颅骨组织工程复合支架动态灌注装置的设计
李 佳,张崇越,郑淑贤,亓 剑,付志明
颅骨组织工程复合支架动态灌注装置的设计
李 佳,张崇越,郑淑贤,亓 剑,付志明
(天津大学装备设计与制造技术天津市重点实验室,天津 300350)
为了解决颅骨组织工程支架中存在的中心处细胞存活率低的问题,针对具有血管嵌入的颅骨复合支架研制动态灌注培养装置,为多孔支架内部细胞提供营养物质以提高细胞存活率,使复合支架达到体内整合的要求.基于颅骨复合支架结构特点,设计双通道循环分别对多孔支架和血管进行灌注,为颅骨复合支架中成骨细胞和内皮细胞提供物质传输,气体循环为循环培养液提供氧气、二氧化碳,保证了培养液氧气充足和pH稳定;模拟人体自然颅骨组织中机械刺激,建立多孔支架、血管流体模型,根据灌注室和血管入口流量计算流体所需的剪切应力,据此确定流体流量和压力的控制范围与精度,为复合支架提供合适的流量;设计三因素三水平正交试验对灌注室的结构尺寸进行优选,确定关键部位灌注室的结构,通过硅胶管连接灌注室、储液瓶、蠕动泵形成循环系统,实现动态灌注装置的总体结构安装;建立流体仿真分析有限元模型,分析不同流速的流体培养液分别对颅骨多孔支架和血管结构的影响,结果表明灌注室和血管结构内流体速度、剪切应力、压力分布均匀,满足骨组织和内皮细胞的培养生理环境,验证了装置设计的合理性.该装置可用于骨组织体外细胞的动态培养,为骨组织工程支架体外构建及血管化奠定了基础.
颅骨;组织工程;复合支架;动态灌注;流体模型
在骨组织工程中,支架作为载体,其外形可以提供形态支撑,内部多孔结构可为细胞的贴附以及营养物质的传输提供保障,从而维持或改善组织功能和结构[1-2].目前,体外支架与细胞的整合通常采用静态方式进行,但存在营养物质供应不足、代谢废物无法排除导致细胞坏死、无法在支架中心形成细胞基质等问题[3-4].动态培养可解决上述问题,它通过动态灌注方式,使培养液持续流动,加快营养物质的输送和交换,有利于支架中心的细胞生长[5].此外,动态灌注所产生的流体剪切应力加强细胞信号通道的活跃,促进碱性磷酸酶、骨钙蛋白、collagen I、骨桥蛋白等特殊基因表达,可提高细胞的增殖和分化[6-9].在血管支架的动态培养中,流体剪切应力可促进内皮细胞标志物的表达[10].
近年来,各式动态灌注设备已经被应用到骨组织工程修复当中.Santoro等[11]和Bhaskar等[12]为培养大面积骨组织,采用了多出入口的方式进行动态灌注,促进了细胞在3D支架中的增殖与分化.Jagodzinski等[13]采用周期性压力培养多孔支架中的骨细胞,并分析了周期性机械刺激对成骨细胞增殖与分化的影响.但是,在动态灌注培养支架-细胞的体外整合研究中,仍发现其骨细胞的存活率低,成骨缓慢.研究表明由于支架中没有血管结构,导致细胞营养物质的供给受到限制,骨细胞不能深入支架内部,造成成骨能力下降[14-15].
针对这一问题,笔者基于前期颅骨板障及其内部静脉的仿生研究[16],设计了具有血管嵌入式的颅骨复合支架,其三维结构如图1所示.在此,提出了一种颅骨血管复合支架的动态灌注方法,研制新型的动态灌注装置以解决常规静/动态培养中出现的骨细胞存活率低、成骨缓慢等问题,并通过流体仿真分析,验证装置设计的可行性,为骨组织工程体外血管化的实现提供技术基础.

图1 颅骨复合支架结构示意
1 动态灌注装置设计
为模拟自然颅骨组织的物质交换过程,在此提出双通道动态灌注方式,即分别对颅骨多孔支架通道和血管结构通道独立进行灌注.以下主要从循环系统、灌注室流体模型、仿真分析等3个方面阐述该动态灌注系统的研制过程.
1.1 循环系统的设计
双通道灌注循环系统如图2所示,多孔支架灌注通道为黄色线,代表支架培养液,采用硅胶管通过接口C、D连接灌注室,与灌注室形成灌注循环回路;血管灌注通道为红色线,代表血管培养液,硅胶管通过接口A、B与血管结构出入口连接,与血管形成灌注循环回路.循环系统分为气体循环和液体循环,气体循环是棕色线,为动态培养提供二氧化碳、氧气,维持培养液的pH值和氧气浓度的稳定;液体循环为细胞提供营养液、排除代谢废物,并产生一定的剪切应力、压应力,刺激细胞的生长.整个循环过程中,由蠕动泵提供动力,输送培养液和混合气体,实现灌注循环.

图2 灌注循环系统示意
1.2 灌注流体模型的建立

1.2.1 支架剪切应力的计算


(1)

(2)

(3)
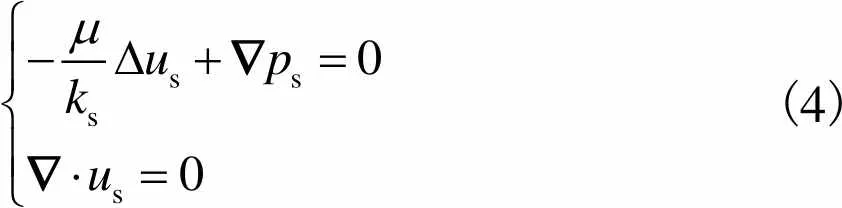
(4)
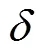
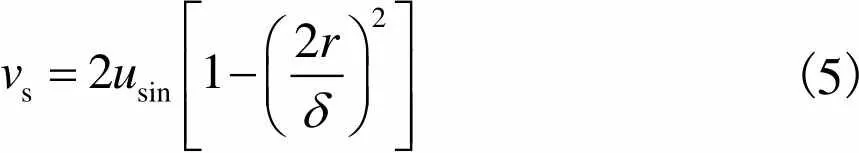
(5)
因此,细胞在多孔支架孔隙中受到的剪切应力可以通过Darcy速度公式进行计算,多孔支架空隙剪切应力为
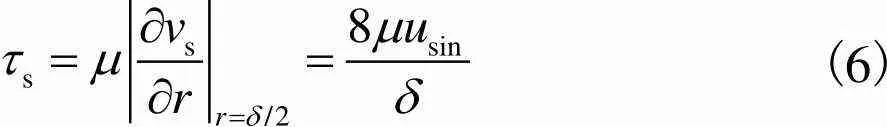
(6)
得

(7)
由此得到多孔支架的剪切应力计算公式,为后面多孔支架通道流量提供依据.
1.2.2 血管剪切应力的计算

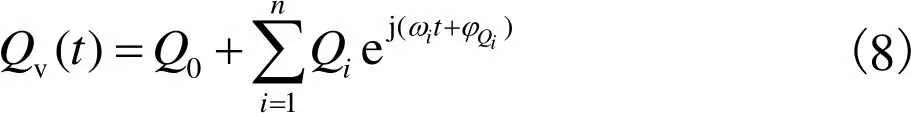
(8)
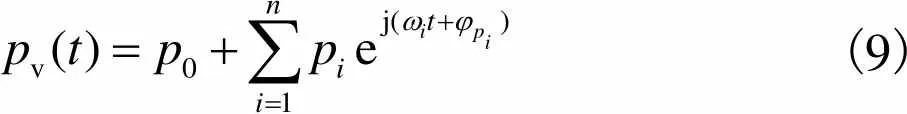
(9)
静脉血管血流量、脉动小,所以可以近似认为满足Poiseuille流动[19].
体积流量

(10)
血管壁剪切应力

(11)
血管最大流速
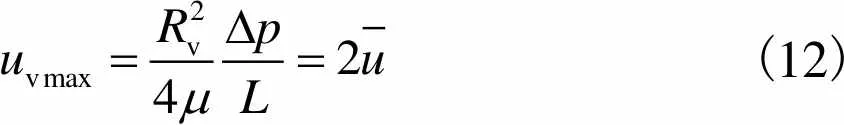
(12)
血管平均流速

(13)
综上可知
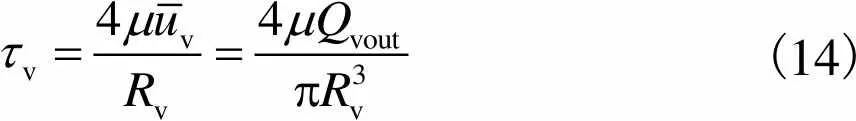
(14)
通过建立灌注流体模型,得到了血管剪切应力计算公式,为第2.1节血管流量计算提供依据.
1.3 灌注室的设计
复合支架放置在灌注室中进行灌注,灌注室的结构尤为重要,因此,需要对灌注室结构进行详细设计.针对血管嵌入的复合支架的特殊结构及其双通道灌注方式,设计了如图3(a)所示的灌注室.灌注分为Ⅰ和Ⅱ两个对称的腔室,采用圆柱和半球组合的结构,以使流体流速分布更加均匀.

图3 灌注室结构与尺寸示意
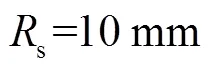
表1 因素水平表
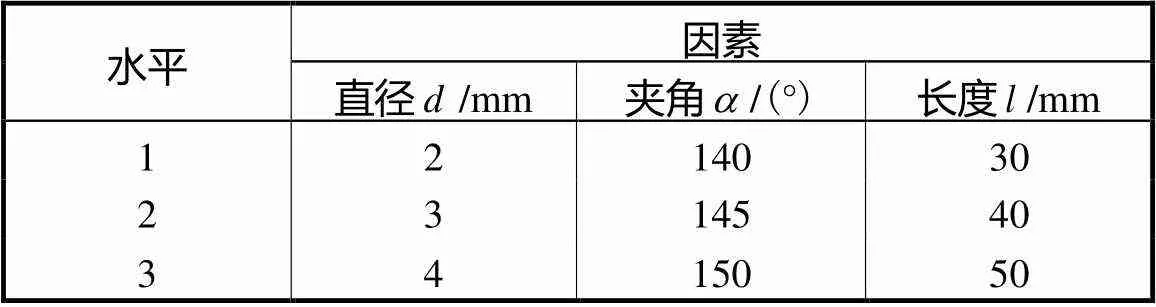
Tab.1 Factor and level table
表2 正交试验结果

Tab.2 Orthogonal experimental results

表3 极差法分析结果
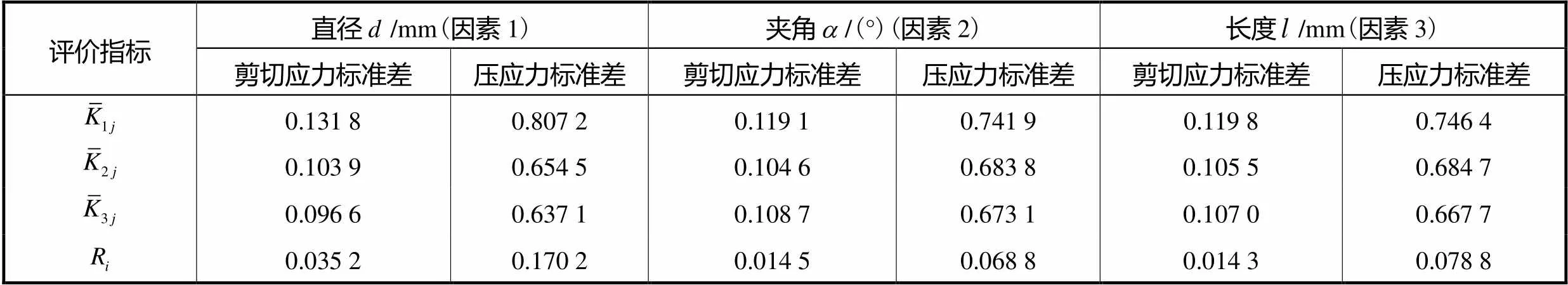
Tab.3 Analysis results of step method
将灌注室设计为Ⅰ和Ⅱ两部分,如图4所示,并设计凹槽安放颅骨复合支架.灌注室Ⅰ和Ⅱ部分用螺栓固定法兰盘形成密闭的灌注室,作为培养液灌流、储存的结构.

图4 灌注室模型
2 灌注系统流量计算与装置加工
2.1 流体流量计算
在动态灌注装置中,流量是系统最重要的输入量,不仅为细胞提供合适范围的营养物质,也为细胞提供剪切应力促进细胞的增殖,因此流量的计算非常必要.下面分别计算支架和血管入口的流量.
对于血管流量,参考直径约为2mm的下矢状窦静脉流速和压力[25]类比人体颅骨板障静脉,得到血管平均流速为0.10m/s,出口平均压力为1.5kPa,周期为0.4~1.0s,平均流量为0.08mL/s.因此,根据式(8)和(9),得到血流流量和压力分别为
表4 颅骨多孔支架参数
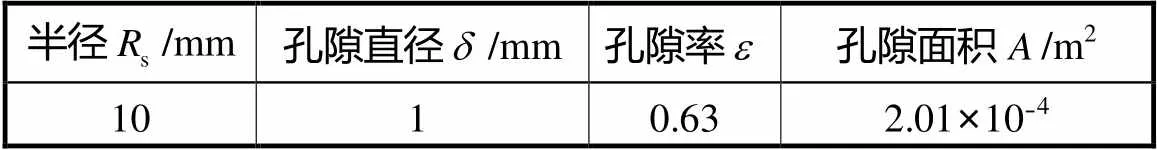
Tab.4 Parameters of porous scaffold for skull

(15)
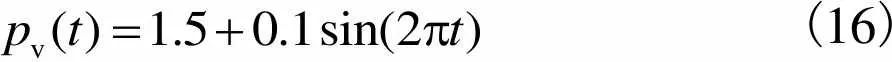
(16)
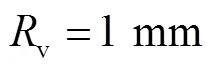


图5 血管压力和流量曲线

(17)
控制柜中PLC控制频率随时间变化,得到恒定或者脉动转速,可实现恒定或脉动流量灌注支架.
2.2 灌注装置加工
根据上述设计结果采用无毒的有机玻璃制造灌注室,在灌注室Ⅰ和Ⅱ之间采用O型密封圈密封,通过6个螺栓连接法兰盘形成灌注室,如图6(a)所示.动态灌注装置实物如图6(b)所示,整个灌注装置主要由控制柜和灌注循环柜组成.该装置的特性在于可同时对多孔支架和血管实现动态灌注;还可对多灌注室同时灌注,方便进行对比试验.

图6 灌注室和动态灌注装置实物图
3 仿真分析
利用ANSYS Workbench建立流体力学有限元模型,分析灌注流量对于复合支架及血管的影响,确定最优流量,得到恰当的应力分布,验证装置的可行性.课题组前期采用具有较好生物相容性的羟基磷灰石作为支架材料,验证了该材料的可行性[27],并且分析得到复合支架的变形量相对较小,所以本文不考虑支架变形对流体应力的影响,而主要分析流体对支架及血管应力影响,为后续流量设定提供指导作用.
3.1 灌注室-支架仿真分析
3.1.1 灌注室-支架流体模型
(1) 几何模型.建立支架灌注室模型,导入到Workbench中.
(3) 网格划分.对流体采用四面体网格划分,在支架孔隙处加密网格.

(5) 流体模型与求解器.在光滑圆管中,雷诺数决定了层流过渡为湍流的条件,即

(18)
3.1.2 结果与讨论


图7 不同入口速度对支架应力影响
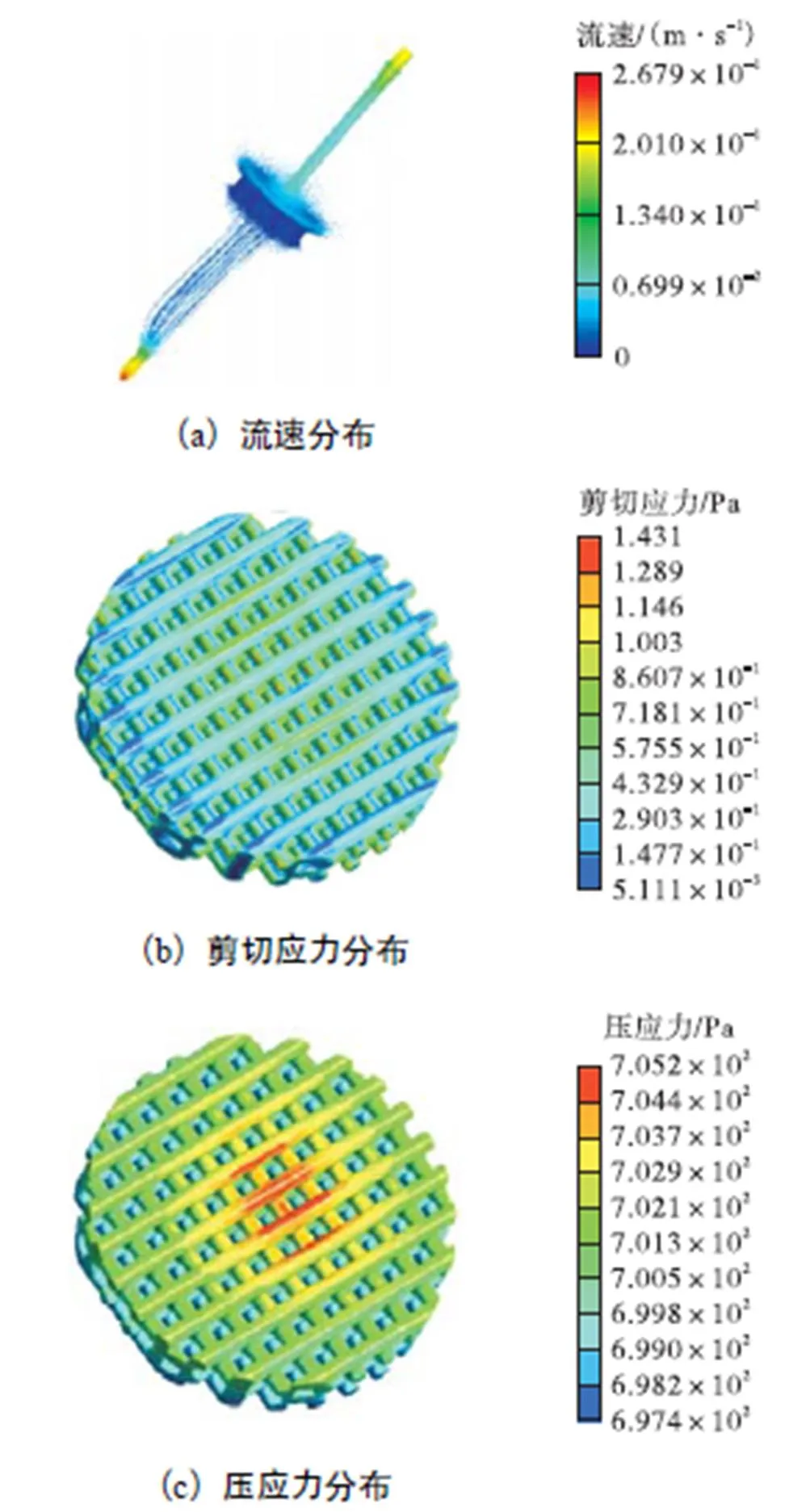
图8 颅骨多孔支架应力分布
(2) 支架剪切应力分布基本均匀,均值为0.36Pa,最大值在入口侧中心处为1.431Pa,从支架中心到边缘,速度明显减小,由式(6)可知,剪切应力逐渐减小.满足成骨细胞剪切应力生长的需求,即0.30~2.86Pa[22-24].
(3) 培养液对支架的压力分布均匀,均值为700Pa,最大值在入口处为705Pa,适合骨细胞生长的范围0~20kPa[28].压力从入口方向向出口方向逐渐减小,产生压力梯度,是培养液流动的动力.另外,压力在支架中心处局部增大,原因是培养液对中心处的支架产生一定的冲击,使得局部压力增大.
3.2 血管仿真分析
3.2.1 血管流体模型

在光滑圆管中,雷诺数决定了层流过渡为湍流的条件,即

(20)
3.2.2 结果与讨论
(1) 血管中流体流速分布均匀且大部分位置流速在0.10m/s左右,同时中间主血管的流速大于周围血管,符合人体血管流速特征[25].
(2) 血管内剪切应力分布均匀,均值为1.7Pa,入口处最大为11.9Pa,满足内皮细胞生长的需求[26].

图9 不同入口速度对血管应力的影响

图10 血管应力分布
(3) 培养液对血管的压力分布比较均匀,最大值在入口处为1760Pa,最小在出口处为1500Pa,最值差较小,均值为1640Pa,适合内皮细胞生长[25].压力从入口方向向出口方向逐渐减小,产生压力梯度,是培养液流动的动力.
由上述灌注室中支架和血管的流体仿真分析和优化结果,得到了最优流量入口和压力出口,分析表明复合支架表面流速、剪切应力、压应力分布均匀,符合灌注装置的设计要求,满足颅骨复合支架的生长环境的要求.验证了灌注室设计和灌注装置提供的流量、压力的合理性.
4 结 语
本文研制了一种动态血液灌注装置.该装置具有双通道流体、气体循环,能用于颅骨复合支架的细胞灌流培养,可模拟人体生理环境相符的体外培养环境.根据细胞生长的需要,建立了流体模型计算剪切应力,为流量提供依据,所得流量可通过PLC对蠕动泵进行精确控制.通过对灌注系统进行有限元优化分析,得出流体的压力、速度和剪切力符合静脉层流的血流特性,血管应力分布较为均匀,同时多孔支架中剪切应力、压力、速度符合颅骨细胞的生长要求.该装置可系列化设计,易于装配,通过修改不同参数可调整培养条件,为组织工程支架的动态培养与体外血管化提供技术支持.
[1] Venugopal J,Ramakrishna S. Applications of polymer nanofibers in biomedicine and biotechnology[J]. Appl Biochem Biotechnol,2005,125(3):147-158.
[2] Ben-David D,Kizhner T A,Kohler T,et al. Cell-scaffold transplant of hydrogel seeded with rat bone marrow progenitors for bone regeneration[J]. Journal of Cranio-Maxillofacial Surgery,2011,39(5):364-371.
[3] Ding M,Henriksen S S,Theilgaard N,et al. Assessment of activated porous granules on implant fixation and early bone formation in sheep[J]. Journal of Orthopaedic Translation,2016,5:38-47.
[4] Goldstein A S,Juarez T M,Helmke C D,et al. Effect of convection on osteoblastic cell growth and function in biodegradable polymer foam scaffolds[J]. Biomate-rials,2001,22(11):1279-1288.
[5] Yeatts A B,Fisher J P. Bone tissue engineering bioreactors:Dynamic culture and the influence of shear stress[J]. Bone,2011,48(2):171-181.
[6] Liu L,Yuan W,Wang J. Mechanisms for osteogenic differentiation of human mesenchymal stem cells induced by fluid shear stress[J]. Biomechanics and Modeling in Mechanobiology,2010,9(6):659-670.
[7] Volkmer E,Drosse I,Otto S,et al. Hypoxia in static and dynamic 3D culture systems for tissue engineering of bone[J]. Tissue Engineering Part A,2008,14(8):1331-1340.
[8] Du D,Furukawa K S,Ushida T. 3D culture of osteoblast-like cells by unidirectional or oscillatory flow for bone tissue engineering[J]. Biotechnol Bioeng,2009,102(6):1670-1678.
[9] Frohlich M,Grayson W L,Marolt D,et al. Bone grafts engineered from human adipose-derived stem cells in perfusion bioreactor culture[J]. Tissue Eng Part A,2010,16(1):179-189.
[10] Kim D H,Heo S J,Kang Y G,et al. Shear stress and circumferential stretch by pulsatile flow direct vascular endothelial lineage commitment of mesenchymal stem cells in engineered blood vessels[J]. Journal of Materials Science:Materials in Medicine,2016,27:DOI 10.1007/s10856-016-5670-0.
[11] Santoro R,Olivares A L,Brans G,et al. Bioreactor based engineering of large-scale human cartilage grafts for joint resurfacing[J]. Biomaterials,2010,31(34):8946-8952.
[12] Bhaskar B,Owen R,Bahmaee H,et al. Design and assessment of a dynamic perfusion bioreactor for large bone tissue engineering scaffolds[J]. Applied Biochemistry and Biotechnology,2018,185(2):555-563.
[13] Jagodzinski M,Breitbart A,Wehmeier M,et al. Influence of perfusion and cyclic compression on proliferation and differentiation of bone marrow stromal cells in 3-dimensional culture[J]. Journal of Biomechanics,2008,41(9):1885-1891.
[14] Berner A,Woodruff M A,Lam C X F,et al. Effects of scaffold architecture on cranial bone healing[J]. International Journal of Oral and Maxillofacial Surgery,2014,43(4):506-513.
[15] Sinikovic B,Schumann P,Winkler M,et al. Calvaria bone chamber—A new model for intravital assessment of osseous angiogenesis[J]. Journal of Biomedical Materials Research Part A,2011,99A(2):151-157.
[16] 刘洁,郑淑贤,李佳. 颅骨组织工程血管支架的参数化设计[J]. 机械工程学报,2018,54(1):178-187.
Liu Jie,Zheng Shuxian,Li Jia. Parametric design for skull tissue engineering vascular scaffold[J]. Chinese Journal of Mechanical Engineering,2018,54(1):178-187(in Chinese).
[17] Whittaker R J,Booth R,Dyson R,et al. Mathematical modelling of fibre-enhanced perfusion inside a tissue-engineering bioreactor[J]. Journal of Theoretical Biology,2009,256(4):533-546.
[18] Guyot Y,Papantoniou I,Luyten F P,et al. Coupling curvature-dependent and shear stress-stimulated neotissue growth in dynamic bioreactor cultures:A 3D computational model of a complete scaffold[J]. Biomechanics and Modeling in Mechanobiology,2016,15(1):169-180.
[19] 邹盛铨. 血流动力学与心血管人工器官[M]. 成都:成都科技大学出版社,1991.
Zou Shengquan. Hemodynamics and Cardiovascular Artificial Organ[M]. Chengdu:Chengdu University of Science and Technology Press,1991(in Chinese).
[20] Freitas D,Almeida H A,Bártolo P J. Perfusion bioreactor fluid flow optimization[J]. Procedia Technology,2014,16:1238-1247.
[21] 喀蔚波. 医用物理学[M]. 北京:高等教育出版社,2008.
Ka Weibo. Medical Physics[M]. Beijing:Higher Education Press,2008(in Chinese).
[22] Jeon J H,Jeong O C. Effect of shear stress magnitude on intracellular calcium expression in bone cells[J]. Microelectronic Engineering,2012,97:329-332.
[23] Sakai K,Mohtai M,Iwamoto Y. Fluid shear stress increases transforming growth factor beta 1 expression in human osteoblast-like cells:Modulation by cation channel blockades[J]. Calcif Tissue Int,1998,63(6):515-520.
[24] Xu H,Duan J,Ren L,et al. Impact of flow shear stress on morphology of osteoblast-like IDG-SW3 cells[J]. Journal of Bone and Mineral Metabolism,2018,36:529-536.
[25] Ho H,Mithraratne K,Hunter P. Numerical simulation of blood flow in an anatomically-accurate cerebral venous tree[J]. IEEE Trans on Med Imaging,2013,32(1):85-91.
[26] McCormick S M,Whitson P A,Wu K K,et al. Shear stress differentially regulates PGHS-1 and PGHS-2 protein levels in human endothelial cells[J]. Ann Biomed Eng,2000,28(7):824-833.
[27] 毕振庆. 颅骨多孔支架的设计及其增材制造工艺研究[D]. 天津:天津大学机械工程学院,2018.
Bi Zhenqing. Design and Additive Manufacturing for Skull Porous Scaffold[D]. Tianjin:School of Mechanical Engineering,Tianjin University,2018(in Chinese).
[28] Hu K,Wang C,Zhang X. High pressure may inhibit periprosthetic osteogenesis[J]. Journal of Bone and Mineral Metabolism,2010,28(3):289-298.
Dynamic Perfusion Device Design for Skull Tissue-Engineered Composite Scaffold
Li Jia,Zhang Chongyue,Zheng Shuxian,Qi Jian,Fu Zhiming
(Tianjin Key Laboratory of Equipment Design and Manufacturing Technology,Tianjin University,Tianjin 300350,China)
To solve the problem of low survival rate of cells at the center of a scaffold,a dynamic perfusion culture device is developed for skull composite scaffold with embedded vessels. The device improves cell survival rate and makes composite scaffolds meet the integration requirement in vivo. Based on the structural features of the skull composite scaffold,a double-channel perfusion circulation is designed to separately perfuse porous scaffold and vessels to provide material transmission for osteoblasts and endothelial cells.Moreover,gas circulation provides oxygen and carbon dioxide for circulating culture medium,which ensures sufficient oxygen and stable pH. To simulate the mechanical environment in a natural human skull,a porous scaffold and a vascular fluid model are established,and the shear stress is calculated according to the inlet flow rate of perfusion chamber and vessel. Therefore,the control range and accuracy of fluid flow and pressure are determined,and the suitable flow rate for composite scaffolds are obtained. An orthogonal test with three factors and three levels is designed to optimize the size and determine the key parts of the perfusion chamber structure. The whole structure of the dynamic perfusion device is installed by connecting the perfusion chamber,the liquid storage bottle,and the peristaltic pump with a silica gel tube to form a circulating system. A finite element fluid analysis model is established to analyze the effect of flow with different velocities on composite scaffold. The results show that the fluid velocity,shear stress,and pressure are uniformly distributed in the perfusion chamber and vessel,and the physiological environment of bone tissue and endothelial cells is satisfactorily simulated;therefore,the rationality of the device is verified. The device can be used for dynamic cell culture of bone tissue in vitro. It lays a foundation for the construction and vascularization of bone tissue-engineered scaffolds.
skull;tissue engineering;composite scaffold;dynamic perfusion;fluid model
10.11784/tdxbz201808026
TH79
A
0493-2137(2019)05-0459-09
2018-08-06;
2018-10-05.
李佳(1955— ),男,博士,教授,jli@tju.edu.cn.
郑淑贤,sxzheng@tju.edu.cn.
国家自然科学基金资助项目(51575380).
the National Natural Science Foundation of China(No. 51575380).
(责任编辑:金顺爱)

