紫外耦合游离亚硝酸强化剩余污泥厌氧发酵产酸研究
殷霄云,刘芝宏,周爱娟,李亚男,岳秀萍,崔芷瑄
紫外耦合游离亚硝酸强化剩余污泥厌氧发酵产酸研究
殷霄云,刘芝宏*,周爱娟,李亚男,岳秀萍,崔芷瑄
(太原理工大学环境科学与工程学院,山西 太原 030024)
为打破传统厌氧发酵过程中污泥破壁溶胞困难和产酸效能低的瓶颈,探究了紫外光(UV)耦合游离亚硝酸(FNA)预处理对污泥发酵产酸的影响,并与热(H)和超声法(US)耦合FNA预处理进行了对比分析.结果表明,UV辅助FNA联合预处理(FNA-UV)对细胞破碎和胞外聚合物的剥离具有协同效应,・OH和・O2-作为反应中间体,其强度远高于其他预处理组(FNA-US和FNA-H),与・NO,・NO2及ONOO-等中间产物共同促进了溶解性有机物的释放,溶解性碳水化合物和蛋白质含量相比FNA组分别提升60%和90%,进而为后续水解产酸过程提供了充足的底物.FNA-UV组短链脂肪酸(SCFAs)浓度于第4d达到峰值,为(201.8±4.8)mg COD/g VSS,相比FNA组提升67%,乙酸占比高达56.8%.通过发酵末期对各体系进行碳平衡分析表明,紫外耦合FNA预处理在污泥减量、溶解性有机物的释放与转化、SCFAs的产生方面具有重要作用.微生物群落分析表明,FNA-UV对功能菌群的富集发挥重要作用,表现为厌氧发酵菌和反硝化菌的有效增强,相比其他各组提升了23.7%~270.6%.
游离亚硝酸;剩余污泥;短链脂肪酸;厌氧发酵;紫外;高通量测序
近年来,随着我国城镇化进程的日益加速,剩余污泥作为市政污水处理厂生物处理段的伴生产物,其产量随污水处理能力的提升而飞速增长[1].据报道,剩余污泥可作为能源和资源的载体,回收污泥中的能源和资源成为目前研究的重点和难点[2-3].厌氧发酵技术(AF)通过微生物代谢进行水解酸化,进而实现污泥减量和资源回收(如短链挥发性脂肪酸SCFAs、甲烷CH4),成为污泥处理的重要途径[4]. SCFAs具有高附加值,不仅可以作为各种化工产品生产的原材料,还可以作为污水处理厂的有机碳源[5].然而,由于污泥中有机物受细胞壁和胞外聚合物(EPS)包裹,导致其释放困难,水解过程成为剩余污泥AF的限速步骤.因此,寻找高效的预处理技术成为污泥资源化的先决条件[6].
游离亚硝酸(FNA)作为亚硝酸盐的质子化形式,是一种绿色可再生的抗菌剂[7-8].在百万分之一水平下,FNA及其衍生物可与细胞/EPS中的脂质、蛋白质、碳水化合物和脱氧核糖核酸(DNA)反应,有效破坏微生物细胞膜,分解胞内大分子有机物,为产酸菌提供底物[9].单独采用FNA预处理可实现污泥有效溶胞(1~2mg N/L预处理污泥24~48h,可灭活50%~ 80%的细胞[10]).但相关研究表明,经FNA预处理后仍有大量耐受细胞处于稳态,且其对EPS的破坏作用极为有限,进而限制水解产酸效能(3.04mg N/L FNA预处理污泥,EPS中腐殖质等大分子物质不能被水解[11])[12-13].因此,越来越多学者采用物理/机械法(冷冻[14]、热[15]、超声[16])和投加化学试剂(Fenton法[13]、过氧化氢[17]、鼠李糖脂[18])与FNA预处理耦合,有效强化了水解、产酸和产甲烷过程.然而,化学法存在反应条件苛刻,易造成二次污染等缺陷,限制了其大规模应用[19].相比其他物理预处理方法,紫外预处理具有处理效率高、成本低廉和无二次污染等优点,通过损伤和破坏生物活性,分解胞内物质,导致细胞破裂,成为重要的污泥预处理手段[20].研究发现紫外法(UV)与其它预处理方式联合能有效破坏污泥EPS,使污泥絮体分解(UV辅助零价铁活化过硫酸钠(PDS)氧化法、UV耦合CaO2法)[21-22].已有研究表明亚硝酸盐在UV照射下可能产生活性氮物种(×NO、×NO2、ONOO-、ONOOH)和活性氧物种(×OH、×O2-),通过电子转移、双键加成或氢抽提等方式破坏细胞内各种蛋白质、脂质和DNA结构[11,20],但UV联合FNA预处理对污泥细胞破裂、EPS分解、厌氧发酵产酸效能及作用机理尚不明晰.
基于此,本研究通过超声、热和紫外照射3种方式强化FNA预处理污泥,考察不同预处理方式对污泥发酵产酸性能的影响,以及对污泥微生物多样性的影响,旨在为剩余污泥资源化利用提供理论参考.
1 材料和方法
1.1 实验材料
本实验所用污泥取自山西省太原市杨家堡污水处理厂的污泥浓缩池,所取污泥首先用200目筛子过滤,静置24h后除去上清液,置于4℃冰箱备用.污泥浓缩后10000r/min离心,经0.45µm滤膜过滤后测定其相关性质,剩余污泥初始性质如表1所示.

表1 剩余污泥初始性质
1.2 实验设计
为了考察3种物理法辅助FNA预处理对污泥破壁及厌氧发酵效果的影响,共设置5组实验:未预处理污泥作为空白对照组,FNA预处理组,超声、热和紫外照射强化FNA预处理实验组(FNA-H,FNA-US,FNA-UV).FNA投加量设置2.13mg N/L[2],FNA预处理12h后进行1h的物理强化处理,具体参数如表2所示[23-26].预处理后,测定EPS中溶解性碳水化合物及蛋白质含量.发酵实验采用厌氧发酵瓶,工作容积为400mL,取350mL预处理污泥和50mL新鲜污泥加入发酵瓶中,利用1.0mol/L NaOH或10% HCl调节pH值至(7.0±0.1),并连续充氮15min以保证厌氧环境.每组设置3个平行,反应器置于35 ℃,120r/min的恒温摇床中进行为期10d的发酵实验.每隔24h取定量发酵污泥混合液,10000r/min离心取上清液,经0.45µm滤膜过滤后测定上清液中的NH4+-N,SCFAs,溶解性蛋白质,溶解性碳水化合物的含量.

表2 实验组参数设置
1.3 分析方法
采用热提取法对预处理后EPS进行提取,pH值采用pH计测定,氨氮,SCOD,VSS,TSS采用国标法测定,溶解性碳水化合物采用苯酚-硫酸法,溶解性蛋白采用改良型BCA法蛋白质浓度测定试剂盒测定,SCFAs采用配有氢火焰离子化检测器(FID)的安捷伦6890气相色谱仪测定.
预处理后用电子顺磁共振(EPR)法鉴定自由基种类,以5,5-二甲基-1-吡咯啉-N-氧化物(DMPO)作为自旋捕获剂对体系内自由基进行捕获,通过JES- FA300光谱仪进行分析.对发酵末期污泥样品进行Illumina MiSeq高通量测序.污泥样品经DNA提取后,进行PCR扩增.选取Miseq测序平台V3-V4区域的通用引物338F和806R进行Illumina MiSeq测序.
为便于比较分析,上述所测得的物质浓度(mg/L)均换算为COD浓度(mg COD/L),其转化因子分别为:1.06g COD/g碳水化合物,1.50g COD/g 蛋白,1.07g COD/g乙酸,1.51g COD/g 丙酸,1.82g COD/g丁酸和2.04g COD/g戊酸[2].
2 结果与讨论
2.1 EPS的剥离与释放
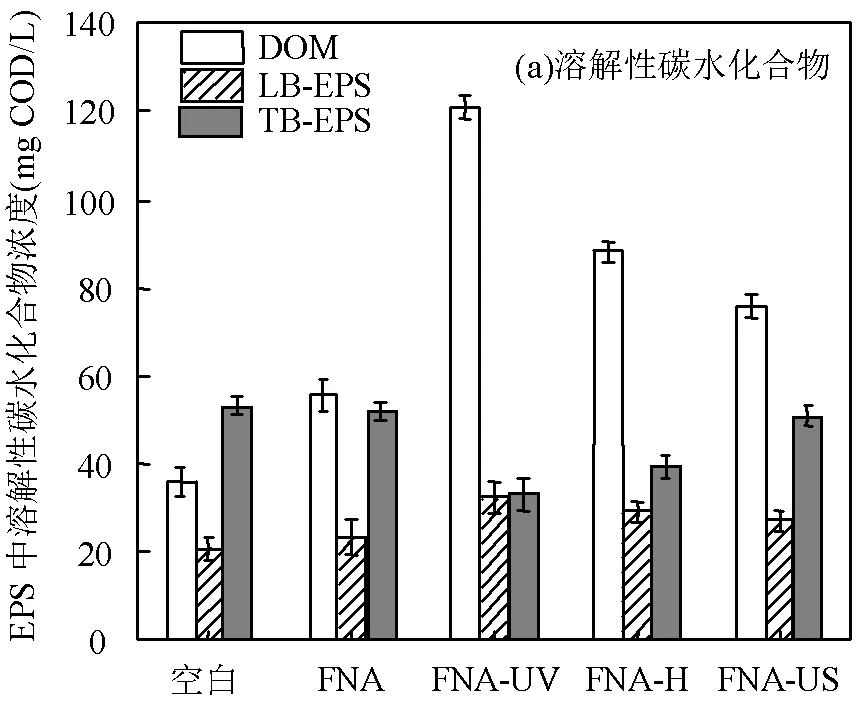
EPS有机物的剥离和释放可直观反映不同预处理方式的处理效能.一般地,EPS可分为溶解性有机物(DOM)、紧密附着型EPS(TB-EPS)和松散附着型EPS(LB-EPS)3种[27].如图1所示,相比空白组,FNA组强化了DOM中有机物的释放,溶解性碳水化合物和蛋白质的含量分别提升了54%和36%.3组联合预处理均有效强化了EPS的剥离,DOM在FNA-UV组的含量相比FNA-H和FNA-US分别提升了29.9%和62.0%,溶解性碳水化合物和蛋白质含量分别达到最大值(121.1±4.3)和(202.9±6.8) mg COD/L,比FNA单独预处理提升了2.2和2.3倍.同时,经FNA预处理,TP-EPS中溶解性碳水化合物和蛋白质含量相比空白组分别下降了20.0%和11.9%,而FNA-UV组的溶解性碳水化合物和蛋白质相比FNA组下降了29.8%和36.7%.说明UV耦合FNA能明显促进污泥增溶,利于溶解性有机物从TB-EPS释放到液相.这是由于FNA-UV预处理过程生成的活性氮自由基(×NO、×NO2)和过氧亚硝酸盐(ONOO-、ONOOH)可以破坏污泥絮体结构,促进EPS剥离及污泥细胞壁破碎,表现为溶解性有机物的有效释放.
相比空白组,FNA预处理及联合预处理后,LB- EPS中有机物含量仅有少量积累,其原因是LB-EPS处于EPS外层,结构松散且流动性强,可以吸附细胞内及TP-EPS中有机物,因此不会有明显积累[11].
2.2 不同预处理方式对污泥厌氧发酵的影响
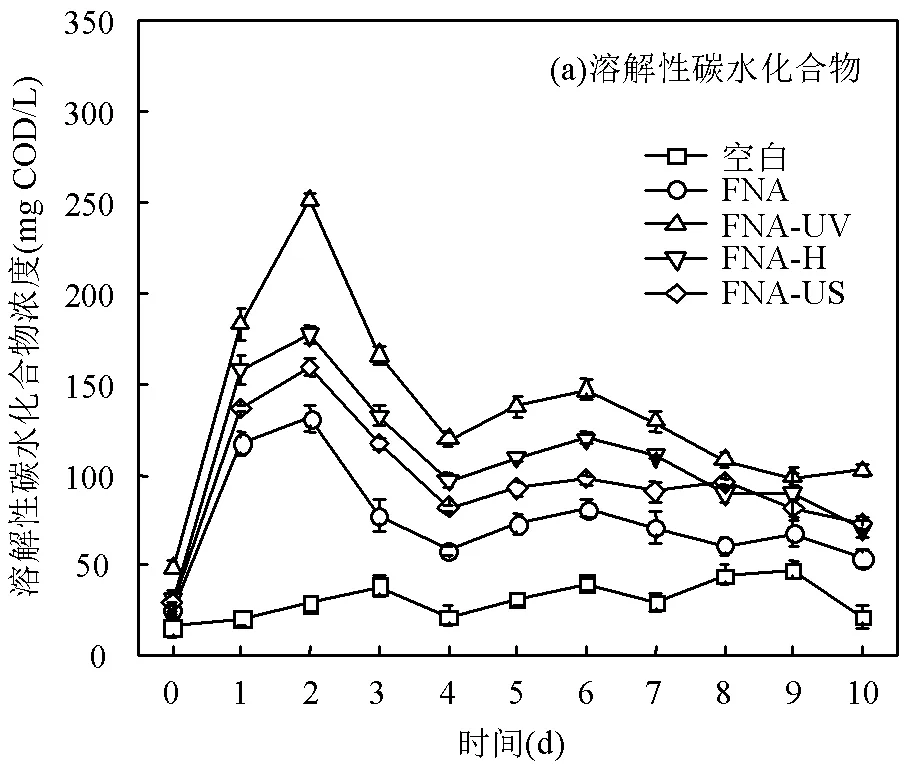
2.2.1 溶解性有机物的变化 溶解性碳水化合物和蛋白质是污泥厌氧发酵过程中主要可利用有机物,因此剩余污泥水解效果可以通过液相中溶解性碳水化合物和蛋白质的浓度来衡量.如图2所示,各组中溶解性碳水化合物和蛋白质总体呈现先增加后减小的趋势,其峰值分别在发酵第2d和第4d达到.FNA组中溶解性碳水化合物和蛋白质含量可以达到(131.1±5.1)和(684.8±7.6) mg COD/L,相比空白组分别提升了2.8和2.1倍.物理法辅助的FNA预处理进一步提升了溶解性有机物的溶出.其中FNA- UV组效果最为明显,溶解性碳水化合物和蛋白质分别高达(251.1±6.3)和(1117.9±12.7) mg COD/L,分别是FNA组的1.9和1.6倍.这主要是由于UV辅助FNA明显促进污泥中EPS水解及污泥细胞破裂,释放大量胞内外有机质到液相中.各实验组溶解性碳水化合物和蛋白质含量达到最大值后出现下降趋势,主要是由于溶解性有机物作为发酵产酸菌的底物,逐步转化为SCFAs[28].
2.2.2 挥发酸的产量及成分 污泥厌氧发酵过程中,发酵菌群利用污泥中多种有机成分代谢产生SCFAs. SCFAs含量随时间变化情况如图3a所示,不同发酵条件下,挥发酸浓度随时间呈现先增加后快速下降的趋势,其峰值均在发酵第4d达到.FNA预处理组中的SCFAs产量((115.3±2.9) mg COD/g VSS)比未预处理污泥((69.1±4.1) mg COD/g VSS)提升了67%.联合预处理进一步强化了SCFAs的产生,其中FNA-UV组SCFAs浓度高达(201.8±4.8)mg COD/g VSS,是FNA组的1.7倍,相比其他联合预处理提升了16%~35%.与类似预处理方法相比,产酸效果明显升高(Wu等[29]采用冷冻-FNA预处理(1.07mg N/L FNA,-5℃ 48h,SCFAs产量124.0mg COD/g VSS)),且明显高于Gao等[30]中试发酵产酸效果(pH值10.0,连续搅拌反应器,SCFAs产量1248.6mg COD/L,约113.5mg COD/ g VSS).其原因是紫外照射与FNA协同作用产生更多自由基,促进污泥解体,破坏污泥细胞结构,释放蛋白质、糖、脂类等大分子有机物,为水解菌和产酸菌提供充足基质.各组中SCFAs第4d的组分分布如图3b所示,各实验组中乙酸和丙酸占比达64%~73%,可作为污水厂外加碳源,为反硝化菌和聚磷菌提供最理想的基质,强化脱氮除磷[31].其中,单独FNA预处理组乙酸占比43.8%,比未预处理实验组提高20%,而US、H、UV耦合FNA预处理污泥体系中乙酸占比进一步提高,相比FNA组提高了9%~29%,FNA-UV组乙酸占比达到最高值(56.8± 0.2)%.同时UV耦合FNA预处理强化了丁酸型发酵,正丁酸含量较FNA组提高24.3%,该结果可由溶解性碳水化合物的降解来佐证(图2a).此外,各联合预处理组中的戊酸含量相比FNA预处理降低12.6%~29.8%,尤其是紫外照射联合组(29.8%)达到最高值,表明该预处理强化了戊酸向小分子挥发酸的转化.

2.2.3 发酵过程中氨氮的释放效果 污泥发酵过程中,蛋白质分解为氨基酸后,可进一步进行脱氨基作用生成氨氮,因此,氨氮含量的变化可间接反映细胞的死亡情况及有机物的水解效果[32-33].如图4所示,各实验组中的NH4+-N浓度都随着发酵时间呈现逐渐升高的趋势,表明在发酵过程中溶解性蛋白不断水解转化生成小分子有机物,为产酸菌提供所需基质[34].以第4d为例,FNA处理组的NH4+-N浓度是空白组的1.2倍,而联合预处理组的NH4+-N浓度又有了进一步上升,FNA-UV组NH4+-N浓度高达(267±21)mg/L,是FNA组的1.6倍.与溶解性蛋白含量变化一致,各实验组NH4+-N含量高低顺序为: FNA-UV组(267±21) mg/L>FNA-H组(227±11) mg/L>FNA-US组(204±19)mg/L>FNA组(165±14) mg/L>空白组(137±12) mg/L.
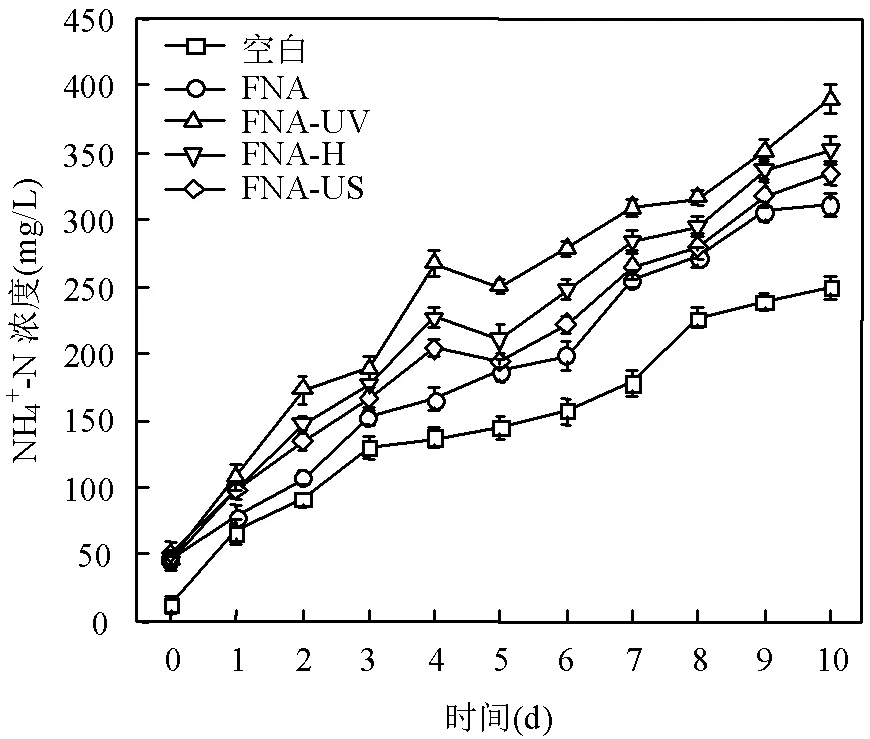
图4 不同预处理方式下氨氮浓度变化
2.3 微生物群落结构分析
剩余污泥厌氧发酵过程涉及多种微生物的共同参与,图5反映了不同反应器内微生物菌落在属水平下的相对丰度.对于门水平,主导菌群为变形菌门(Proteobacteria)、拟杆菌门(Bacteroidetes)、厚壁菌门(Firmicutes),均为最常见的发酵菌门,各处理组常见的发酵菌门占比高达80%[35-37].从纲水平分析,-变形菌纲(Alphaproteobacteria)、-变形菌纲(Betaproteobacteria)及-变形菌纲(Gammaproteobacteria)都属于变形菌门,在水解酸化阶段发挥重要作用.其中-变形菌纲主要参与污泥中溶解性碳水化合物的降解和有机酸的积累,FNA预处理组占比相对空白组提高2.0倍,其在FNA-UV组占比高达36.6%,是FNA组的1.2倍.拟杆菌纲(Bacteroidia)主要参与污泥中大分子有机物的降解和有机酸的积累.
根据属水平上的群落分布,可以发现微生物菌落结构发生了较大变化.以等为主的碳水化合物降解菌在FNA预处理条件下得到了明显富集,相对丰度为13.7%,是空白组的2.3倍,并在物理辅助下进一步积累,相对丰度达15.3%~21.6%,是FNA组的1.1~1.6倍,其中FNA-UV组占比高达21.6%[38-39].可以利用多种碳水化合物产生乙酸,丁酸等,在FNA组含量为11.2%,是空白组的21.0倍,在FNA-UV组含量进一步增至14.1%[40].以、为首的蛋白质降解菌,在 FNA预处理组和空白组的累积丰度分别为1.2%和2.1%,而超声、热、紫外辅助FNA组相对丰度达4.8%~9.8%.其中可有效降解蛋白质产生乙酸和丙酸,在FNA组占比为1.1%,是空白组的1.1倍,而在FNA-UV组丰度增至6.9%[41].此外,、和等主要的反硝化菌在FNA组累积丰度为6.9%,是空白组的1.9倍,而FNA-UV组丰度高达8.2%,是FNA组的1.2倍[42-44].其中在FNA-UV组含量高达5.9%,是FNA组的18.8倍.说明FNA-UV预处理可以有效富集反硝化菌,强化了反硝化过程.

图5 功能微生物在属水平的相对丰度
2.4 紫外光耦合FNA预处理强化污泥厌氧发酵产酸潜在机理分析
如图6所示,对预处理后污泥进行EPR分析,各组均检测出DMPO-OH和DMPO-O2-信号,联合预处理组中DMPO-OH和DMPO-O2-信号强度均强于FNA预处理组,其中FNA-UV组信号强度明显强于FNA-US组和FNA-H组,表明3种物理预处理,尤其是紫外预处理与FNA之间存在协同作用,强化了两种自由基的产生与释放,与预处理后EPS中DOM释放效果相吻合,进一步为上述分析提供了依据[45].其潜在的强化产酸机理如图7所示,FNA预处理过程中产生的活性氮自由基(×NO、×NO2)及活性氮中间体(N2O3和N2O4)通过与胞内或EPS发生反应,改变蛋白质、脂质、碳水化合物和DNA的结构,从而促进EPS水解及污泥破碎.但FNA对分子结构的破坏作用有限,细胞内容物溶出较少[46-48]. FNA在UV照射下强化了×NO和×NO2释放,同时产生过氧亚硝酸盐(ONOO-和ONOOH),ONOO-和ONOOH作为内源氧化剂和亲核试剂,破坏细胞结构,导致细胞解体[49]. ONOO-在FNA预处理的酸性条件下产生×NO和×O2-自由基,ONOOH可分解为×NO2和×OH自由基(式(1 ~ 10)).因此,FNA和UV协同作用产生的各种活性自由基及中间体(×NO、×NO2、ONOO-、ONOOH、×O2-、×OH),促进细胞裂解,释放EPS及胞内外有机物释放到液相中为后续发酵产酸菌提供更多底物[50-52].


图7 UV辅助FNA促进污泥厌氧发酵效能机理
3HNO2⇄HNO3+ 2×NO+ H2O (1)
2HNO2⇄×NO +×NO2+ H2O (2)
2NO2→ N2O4(3)
N2O4+ H2O → HNO3+ HNO2(4)
NO3-×NO +×O2-(< 280nm) (5)
NO3-×NO2+×OH (< 280nm) (6)

ONOOH →×NO2+×OH → ONOO-(8)
ONOO-+×OH →H++×NO+×O2-(9)
×O2-+ H2O ⇄×OH + OH-(10)

表3 发酵10d不同发酵体系碳平衡分析
注: COD转换因子分别为:1.42g COD/g VSS,8g COD/g H2,4g COD/g CH4,1.5g COD/g 溶解性蛋白,1.06g COD/g 溶解性碳水化合物,1.07g COD/g 乙酸,1.51g COD/g 丙酸,1.82g COD/g丁酸,2.04g COD/g戊酸[53].
众所周知,VSS、H2、CH4、溶解性碳水化合物、溶解性蛋白和SCFAs是发酵系统中典型的中间产物或最终产物.为进一步剖析联合预处理对剩余污泥厌氧发酵产酸的影响机制,对不同体系进行了碳平衡分析.VSS的变化可直观反映污泥中有机质的降解及其减量化的程度.由表3可知,各预处理均不同程度实现了污泥的减量,相比空白组(21.94%),FNA组VSS下降至20.92%,进一步在FNA-UV、FNA-H和FNA-US组中降至19.48%,19.25%和20.23%.与VSS变化趋势相反,溶解性碳水化合物和蛋白质含量相比空白组均有不同程度的提升,并在FNA-UV组中占比达到最大,分别为3.07%和24.03%,表明紫外耦合FNA预处理最大程度地强化了溶解性有机物的释放.同时,各组中溶解性蛋白质占比均高于溶解性碳水化合物,表明蛋白质类物质相较于碳水化合物更难被微生物所降解,该结果与Arshad等[54]的研究结果相一致[54].相应地,SCFAs在各预处理组中的占比均高于空白组,且在整个体系碳分布中占比高达28.52%~38.27%,且在FNA-UV组中达到最高,证实了紫外光的引入强化了溶解性有机物的溶出,并在后续产酸过程中强化了其向SCFAs的大量转化.
2.5 讨论及展望
剩余污泥(WAS)含有丰富的有机化合物,为能源回收和SCFAs生产带来巨大潜力.紫外光和FNA预处理均可破坏污泥絮状物和细胞结构,促进胞内外有机物释放[55].研究表明紫外光通过对微生物的辐射损伤和破坏DNA中各种结构键致使微生物破裂,同时可改变腐殖酸的结构特性,使得大分子腐殖酸脱稳并分解为小分子.相比紫外照射驱动的光催化氧化技术,FNA预处理污泥通过FNA及其衍生物(如×NO、×NO2和N2O3)等毒性作用,导致细胞破裂、EPS剥离.FNA与紫外照射联合技术强化并产生活性自由基及中间体(×NO、×NO2、ONOO-、ONOOH),同步强化EPS剥离及污泥细胞破裂.与类似的化学预处理、物理预处理及联合预处理相比,紫外光耦合FNA预处理法对WAS瓦解和发酵产酸效果具有优越性.如Luo等[57]发现,Ca(OCl)2用量为0.025g/g TSS,SCFAs最大产量为192.8mg COD/g VSS,Zheng等[52]采用紫外照射驱动的光催化氧化技术进行厌氧消化(254nm 1h),获得SCFAs产量约150mgCOD/ gVSS,Wu等[29]采用冷冻-FNA预处理(1.07mg N/L FNA,-5℃ 48h),SCFAs产量124.0mg COD/g VSS,低于本研究中使用紫外耦合FNA预处理获得的SCFAs最大浓度(201.8±4.8) mg COD/g VSS[29,55,57].据报道,FNA可从污泥厌氧发酵液中原位合成,且采用FNA预处理在发酵过程中FNA会反硝化为氮气,无二次污染的风险,处理成本约3.60元/m[2].本研究证实了紫外光耦合FNA预处理污泥产酸的可行性,然而目前紫外光耦合FNA预处理技术的耦合条件及机理尚不明晰,之后将对耦合方式、照射时间、FNA浓度和紫外线照射强度,以及处理时间等进一步优化.
3 结论
3.1 紫外光耦合FNA预处理可有效促进污泥溶胞.其DOM中溶解性蛋白及碳水化合物的含量高达(202.9±6.8)和(121.1±4.3) mg COD/L,为其他实验组的1.3~3.1和1.4~3.4倍.
3.2 紫外光耦合FNA预处理有效提升了污泥厌氧发酵过程中SCFAs的产量,在第4d达(201.8±4.8) mg COD/g VSS,相比其他组提升16%~192%.乙酸和丙酸占比高达64%,均高于其他各组.
3.3 紫外光耦合FNA预处理强化了污泥中发酵菌和反硝化菌的生长和富集,其丰度分别为31.3%和8.2%,为其他各实验组的1.2~4.4倍和1.3~2.3倍.
3.4 紫外光耦合FNA预处理有效强化了×OH和×O2-的产生,与活性自由基及中间体(×NO、×NO2、ONOO-、ONOOH)共同促进了污泥的溶胞和胞外聚合物的破解,发酵末期有效实现了污泥减量(VSS占比19.48%)和溶解性有机物的释放与利用.
[1] Bao H X,Yang H,Zhang H,et al. Improving methane productivity of waste activated sludge by ultrasound and alkali pretreatment in microbial electrolysis cell and anaerobic digestion coupled system [J]. Environmental Research,2020,180:108863.
[2] Liu Z H,Zhou A J,Zhang J G,et al. Hydrogen recovery from waste activated sludge: Role of free nitrous acid in a prefermentation– microbial electrolysis cells system [J]. ACS Sustainable Chemistry & Engineering,2018,6(3):3870-3878.
[3] Zhou A J,Liu H Y,Varrone C,et al. New insight into waste activated sludge acetogenesis triggered by coupling sulfite/ferrate oxidation with sulfate reduction-mediated syntrophic consortia [J]. Chemical Engineering Journal,2020,400:125885.
[4] Zurzolo F,Yuan Q,Oleszkiewicz J. Increase of soluble phosphorus and volatile fatty acids during Co-fermentation of wastewater sludge [J]. Waste and Biomass Valorization,2016,7(2):317-324.
[5] Liu X R,Du M T,Yang J N,et al. Sulfite serving as a pretreatment method for alkaline fermentation to enhance short-chain fatty acid production from waste activated sludge [J]. Chemical Engineering Journal,2020,385,123991.
[6] 金宝丹,王淑莹,邢立群,等.不同发酵方式对污泥厌氧发酵性能的影响及其发酵液利用 [J]. 中国环境科学,2016,36(7):2079-2089.
Jin B D,Wang S Y,Xing L Q,et al. The effect of different fermentation methods on the sludge anaerobic fermentation performance and the utilization of fermentation liquor [J]. China Environmental Science,2016,(36)7:2079-2089.
[7] Law Y Y,Ye L,Wang Q L,et al. Producing free nitrous acid – A green and renewable biocidal agent – From anaerobic digester liquor [J]. Chemical Engineering Journal,2015,259:62-69.
[8] Liu Z H,Zhou A J,Liu H Y,et al. Extracellular polymeric substance decomposition linked to hydrogen recovery from waste activated sludge: Role of peracetic acid and free nitrous acid co-pretreatment in a prefermentation-bioelectrolysis cascading system [J]. Water Research,2020,176:115724.
[9] Li X M,Zhao J W,Wang D B,et al. An efficient and green pretreatment to stimulate short-chain fatty acids production from waste activated sludge anaerobic fermentation using free nitrous acid [J]. Chemosphere,2016,144:160-167.
[10] Wang Q L,Ye L,Jiang G M,et al. Free nitrous acid (FNA)-based pretreatment enhances methane production from waste activated sludge [J]. Environmental Science and Technology,2013,47(20): 11897-11904.
[11] Chislett M,Guo J H,Bond P L,et al. Structural changes in model compounds of sludge extracellular polymeric substances caused by exposure to free nitrous acid [J]. Water Research,2021,188:116553.
[12] Wang J S,Zhang Z J,Ye X,et al. Enhanced solubilization and biochemical methane potential of waste activated sludge by combined free nitrous acid and potassium ferrate pretreatment [J]. Bioresource Technology,2020,297:122376.
[13] Karimi R,Hallaji S M,Siami S,et al. Synergy of combined free nitrous acid and Fenton technology in enhancing anaerobic digestion of actual sewage waste activated sludge[J]. Scientific Reports,2020,10(1):5027.
[14] Wu Y Q,Song K,Sun X Y,et al. Mechanisms of free nitrous acid and freezing co-pretreatment enhancing short-chain fatty acids production from waste activated sludge anaerobic fermentation [J]. Chemosphere,2019,230:536-543.
[15] Wang Q L,Jiang G M,Ye L,et al. Enhancing methane production from waste activated sludge using combined free nitrous acid and heat pre-treatment [J]. Water Research,2014,63:71-80.
[16] Niu Q Q,Xu Q X,Wang Y L,et al. Enhanced hydrogen accumulation from waste activated sludge by combining ultrasonic and free nitrous acid pretreatment: Performance,mechanism,and implication [J]. Bioresource Technology,2019,285:121363.
[17] Zhang T T,Wang Q L,Ye L,et al. Combined free nitrous acid and hydrogen peroxide pre-treatment of waste activated sludge enhances methane production via organic molecule breakdown [J]. Scientific Reports,2015,5:16631.
[18] Wu Q L,Guo W Q,Bao X,et al. Enhanced volatile fatty acid production from excess sludge by combined free nitrous acid and rhamnolipid treatment [J]. Bioresource Technology,2017,224:727- 732.
[19] 张旭光,陈 宇,张 龙.游离亚硝酸偶联生物表面活性剂强化污泥厌氧发酵产酸[J]. 环境工程,2019,37(1):56-60,87.
Zhang X G,Chen Y,Zhang L. Enhancement of acid production from sludge anaerobic fermentation by Free nitrous bio-surfactant [J]. Environmental Engineering,2019,37(1):56-60,87.
[20] Erwan C,Jean P ,Vincent J,et al. Impact of suspended particles on UV disinfection of activated-sludge effluent with the aim of reclamation [J]. Journal of Water Process Engineering,2018,22:87-93.
[21] Zheng M,Ping Q,Wang L,et al. Pretreatment using UV combined with CaO2for the anaerobic digestion of waste activated sludge: Mechanistic modeling for attenuation of trace organic contaminants [J]. Journal of Hazardous Materials,2021,402:123484.
[22] Zhang Y P,Li T T,Tian J Y,et al. Enhanced dewaterability of waste activated sludge by UV assisted ZVI-PDS oxidation [J]. Journal of Environmental Sciences,2022,13(3):152-164.
[23] Sun J,Guo L,Li Q Q,et al. Structural and functional properties of organic matters in extracellular polymeric substances (EPS) and dissolved organic matters (DOM) after heat pretreatment with waste sludge [J]. Bioresource Technology,2016,219:614-623.
[24] Yu H W,Anumol T,Park M,et al. On-line sensor monitoring for chemical contaminant attenuation during UV/H2O2advanced oxidation process [J]. Water Research,2015,81:250-260.
[25] Zhang Y P,Li T T,Tian J Y,et al. Enhanced dewaterability of waste activated sludge by UV assisted ZVI-PDS oxidation [J]. Journal of Environmental Sciences,2022,113:152-164.
[26] 杨春雪.嗜热菌强化剩余污泥水解及短链脂肪酸积累规律研究 [D]. 哈尔滨:哈尔滨工业大学,2015.
Yang C X. Enhanced effects of thermophiles on waste activated sludge hydrolysis and short-chain fatty acids production [D]. Harbin: Harbin Institute of Technology,2015.
[27] Donlan R M,Costerton J W. Costerton. Biofilms: survival mechanisms of clinically relevant microorganisms [J]. Clinical Microbiology Reviews,2002,15(2):167-193.
[28] Jan T W,Adav S S,Lee D J,et al. Hydrogen fermentation and methane production from sludge with pretreatments [J]. Energy Fuels,2008,22(1):98-102.
[29] Wu Y Q,Song K,Sun X Y,et al. Effects of free nitrous acid and freezing co-pretreatment on sludge short-chain fatty acids production and dewaterability [J]. Science of the Total Environment,2019,669: 600-607.
[30] Gao Y Q,Peng Y Z,Zhang J Y,et al. Biological sludge reduction and enhanced nutrient removal in a pilot-scale system with 2-step sludge alkaline fermentation and A2O process [J]. Bioresource Technology,2011,102(5):4091-4097.
[31] He Z W,Tang C C,Liu W Z,et al. Enhanced short-chain fatty acids production from waste activated sludge with alkaline followed by potassium ferrate treatment [J]. Bioresource Technology,2019,289: 121642.
[32] Pijuan M,Wang Q L,Liu Y,et al. Improving secondary sludge biodegradability using free nitrous acid treatment [J]. Bioresource Technology,2012,116:92-98.
[33] 樊雅欣,刘红燕,潘凌峰,等.活化方式对过硫酸盐强化剩余污泥发酵的影响 [J]. 中国环境科学,2019,39:2460-2466.
Fan Y X,Liu H Y,Pan L F,et al. Enhancement of waste activated sludge acidification by persulfate: Role of activation methods [J]. China Environmental Science,2019,39:2460-2466.
[34] 冯宇杰,魏瑶丽,李虹瑶,等.醋糟与剩余污泥共发酵体系中底物配比对挥发性脂肪酸产量的影响 [J]. 科学技术与工程,2020,20(34): 14332-14306.
Feng Y J,Wei Y L,Li H Y,et al. Effect of Substrate Ratio on the Yield of Volatile Fatty Acids in the Cofermentation System of Vinegar Dregs and Residual Sludge [J]. Science Technology and Engineering,2020,20(34):14332-14306.
[35] 刘芝宏,魏瑶丽,樊雅欣,等.游离亚硝酸预处理对剩余污泥电解及微生物群落结构的影响 [J]. 中国环境科学,2019,39(7):2953-2959.
Liu Z H,Wei Y L,Fan Y X,et al. Role of free nitrous acid on waste activated sludge bio-electrolysis and key microflora shift [J]. China Environmental Science,2019,39(7):2953-2959.
[36] 张 强,李咏梅.投加Na2S对化学强化除磷污泥厌氧发酵释磷的影响 [J]. 中国环境科学,2021,41(3):1219-1227.
Zhang Q,Li Y M. Effects of dosing sodium sulfide on phosphorus release during the anaerobic fermentation of waste sludge produced by chemical enhanced phosphorus removal [J]. China Environmental Science,2021,41(3):1219-1227.
[37] 康晓荣.超声联合碱促进剩余污泥水解酸化及产物研究 [D]. 哈尔滨:哈尔滨工业大学,2013.
Kang X R. Study on hydrolysis and acidification of activated sludge enhanced by ultrasound combined with alkaline [D]. Harbin: Harbin Institute of Technology,2013.
[38] Donachie S P,Bowman J P,Stephen L W,et al. Arcobacter halophilus sp. nov.,the first obligate halophile in the genus Arcobacter [J]. International Journal of Systematic and Evolutionary Microbiology,2005,55:1271-1277.
[39] Gerritsen J,Fuentes S,Grievink W,et al. Characterization of..,sp..,isolated from the gastro- intestinal tract of a rat,and proposal for the reclassification of five closely related members of the genusinto the genera..,..,.. and.[J]. International Journal of Systematic and Evolutionary Microbiology,2014,64(Pt 5):1600-1616.
[40] 贺张伟.预处理方法对污泥厌氧耦合微生物电解及厌氧消化产能的影响 [D]. 哈尔滨:哈尔滨工程大学,2014.
He Z W. Study on influence factor and mechanisms of organic matters conversion and phosphorus release during waste activated sludge anaerobic fermentation process [D]. Harbin: Harbin Engineering University,2014.
[41] Mielczarek A T,Kragelund C,Eriksen P S,et al. Population dynamics of filamentous bacteria in Danish wastewater treatment plants with nutrient removal [J]. Water Research,2012,46(12):3781-3795.
[42] He Q C,Feng C P,Chen N,et al. Characterizations of dissolved organic matter and bacterial community structures in rice washing drainage (RWD)-based synthetic groundwater denitrification [J]. Chemosphere,2019,215:142-152.
[43] Simona Č,Jakub K,TomášJ. Biological water denitrification-A review [J]. Enzyme and Microbial Technology,1992,14(3):170-183.
[44] Pervin H M,Batstone D J,Bond P L. Previously unclassified bacteria dominate during thermophilic and mesophilic anaerobic pre-treatment of primary sludge [J]. Systematic and Applied Microbiology,2013,36(4):281-290.
[45] Wang J,Lou Y,Feng K,et al. Enhancing the decomposition of extracellular polymeric substances and the recovery of short-chain fatty acids from waste activated sludge: Analysis of the performance and mechanism of co-treatment by free nitrous acid and calcium peroxide [J]. J Hazard Mater,2022,423(Pt A):127022.
[46] Chislett M,Guo J H,Bond P L,et al. Structural changes in cell-wall and cell-membrane organic materials following exposure to free nitrous acid [J]. Environmental Science and Technology,2020,54(16): 10301-10312.
[47] Wang J S,Zhang Z J,Ye X,et al. Performance and mechanism of free nitrous acid on the solubilization of waste activated sludge [J]. RSC Advances,2018,8(29):15897-15905.
[48] Sang S Y,Coakley R,Lau G W,et al. Anaerobic killing of mucoid Pseudomonas aeruginosa by acidified nitrite derivatives under cystic fibrosis airway conditions [J]. Journal of Clinical Investigation,2006,116(2):436-446.
[49] Huang Y,Kong M,Westerman D,et al. Effects of HCO3-on degradation of toxic contaminants of emerging concern by UV/NO3(.) [J]. Environmental Science and Technology,2018,52(21):12697- 12707.
[50] 徐慧敏,秦卫华,何国富,等.超声联合热碱技术促进剩余污泥破解的参数优化 [J]. 中国环境科学,2017,37(9):3431-3436.
Xu H M,Qin W H,He G F,et al. Optimization of combined ultrasonic and thermo-chemical pretreatment of waste activated sludge for enhanced disintegration [J]. China Environmental Science,2017,37(9): 3431-3436.
[51] Thorn K A,Cox L G. Ultraviolet irradiation effects incorporation of nitrate and nitrite nitrogen into aquatic natural organic matter [J]. Journal of Environmental Quality,2012,41(3):865-881.
[52] Zheng M,Daniels K D,Park M,et al. Attenuation of pharmaceutically active compounds in aqueous solution by UV/CaO2process: Influencing factors,degradation mechanism and pathways [J]. Water Research,2019,164:114922.
[53] Yang J,Liu X,Wang D,et al. Mechanisms of peroxymonosulfate pretreatment enhancing production of short-chain fatty acids from waste activated sludge [J]. Water Research,2019,148:239-249.
[54] Arshad Z,Maqbool T,Shin K H,et al. Using stable isotope probing and fluorescence spectroscopy to examine the roles of substrate and soluble microbial products in extracellular polymeric substance formation in activated sludge process [J]. Science of the Total Environment,2021,788:147875.
[55] 孙珮石,江映翔,刘安文,等.紫外线辐射对活性污泥除磷性能的增强作用 [J]. 中国环境科学,2003,23(2):184-188.
Sun P S,Jiang Y X,Liu A W,et al. Enhancement of phosphorus removal property of activated sludge with UV ray irradiation [J]. China Environmental Science,2003,23(2):184-188.
[56] 黄 芳.高温热水解过程中污泥腐殖酸的演变及其对厌氧消化效果的影响研究 [D]. 无锡:江南大学,2021.
Huang F. Evolutions of humic acids during slusge thermal hydrolysis and their effects on anaerobic digestion [D]. Wuxi: Jiangnan University,2021.
[57] Luo J Y,Huang W X,Zhang Q,et al. Effects of different hypochlorite types on the waste activated sludge fermentation from the perspectives of volatile fatty acids production,microbial community and activity,and characteristics of fermented sludge [J]. Bioresource Technology,2020,307:123227.
[58] Zahedi S,Icaran P,Yuan Z,et al. Effect of free nitrous acid pre- treatment on primary sludge at low exposure times [J]. Bioresource Technology,2017,228:272-278.
[59] 韩 兴.污水紫外消毒装置设计及工艺参数优化研究[D]. 长春:吉林农业大学,2006.
Han X. Research on the design of sewage ultraviolet disinfection equipment and optimization of the craft parameter [D]. Changchun: Jilin Agricultural University. 2006.
致谢:感谢山西省太原市杨家堡污水处理厂的工作人员协助完成.
Enhancement of acidification from waste activated sludge via anaerobic fermentation by free nitrous acid (FNA) pretreatment assisted by ultraviolet.
YIN Xiao-yun,LIU Zhi-hong*,ZHOU Ai-juan,LI Ya-nan,YUE Xiu-ping,CUI Zhi-xuan
(College of Environmental Science and Engineering,Taiyuan University of Technology,Taiyuan 030024,China).,2022,42(8):3770~3779
In order to break the bottleneck of the limited hydrolysis performance and low short chain fatty acids (SCFAs) production efficiency from waste activated sludge (WAS) during the traditional anaerobic fermentation,this work investigated the effect of the ultraviolet (UV) assisted free nitrous acid (FNA) pretreatment on WAS disintegration and acidification,and compared with thermal (H) and ultrasonic (US) coupled with FNA pretreatment. Results revealed that UV assisted FNA co-pretreatment (FNA-UV) had a synergistic effect on disruption of both extracellular polymeric substances and cell envelope.・OH and・O2-,as the main reaction intermediates,their intensities in FNA-UV group were much stronger than that obtained in other pretreatment groups (FNA-US and FNA-H). Moreover,these two free radicals,with the intermediates such as ・NO,・NO2andONOO-,further promoting the release of soluble organics.The contents of soluble carbohydrates and protein were 60% and 90% higher than that obtained in FNA group respectively,which served more soluble substrates for SCFAs generation. Accordingly,SCFAs concentration peaked at 4d in FNA-UV group (201.8±4.8)mg COD/g VSS with 56.8% acetic acid (HAc)),which was 67% higher than that of FNA group. Carbon balance analysis at the final stage of the fermentation showed that UV assisted FNA pretreatment played an important role in sludge reduction,release and transformation of soluble substrates,and finally SCFAs production. The functional microbial consortia analysis indicated the anaerobic fermentation bacteria and nitrate-reducing bacteria were obviously enriched in FNA-UV group,which were 23.7%~270.6% higher than that obtained in other groups.
free nitrous acid (FNA);waste activated sludge (WAS);short chain fatty acids (SCFAs);anaerobic fermentation;physical method;high-throughput sequencing
X703.1
A
1000-6923(2022)08-3770-10
2021-12-28
国家自然科学基金资助项目(52100155,52070139);山西省基础研究计划项目(20210302124347);山西省重点研发项目(社发领域) (201903D321055)
* 责任作者,讲师,liuzhihong0227@163.com
殷霄云(1998-),女,河南漯河人,太原理工大学硕士研究生,主要从事废弃生物质资源化.

