度高;荧光寿命跨度大(6个数量级);理化性质稳定,可承受强紫外光、大功率的电子束和高能辐射的作用[4]。然而,由于镧系离子的电子转移受Laporte规则束缚,其4f⁃4f跃迁是禁阻的,当直接对镧系离子进行激发时,不易产生较好的发射。克服Laporte规则引起的发光效率低的问题,最常用且最有效的方法就是寻找一个有机配体与稀土离子结合[5⁃6]。通过有机配体吸收激发光的能量,再将能量传递给稀土离子,敏化稀土离子发光,这一过程被称为“天线效应”[7],即配体⁃金属能量转移(LMET)。
传统杯芳烃是由若干苯酚单元通过亚甲基连接构成的具有空腔结构的大环化合物。其中,具有四个酚单元的叔丁基杯[4]芳烃(H4C4A)合成方法最为简单,同时因上下缘均可功能化且具有多种可能的构象以及良好的热稳定性等特点,是众多亚甲基桥连杯芳烃中应用最广泛的一种[8⁃10],而以硫原子替代亚甲基的叔丁基硫杂杯[4]芳烃(H4TC4A)是近年来发现的第二代杯芳烃化合物[11⁃12]。H4TC4A除了具有与H4C4A相似的结构特点及性能外,还具备许多新的优异特性,如H4TC4A更易发生结构转变、桥连硫的位置也可氧化成亚磺酰基(H4TC4A⁃SO)和磺酰基(H4TC4A⁃SO2)官能团、金属离子可通过与桥连的硫以及相邻的酚氧原子同时配位而结合、较大的空腔结构使其包容性更强、对过渡金属尤其是软金属离子具有更好的识别能力等[13⁃15]。
鉴于H4TC4A的结构特点,研究者以硫杂杯芳烃或其衍生物为多齿配体,与过渡金属离子(3d)、镧系金属离子(4f)或混合金属离子(3d⁃4f、4f⁃4f)组装,制备了大量金属簇化合物。2000年,J.C.G.BUNZLI等[16]综述了双金属镧系杯芳烃超分子体系,讨论了具有简单杯[]芳烃(=5,8)的双金属镧系元素配合物的结构、动力学和光物理性质。2017年,M.MASSI等[17]概述了掺入发光材料中的镧系元素⁃杯芳烃络合物的实例及早期工作,讨论了材料类别(聚合物、纳米粒子和金属簇)。2020年,R.O.FULLER等[18]介绍了部分稀土⁃硫杂杯芳烃配合物的磁性能。本文论述了硫杂杯[4]芳烃及其衍生物与稀土离子形成的配合物及其光学性质的研究进展,并阐述了配合物和发光性能之间的”构效关系”,旨在为将来的科学研究提供有价值的参考。
1 4f⁃硫杂杯[4]芳烃簇合物
H4TC4A的宏量制备方法已十分成熟[19⁃20],这为H4TC4A的深入研究和广泛应用奠定了基础。2000年,J.M.HARROWFIELD等[21]报道了第一例基于硫杂杯[4]芳烃的稀土配合物——Nd4(TC4A)配合物,并通过X⁃射线单晶衍射测定了配合物的晶体结构。该配合物是一个夹心四核簇合物,其结构代表H4TC4A及其衍生物与稀土离子的典型结构(见图1)。Nd4(TC4A)也是被首次报道具有明确单晶结构的稀土⁃硫杂杯[4]芳烃配合物。
在H4C4A与稀土离子所形成的配合物中,金属离子与配体之间存在有效的能量传递,可激发稀土离子发光[22⁃25]。N.IKI等[26]证明了硫桥连的杯芳烃比亚甲基桥连的杯芳烃能更有效地敏化稀土发光,制备了NaSO3⁃H4C4A、NaSO3⁃H4TC4A和NaSO3⁃H4TC4A⁃SO2三种杯芳烃配体,所有配体均能分别与镧系离子(PrⅢ、SmⅢ、EuⅢ、TbⅢ和DyⅢ)形成配合物。其中,TbⅢ配合物出现了较强的能量转移发光现象。三种配体与TbⅢ形成的配合物的发光能力不同。NaSO3⁃H4TC4A配体和NaSO3⁃H4TC4A⁃SO2配体的桥连S和SO2使杯[4]芳烃配位能力更强,可提供更好的环境来匹配TbⅢ的激发,使Tb相关配合物具有更高的发光量子产率。

图1 叔丁基硫杂杯[4]芳烃与NdⅢ形成的夹心四核晶体结构[21]
硫杂杯[4]芳烃与稀土离子形成的配合物的发光性能优于杯[4]芳烃的相关化合物,这一点也逐渐被证实。针对硫杂杯[4]芳烃具有多种构象异构体以及易衍生化的性质,研究者对该类配体形成金属簇的光学性质进行了大量探究。T.KAJIWARA等[27]通过H4TC4A⁃SO2构象控制调控了稀土簇的发光性能;合成了分别含有EuⅢ和TbⅢ的双核和四核的镧系簇配合物。其中,在双核簇合物中,杯芳烃形成压扁的锥式构象,作为四齿配体通过下缘的4个酚羟基氧与LnⅢ结合形成杯芳烃与稀土离子物质的量比为1∶1的配合物;在四核簇合物中,杯芳烃配体采用锥式构象并通过2个酚羟基氧和1个磺酰基氧与LnⅢ配位形成物质的量比为1∶2的配合物(见图2)。在两种配位模式中,配体的单重态S1和三重激发态T1能量存在差异,这导致两种配合物的发光性质发生显著变化,所得的双核金属簇显示出较高效的发光性能。文献[28]的研究结果表明,具有1,2⁃交替构象的H4TC4A⁃SO2配体的三重激发态T1的能量略高于Tb离子的5D4能级,这对于有效的LMET和f⁃f跃迁发光是有利的。
通过检索发现,低核数的单一稀土离子⁃硫杂杯[4]芳烃(TC4AS)配合物的经典结构是夹心型结构。例如,N.IKI等[29⁃30]报道的NaSO3⁃H4TC4A和镧系元素离子自组装形成杯芳烃与金属离子物质的量比为3∶2的Ln3(SO3⁃TC4A)2络合物,其中三核LnⅢ簇夹在两个配体之间,该结构的配合物具有很高的稳定性;廖伍平课题组合成了由H4PTC4A和H4TC4A构筑的四种四核TbⅢ和DyⅢ配合物,这些配合物的特征是呈由2个尾对尾杯芳烃分子和1个平面四方(4⁃OH)Ln4簇构成的三明治状[31]。其中,H4PTC4A形成的镧系配合物的发光性能更优。在NaOH存在的情况下,TbⅢ或EuⅢ和H4TC4A也可形成相似的结构,钠离子是配合物晶体的一部分[32]。该结构中的钠离子吸收一部分激发能量,使LnⅢ配合物的激发波长变小,所需的光致发光的能量变大。
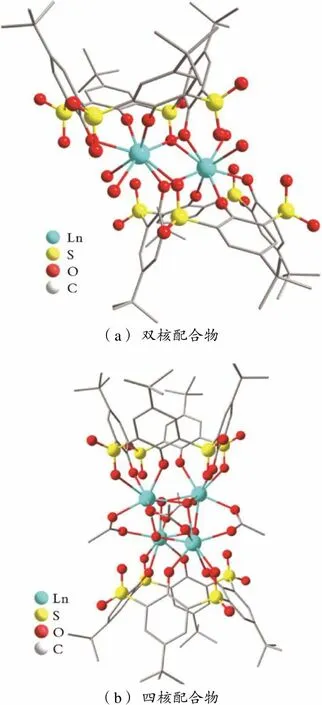
(a)双核配合物 (b)四核配合物
除了上述常见的低核数夹心型配合物外,高核的单一稀土⁃硫杂杯[4]芳烃簇合物也有报道。T.KAJIWARA等[33⁃34]先后报道了基于H4TC4A⁃SO2的八核(Ln8(TC4A⁃SO2)4,Ln=Pr,Nd,Sm,Gd)和十二核(Ln12(TC4A⁃SO2)6,Ln=Ho)轮状稀土簇合物(见图3),并论述了这些簇合物的合成、结构与磁性。

(a)八核配合物 (b)十二核配合物
洪茂椿课题组利用H4TC4A⁃SO2和膦酸酯配体制备了三种高核钕簇,分别是椭圆形的Nd10簇、橄榄球状的Nd11纳米笼和不规则的Nd19簇(见图4)[35]。Nd10由10个Nd、4个(TC4A⁃SO2)4-配体、4个膦酸酯配体以及其他桥连阴离子构成,骨架结构与八核轮状稀土簇合物Ln8(TC4A⁃SO2)4相似;Nd11由11个Nd、3个(TC4A⁃SO2)4-配体及其他阴离子构成,形似橄榄球结构;Nd19由19个Nd、5个(TC4A⁃SO2)4-配体、5个膦酸酯配体以及其他桥连阴离子构成。Nd19簇可看作是1个较大的Nd16簇与1个较小的Nd3簇连接到一起形成的不规则结构。Nd19簇代表迄今为止基于硫杂杯[4]芳烃配体的核数最高的稀土簇。光致发光研究表明,三种化合物均在近红外区显示NdⅢ的特征发射,与其他报道的杯芳烃负载的钕化合物相似,说明三种结构中发生了配体到钕金属的有效能量传递。
(a)十核 (b)十一核 (c)十九核
图4 十核、十一核和十九核钕簇的晶体结构[35]
Fig. 4 Crystal structures of Nd10, Nd11and Nd19clusters[35]
2 3d⁃4f/4f⁃4f⁃硫杂[4]杯芳烃簇合物

已报道的4f⁃4f异稀土硫杂杯[4]芳烃配合物只有两例:异三核镧系簇Tb3-xYb(SO3⁃TC4A)2(=1,2),其结构与Ln3(SO3⁃TC4A)2络合物相同(夹心型结构)。该结构中存在从TbⅢ到YbⅢ的能量转移,可增强以YbⅢ为中心的发光性能。但是,得到的产物是复杂的混合物,存在分离纯化难的问题[53]。异核镧系Tb/Dy的一维梯形配合物的配体为1,3⁃四羧酸硫杂杯[4]芳烃,铽原子作为“T形连接器”,连接3个相邻配体上的3个羧酸基团,形成双链梯状结构[54]。研究表明,1,3⁃构象的杯芳烃羧酸盐衍生物仍具有作为TbⅢ络合物发色团的能力,其中金属Dy能够调整配合物的发光颜色。
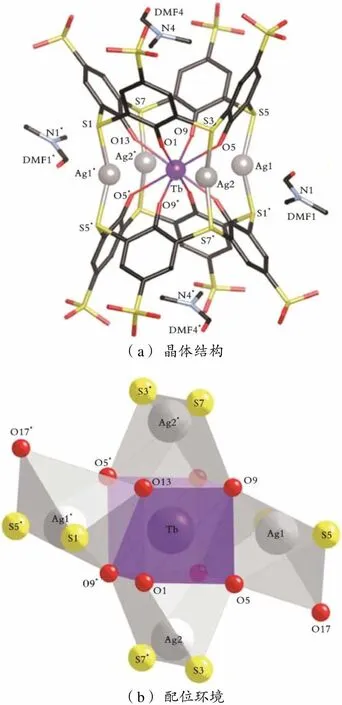
(a)晶体结构 (b)配位环境
3 应 用
4 结论与展望
本文所论述的稀土⁃硫杂杯[4]芳烃配合物一般具有明确的晶体结构,通过结构与发光性质的研究,讨论了结构与发光之间的构效关系。硫杂杯[4]芳烃具有丰富的构象异构结构、可衍生化的特性和优点,最为重要的是硫杂杯[4]芳烃桥连硫原子可与稀土离子形成有效的配位键,能很好地实现从苯酚环到稀土离子的能量转移。硫杂杯[4]芳烃作为配体与稀土离子形成的配合物具有很大的潜在应用价值。今后可以从以下方面,对稀土⁃硫杂杯[4]芳烃簇合物体系进行研究。
1)利用稀土离子的高配位数和多样的配位方式,结合离子的尺寸和性质、杯芳烃配体的配位点和构象,可控地设计出稳定性好、有良好的光学性能的化合物是目前研究的主要方向。
2)根据报道,已应用稀土⁃硫杂杯芳烃配合物多数为水溶性的,因此难回收和易流失问题需要解决。非水溶性的稀土⁃硫杂杯芳烃配合物的应用有待进一步开发。
3)引入不同的官能团、辅助配体或金属离子,对稀土⁃硫杂杯[4]芳烃簇的结构和发光性质都有影响。如何定向构筑特定组分和结构的稀土簇,实现配体与稀土离子发光能级匹配和有效传递,将一直是主要的挑战和难题。
[1] HANG X X, BI Y F. Thiacalix[4]arene⁃supported molecular clusters for catalytic applications[J]. Dalton Transactions, 2021, 50(11): 3749⁃3758.
[2] PAN Z H, WENG Z Z, KONG X J, et al. Lanthanide⁃containing clusters for catalytic water splitting and CO2conversion[J]. Coordination Chemistry Reviews, 2022, 457: 214419.
[3] JIN R C, LI G, SHARMA S, et al. Toward active⁃site tailoring in heterogeneous catalysis by atomically precise metal nanoclusters with crystallographic structures[J]. Chemical Reviews, 2021, 121(2): 567⁃648.
[4] 苏文斌,谷学新,邹洪,等. 稀土元素发光特性及其应用[J]. 化学研究, 2001, 12(4): 55⁃59.
SUN W B, GU X X, ZOU H, et al. Luminescence characteristic and their utility of rare⁃earth elements[J]. Chemical Research, 2001, 12(4): 55⁃59.
[5] 王延梅,王丽. 杯芳烃稀土配合物的光性能及应用[J]. 化学进展, 2010, 22(2⁃3): 427⁃432.
WANG Y M, WANG L. Application and luminescent properties of the complexes of lanthanide ions based on calixarene derivatives[J]. Progress in Chemistry, 2010, 22(2⁃3): 427⁃432.
[6] 黄玲,黄春辉. 稀土配合物的光致发光和电致发光研究[J]. 化学学报, 2000, 58(12): 1493⁃1498.
HUANG L, HUANG C H. Study on photoluminescence and electroluminescence of the rare earth complexes[J]. Acta Chimica Sinica, 2000, 58(12): 1493⁃1498.
[7] CUI Y J, YUE Y F, QIAN G D, et al. Luminescent functional metal⁃organic frameworks[J]. Chemical Reviews, 2012, 112(2): 1126⁃1162.
[8] 张春雷,龚淑玲,陈远荫. 基于杯芳烃的阳离子荧光分子传感器的研究进展[J]. 有机化学, 2007, 27(7): 795⁃805.
ZHANG C L, GONG S L, CHEN Y Y. Progress in fluorescent molecular sensors of cations based on calixarene[J]. Chinese Journal of Organic Chemistry, 2007, 27(7): 795⁃805.
[9] CREAVEN B S, DONLON D F, MCGINLEY J. Coordination chemistry of calix[4]arene derivatives with lower rim functionalisation and their applications[J]. Coordination Chemistry Reviews, 2009, 253(7/8): 893⁃962.
[10] SAMENI S, JEUNESSE C, MATT D, et al. Calix[4]arene daisychains[J]. Chemical Society Reviews, 2009, 38(7): 2117⁃2146.
[11] 胡晓钧,王丽,任杰,等. 硫杂杯芳烃——一类新型的分子受体化合物[J]. 化学通报, 2004, 67(2): 90⁃95.
HU X J, WANG L, REN J, et al. Thiacalixarene——A new kind of molecular receptor[J]. Chemistry, 2004, 67(2): 90⁃95.
[12] 刘莉,韩军,颜朝国,等. 一类新型超分子主体——硫代杯芳烃的研究进展[J]. 有机化学, 2007, 27(8): 907⁃917.
LIU L, HAN J, YAN C G, et al. Progress in study of novel supramolecular host——Thiacalixarene [J]. Chinese Journal of Organic Chemistry, 2007, 27(8): 907⁃917.
[13] MOROHASHI N, NARUMI F, IKI N, et al. Thiacalixarenes[J]. Chemical Reviews, 2006, 106(12): 5291⁃5316.
[14] IKI N, MIYANO S. Can thiacalixarene surpass calixarene?[J]. Journal of Inclusion Phenomena and Macrocyclic Chemistry, 2001, 41(1): 99⁃105.
[15] IKI N. Thiacalixarenes[M]. Cham: Springer, 2016: 335⁃362.
[16] BUNZLI J C G, BESANCON F, IHRINGER F. Calixarenes for separations[M]. Washington:American Chemical Society, 2000.
[17] MASSI M, OGDEN M I. Luminescent lanthanoid calixarene complexes and materials[J]. Materials, 2017, 10(12): 1369.
[18] FULLER R O, KOUTSANTONIS G A, OGDEN M I. Magnetic properties of calixarene⁃supported metal coordination clusters[J]. Coordination Chemistry Reviews, 2020, 402: 213066.
[19] KUMAGAI H, HASEGAWA M, MIYANARI S, et al. Facile synthesis of⁃⁃butylthiacalix[4]arene by the reaction of⁃⁃butylphenol with elemental sulfur in the presence of a base[J]. Tetrahedron Letters, 1997, 38(22): 3971⁃3972.
[20] IKI N, KABUTO C, FUKUSHIMA T, et al. Synthesis of⁃⁃butylthiacalix[4]arene and its inclusion property[J]. Tetrahedron, 2000, 56(11): 1437⁃1443.
[21] BILYK A, HALL A K, HARROWFIELD J M, et al. A unique rare⁃earth cluster within a calixarene sandwich: Parallels in the chemistry of cyclosiloxanes and calixarenes[J]. Australian Journal of Chemistry, 2000, 53(12): 895⁃898.
[22] SATO N, SHINKAI S. Energy⁃transfer luminescence of lanthanide ions encapsulated in sensitizer⁃modified calix[4]arenes[J]. Journal of the Chemical Society, Perkin Transactions 2, 1993, 2(4): 621⁃624.
[23] SHEVCHUK S V, ALEXEEVA E A, RUSAKOVA N V, et al. Some new properties of calixarenes: The luminescence of four lanthanide ions in calixarene complexes[J]. Mendeleev Communications, 1998, 8(3): 112⁃113.
[24] OUDE WOLBERS M P, VAN VEGGEL F C J M, PETERS F G A, et al. Sensitized near⁃infrared emission from Nd3+and Er3+complexes of fluorescein⁃bearing calix[4]arene cages[J]. Chemistry⁃A European Journal, 1998, 4(5): 772⁃780.
[25] WEI X Q, YANG G, CHENG J B, et al. Synthesis of novel light⁃emitting calix[4]arene derivatives and their luminescent properties[J]. Optical Materials, 2007, 29(8): 936⁃940.
[26] IKI N, HORIUCHI T, OKA H, et al. Energy transfer luminescence of Tb3+ion complexed with calix[4]arenetetrasulfonate and the thia and sulfonyl analogue. The effect of bridging groups[J]. Journal of the Chemical Society, Perkin Transactions 2, 2001, 2(11): 2219⁃2225.
[27] KAJIWARA T, KATAGIRI K, HASEGAWA M, et al. Conformation⁃controlled luminescent properties of lanthanide clusters containing⁃⁃butylsulfonylcalix[4]arene[J]. Inorganic Chemistry, 2006, 45(13): 4880⁃4882.
[28] KAJIWARA T, HASEGAWA M, ISHII A, et al. Highly luminescent superparamagnetic diterbium(Ⅲ) complex based on the bifunctionality of⁃⁃butylsulfonylcalix[4]arene[J]. European Journal of Inorganic Chemistry, 2008, 2008(36): 5565⁃5568.
[29] IKI N, TANAKA T, HIRO⁃OKA S, et al. Self⁃assembly of a trilanthanide(Ⅲ) core sandwiched between two thiacalix[4]arene ligands[J]. European Journal of Inorganic Chemistry, 2016, 2016(31): 5020⁃5027.
[30] IKI N, BOROS E, NAKAMURA M, et al. Gd3TCAS2: An aquated Gd3+⁃thiacalix[4]arene sandwich cluster with extremely slow ligand substitution kinetics[J]. Inorganic Chemistry, 2016, 55(8): 4000⁃4005.
[31] BI Y F, WANG X T, LIAO W P, et al. Thiacalix[4]arene⁃supported planar Ln4(Ln=TbⅢ, DyⅢ) clusters: Toward luminescent and magnetic bifunctional materials[J]. Inorganic Chemistry, 2009, 48(24): 11743⁃11747.
[32] GE J Y, DUAN X, MA J P, et al. Thiacalix[4]arene⁃supported tetranuclear TbⅢand EuⅢcompounds: Synthesis, structure, luminescence, and magnetism[J]. Zeitschrift Für Anorganische und Allgemeine Chemie, 2019, 645(4): 416⁃421.
[33] KAJIWARA T, WU H S, ITO T, et al. Octalanthanide wheels supported by⁃butylsulfonylcalix[4]arene[J]. Angewandte Chemie International Edition, 2004, 43(14): 1832⁃1835.
[34] KAJIWARA T, KATAGIRI K, TAKAISHI S, et al. A dodecalanthanide wheel supported by⁃butylsulfonylcalix[4]arene[J]. Chemistry⁃An Asian Journal, 2006, 1(3): 349⁃351.
[35] SU K Z, JIANG F L, WU M Y, et al. Syntheses, structures, luminescence and magnetic properties of three high⁃nuclearity neodymium compounds based on mixed sulfonylcalix[4]arene⁃phosphonate ligands[J]. CrystEngComm, 2016, 18(26): 4921⁃4928.
[36] AMIROV R, MCMILLAN Z, MUSTAFINA A, et al. A first report on ternary complex formation between⁃sulfonatothiacalix[4]arene, tetramethylammonium ion and gadolinium (Ⅲ) ion in aqueous solutions[J]. Inorganic Chemistry Communications, 2005, 8(9): 821⁃824.
[37] HORIUCHI T, IKI N, HOSHINO H, et al. Di⁃ and tetracarboxylate ligands for highly luminescent terbium(Ⅲ) complexes on the basis of sulfonylcalix[4]arene scaffold[J]. Tetrahedron Letters, 2007, 48(5): 821⁃825.
[38] FEDORENKO S V, MUSTAFINA A R, AMIROV R R, et al. Reactions of heteroaromatic chromophores with lanthanide complexes of⁃sulfonatothiacalix[4]arene[J]. Russian Chemical Bulletin, 2008, 57(9): 1905⁃1911.
[39] PODYACHEV S N, SUDAKOVA S N, GIMAZETDINOVA G S, et al. The enhancement of luminescent properties of Tb3+complexes with tetra⁃1,3⁃diketone ligands promoted by the tetrathiacalix[4]arene scaffold[J]. Tetrahedron Letters, 2018, 59(27): 2695⁃2699.
[40] PODYACHEV S N, SUDAKOVA S N, NAGIMOV R N, et al. Structural and photophysical properties of Tb3+⁃tetra⁃1,3⁃diketonate complexes controlled by calix[4]arene⁃tetrathiacalix[4]arene scaffolds[J]. Dalton Transactions, 2019, 48(12): 3930⁃3940.
[41] IKI N, OHTA M, HORIUCHI T, et al. Exceptionally long⁃lived luminescence emitted from TbⅢion caged in an AgI⁃TbⅢ⁃thiacalix[4]arene supramolecular complex in water[J]. Chemistry⁃An Asian Journal, 2008, 3(5): 849⁃853.
[42] TANAKA T, IKI N, KAJIWARA T, et al. One⁃step heterogeneous assembly of terbium(Ⅲ) and silver(I) with thiacalix[4]arene ligands to form a cage including terbium(Ⅲ) in an octa⁃oxygen cube[J]. Journal of Inclusion Phenomena and Macrocyclic Chemistry, 2009, 64(3): 379⁃383.
[43] IKI N, HIRO⁃OKA S, TANAKA T, et al. Highly efficient near⁃infrared⁃emitting lanthanide(Ⅲ) complexes formed by heterogeneous self⁃assembly of AgI, LnⅢ, and thiacalix[4]arene⁃⁃tetrasulfonate in aqueous solution (LnⅢ=NdⅢ, YbⅢ)[J]. Inorganic Chemistry, 2012, 51(3): 1648⁃1656.


[46] BI Y F, LI Y L, LIAO W P, et al. A unique Mn2Gd2tetranuclear compound of⁃butylthiacalix[4]arene[J]. Inorganic Chemistry, 2008, 47(21): 9733⁃9735.
[47] ALDOSHIN S M, SANINA N A, SOLOV'EVA S E, et al. Thiacalix[4]arene⁃containing M2Ln2complexes (M=MnⅡ, CoⅡ; Ln=EuⅢ, PrⅢ): Synthesis, structure, and magnetic properties[J]. Russian Chemical Bulletin, 2014, 63(7): 1465⁃1474.

[49] GE J Y, XIE J Z, ZHAO Z Y, et al. Thiacalix[4]arene⁃supported heterodinuclear NiⅡ⁃LnⅢcomplexes: Slow magnetic relaxation behavior in the dysprosium analogue[J]. RSC Advances, 2016, 6(2): 1143⁃1150.
[50] ZHU X F, LIAO W P. 3d⁃4f coordination compounds of water⁃soluble sulfonylcalix[4]arenetetrasulfonate in cone conformation[J]. Polyhedron, 2016, 111: 185⁃189.
[51] ALDOSHIN S M, ANTIPIN I S, KNIAZEVA M V, et al. Synthesis, structure and magnetic properties of Mn2Tb2tetranuclear complex with⁃butylthiacalix[4]arene[J]. Israel Journal of Chemistry, 2020, 60(5⁃6): 600⁃606.
[52] SU K Z, JIANG F L, QIAN J J, et al. Stepwise construction of extra⁃large heterometallic calixarene⁃based cages[J]. Inorganic Chemistry, 2015, 54(7): 3183⁃3188.
[53] KARASHIMADA R, IKI N. Thiacalixarene assembled heterotrinuclear lanthanide clusters comprising TbⅢand YbⅢenable f⁃f communication to enhance YbⅢ⁃centred luminescence[J]. Chemical Communications, 2016, 52(15): 3139⁃3142.
[54] OVSYANNIKOV A S, KHARIUSHIN I V, SOLOVIEVA S E, et al. Mixed Tb/Dy coordination ladders based on tetra(carboxymethyl)thiacalix[4]arene: A new avenue towards luminescent molecular nanomagnets[J]. RSC Advances, 2020, 10(20): 11755⁃11765.
[55] HORIUCHI T, IKI N, OKA H, et al. Highly selective luminescence determination of terbium at the sub⁃ppb level with sulfonylcalix[4]arene⁃⁃tetrasulfonate[J]. Bulletin of the Chemical Society of Japan, 2002, 75(12): 2615⁃2619.
[56] MUKHAMETSHINA A, MUSTAFINA A, SYAKAEV V, et al. Effect of silica coating and further silica surface decoration by phospholipid bilayer on quenching of Tb(Ⅲ) complexes by adrenochrome[J]. Journal of Molecular Liquids, 2015, 211: 839⁃845.
[57] BURILOV V A, MIRONOVA D A, IBRAGIMOVA R R, et al. Thiacalix[4]arene⁃functionalized vesicles as phosphorescent indicators for pyridoxine detection in aqueous solution[J]. RSC Advances, 2015, 5(122): 101177⁃101185.
[58] ABE N, HOSHINO H, IKI N. Determination method of trace Cd(Ⅱ) in rice using a luminescent Cd(Ⅱ)⁃Tb(Ⅲ)⁃thiacalix[4]arene ternary complex[J]. Bunseki Kagaku, 2015, 64(7): 493⁃499.
[59] OVSYANNIKOV A S, AKHMETZYANOVA Z V, ALLAHVERDILLI G R, et al. Photoswitchable supramolecular systems based on carboxyl derivatives of thiacalix[4]arene and their complexes with Zn(Ⅱ) and Tb(Ⅲ) ions[J]. Macroheterocycles, 2018, 11(2): 173⁃180.
[60] IKI N, OHTA M, TANAKA T, et al. A supramolecular sensing system for AgIat nanomolar levels by the formation of a luminescent AgI⁃TbⅢ⁃thiacalix[4]arene ternary complex[J]. New Journal of Chemistry, 2009, 33(1): 23⁃25.
[61] STEPANOV A S, YANILKIN V V, MUSTAFINA A R, et al. Reversible electrochemical pH⁃switching of luminescence in a⁃sulfonatothiacalix[4]arene⁃ terbium(3+) system[J]. Russian Chemical Bulletin, 2010, 59(8): 1538⁃1542.
[62] FEDORENKO S, GILMANOVA D, MUKHAMETSHINA A, et al. Silica nanoparticles with dual visible⁃NIR luminescence affected by silica confinement of Tb(Ⅲ) and Yb(Ⅲ) complexes for cellular imaging application[J]. Journal of Materials Science, 2019, 54(12): 9140⁃9154.
[63] FEDORENKO S V, BOCHKOVA O D, MUSTAFINA A R, et al. Dual visible and near⁃infrared luminescent silica nanoparticles. Synthesis and aggregation stability[J]. The Journal of Physical Chemistry C, 2010, 114(14): 6350⁃6355.
Recent Advances of Luminescent Lanthanide⁃Thiacalix[4]arene Complexes
LI Ziping, BI Yanfeng
(School of Petrochemical Engineering, Liaoning Petrochemical University, Fushun Liaoning 113001, China)
Thiacalix[4]arene ligands have the advantages of high recognition, derivatization, excellent stability and other advantages. Lanthanide (Ln) ions can coordinate with thiacalix[4]arene ligands to form multi⁃functional coordination clusters, which received increasing attention due to their unique catalytic, magnetic, optical properties. Thiacalix[4]arene can sensitize Ln ions to luminescence by the coordination of phenol and S groups via the "Antenna effect". This paper reviewed recent advances in structures, luminescent properties, and applications of luminescent Ln⁃thiacalix[4]arene complexes.
Thiacalix[4]arene; Lanthanide (Ln) ion; Coordination cluster; Luminescent property
TQ139.2
A
10.12422/j.issn.1672⁃6952.2024.01.001
2023⁃06⁃29
2023⁃08⁃07
国家自然科学基金项目(91961110)。
李子萍(1995⁃),女,硕士研究生,从事稀土配位化合物方面的研究;E⁃mail:lizipinglnpu@163.com。
毕研峰(1979⁃),男,博士,教授,从事功能配位化学方面的研究;E⁃mail:biyanfeng@lnpu.edu.cn。
李子萍,毕研峰.发光稀土⁃硫杂杯[4]芳烃配合物的研究进展[J].辽宁石油化工大学学报,2024,44(1):1-8.
LI Ziping,BI Yanfeng.Recent Advances of Luminescent Lanthanide⁃Thiacalix[4]arene Complexes[J].Journal of Liaoning Petrochemical University,2024,44(1):1-8.
(编辑 宋官龙)

