根灌乙酸及葡萄酒对海水胁迫下葡萄光抑制的影响
王辉,高玉录,于梦,杜远鹏,孙永江,翟衡
根灌乙酸及葡萄酒对海水胁迫下葡萄光抑制的影响
王辉,高玉录,于梦,杜远鹏,孙永江,翟衡
(山东农业大学园艺科学与工程学院/作物生物学国家重点实验室,山东泰安 271018)
【目的】为了扩大盐渍化土壤的高效利用方式,提高葡萄在盐渍化土壤上的生长发育质量,探讨乙酸与葡萄酒对15%海水胁迫下葡萄叶片光抑制的影响,为盐渍化土壤葡萄栽培技术改良提供理论依据和技术参考。【方法】以一年生‘摩尔多瓦’葡萄(‘Moldova’)盆栽苗为试材,定植于直径为20 cm、高度为18 cm的塑料盆内,每盆土和基质的比例为1﹕1,置于日光温室内。培养条件为日均温在25℃左右,温室内透光率不低于自然光照强度的50%,其中最高光强在800 μmol·m-2·s-1,相对湿度在40%—60%,正常肥水管理至8—10片完全展开叶时,进行海水和外源乙酸处理。进行15%海水浇灌的同时,分别浇灌30 mmol·L-1乙酸、2%葡萄酒,以浇灌清水为对照,每个处理重复5盆,各处理均浇灌3次,每隔2 d浇灌一次,浇灌量为基质持水量的2倍,约1/3的溶液流出,每次浇灌后第2天测定叶绿素荧光指标,处理到第10天后,解析植株取样分析二者对海水胁迫下丙二醛(MDA)含量、叶绿素含量以及根系活力的影响。【结果】15%海水灌溉显著增加了葡萄各器官的MDA含量,显著降低了葡萄叶片叶绿素含量和根系活力,其中,与清水对照相比,根、茎、叶中MDA含量分别提高了1.10、0.27、0.41倍,叶绿素含量降低了18.5%,根系活力降低了41.9%,而浇灌30 mmol·L-1乙酸及2%葡萄酒处理显著降低了葡萄各器官中的MDA含量,其中,浇灌乙酸处理的根、茎、叶中MDA含量比海水胁迫分别降低了29.3%、20.6%、15.8%;浇灌葡萄酒处理的根、茎、叶中MDA含量分别比海水胁迫的降低了29.4%、20.2%、25.2%。浇灌乙酸及葡萄酒处理显著提高了葡萄叶片的叶绿素含量和根系活力,其中,根系活力分别比海水胁迫处理的显著提高了68.4%和56.9%,叶绿素含量则分别提高了18.8%和20.3%。叶绿素荧光分析表明,15%海水灌溉导致叶片光系统II(PSII)最大光化学效率(v/m)及最大光氧化P700 (m)明显下降,其中第3、6、9天的m分别比对照下降了5.0%、9.6%、13.0%,光化学淬灭系数(qP)和PSII实际光化学效率Y(II)逐渐下降,而PSII处可调节性能量耗散的量子产额Y(NPQ)及非调节性能量耗散的量子产额Y(NO)均逐渐升高,PSI供体端侧抑制程度Y(ND)未发生明显变化,但是PSI受体侧抑制程度Y(NA)逐渐升高,导致PSI的实际光化学效率Y(I)降低。根灌乙酸及葡萄酒可以显著缓解15%海水胁迫,PSII及PSI实际光化学效率显著升高,而用于热耗散的能量分配明显降低,从而提高了葡萄叶片的光合性能,其中以2%葡萄酒处理缓解效果较明显。【结论】根灌乙酸及葡萄酒显著提高15%海水胁迫下葡萄根系活力及叶绿素含量,缓解叶片光抑制程度,从而提高了葡萄对海水胁迫环境下的适应性,研究结果为残次果加工果酒、果渣及醋等的综合利用改良盐渍化土壤提供了理论依据。
葡萄;海水;光抑制;乙酸;葡萄酒;叶绿素荧光
0 引言
【研究意义】我国耕地中盐渍化面积已达到920.9万公顷[1]。土壤盐渍化是影响农业生产和生态环境的全球性问题[2],盐渍化耕地中积累的盐主要来自海水、盐碱湖或灌溉水中的氯化钠[3]。随着国家把黄河三角洲开发上升为国家战略,滨海盐渍化土壤改良与高效经济作物的适应性栽培驯化又重新提上研究日程,采用工程技术进行排盐和洗盐地,降低地下水位,避免海水倒灌是滨海盐渍化土地改良及利用的基础,选择耐盐的经济作物,进行耐盐育种及采用一定的技术措施等成为改良土壤盐渍化的发展方向[4]。研究乙酸及葡萄酒缓解海水胁迫的响应机制对改良盐碱地具有重要意义,并可为充分利用各种残次水果及酒糟浸提液来改良盐碱地提供理论依据。【前人研究进展】盐胁迫中产生的离子毒害会降解叶绿素[5-6],造成植物光合电子传递和光系统活力受到抑制[7],研究也发现盐胁迫可以下调参与光反应和卡尔文循环的酶和蛋白活性[8],导致光合受阻,严重的甚至导致植株死亡[9]。对于盐渍化土地的改良,研究者也采取了一定的措施,如袁隆平培育成的‘海水稻’;利用栽培技术措施进一步提高了经济作物在盐渍化土壤上的生长发育,从而提高商品生产水平,如设施栽培、局部改良土壤的根域限制等。生长季节施加外源物质是缓解盐胁迫的一种有效栽培技术方式,较为常用的外源物质有渗透调节物质、激素类等[10-11]。Kim等[12]最新研究发现,通过浇灌乙酸可以显著缓解逆境对拟南芥、水稻、小麦、玉米和油菜生长发育的抑制,其结果为研究者提供了一个新思路,是否乙酸以及含有较高乙酸含量的葡萄酒也能够缓解盐胁迫?【本研究切入点】外源乙酸能够缓解植物受到的非生物胁迫,但乙酸及葡萄酒对海水胁迫下葡萄叶片光合作用的影响未见报道。【拟解决的关键问题】探讨乙酸以及含有较高乙酸含量的葡萄酒缓解葡萄盐胁迫的机制,以期为盐渍化土壤葡萄园栽培管理提供新思路。
1 材料与方法
试验于2017年在山东农业大学葡萄园内进行。
1.1 试材及处理
以一年生‘摩尔多瓦’葡萄(‘Moldova’,×)自根苗为试材,定植于直径为20 cm、高度为18 cm的塑料盆内,每盆土和基质的比例为1﹕1,置于日光温室内。培养条件:日均温在25℃左右,温室内透光率不低于自然光照强度的50%,其中最高光强在800 μmol·m-2·s-1,相对湿度在40%—60%,正常肥水管理至8—10片完全展开叶,此时叶片光合作用的饱和光强在1 100 μmol·m-2·s-1左右,进行海水和外源乙酸处理。选取无病虫害、长势一致的苗子分成4组,处理1为浇灌海水(取自渤海湾,用清水稀释到15% v/v,各种离子以mg·L-1为单位,稀释后分别是Na+1 584,Cl-2 847,SO42-384,HCO3-21.3,Ca2+45,Mg2+253.5,pH 7.88,电导率为12.32 ms·cm-1,盐离子浓度为8.1 mg·L-1);处理2为15%海水+30 mmol·L-1乙酸(购于天津市凯通化学试剂有限公司,预试验确定适宜浓度);处理3为15%海水+2% v/v葡萄酒(葡萄酒产自笔者实验室葡萄园,其中乙酸的含量为0.43 g·L-1,酸度为7.3 g·L-1),对照为清水。每个处理重复5盆,各处理均浇灌3次,每隔2 d浇灌一次,浇灌量为基质持水量的2倍,约1/3的溶液流出,每次浇灌后第2天测定叶绿素荧光指标(从上数第5片展开叶),即第0、3、6、9天测定;处理到第10天,解析植株取样测定丙二醛(malondialdehyde,MDA)含量、根系活力和叶绿素含量,同时取土测定土壤pH等指标。
1.2 测定指标及方法
1.2.1 土壤pH的测定 用土壤水浸液法,称取通过2 mm孔径筛的风干试样20 g(精确至0.1 g)于50 ml高型烧杯中,加入超纯水20 ml,以搅拌器搅拌1 min,使土粒充分分散,放置30 min用PB-10型pH计(北京赛多利斯科学仪器有限公司)进行测定。
1.2.2 土壤容重的测定 用环刀法测定,100 cm3取样烘干后,按公式容重=干土重(g)/体积(cm3)计算。
1.2.3 土壤含水量的测定 采用HBN-BXSF型土壤水分速测仪进行测定。
1.2.4 丙二醛含量、根系活力和叶绿素含量的测定 参照赵世杰等[13]的硫代巴比妥酸比色法测定MDA含量;利用氯化三苯基四氮唑法测定根系活力;按照赵世杰等[13]方法计算叶绿素含量。
1.2.5 叶绿素荧光参数的测定 使用Dual-PAM100叶绿素荧光仪(Walz,Germany)进行叶绿素荧光的活体测定,测定前对叶片进行15 min的暗适应。先测定初始荧光产量(o)与最大荧光产量(m),之后远红光照射10 s后,测定P700最大量子产量(m),对光下完全光启动的叶片打光强度为600 μmol·m-2·s-1的光化光,待荧光信号达到稳态后(大约4—5 min)打开饱和脉冲光(8 000 μmol·m-2·s-1),测定任意时间的实际荧光产量(t)、光下最大量子产量(m′)和光适应下的最大荧光产量(m′)。根据以下公式进行各参数的计算:PSII最大光化学效率v/m=(m-o)/m,PSII光化学量子产量Y(II)=(m′-s)/m′,光化学淬灭系数qP=(m′-s)/(m'-o'),非调节性能量耗散Y(NO)=s/m,调节性能量耗散Y(NPQ)=s/m′-s/m,PSI光化学量子产量Y(I)=(m′-)/m,PSI供体测抑制程度Y(ND)=/m,受体测抑制程度Y(NA)=(m-m')/m[14]。
1.3 数据统计与分析
数据测定取3次生物学重复,分别用Microsoft Excel和Sigma Plot处理数据和作图,用DPS软件对数据进行单因素方差分析。数据以平均值±标准误表示。
2 结果
2.1 浇灌乙酸及葡萄酒对海水胁迫下土壤理化性状的影响
土壤pH是衡量土壤酸碱性的重要指标,由表1可以看出,浇灌15%海水的土壤pH与清水对照相比升高了0.67%,但没有显著性影响,浇灌乙酸和葡萄酒的处理土壤pH仅分别比海水处理降低了1.5%和1.2%,也未出现显著性降低。对土壤容重以及土壤含水量的测定表明,浇灌15%海水后,与对照相比并未出现显著性变化,浇灌乙酸和葡萄酒后,也未出现显著性变化。
2.2 浇灌乙酸及葡萄酒对海水胁迫下葡萄根、茎、叶中丙二醛含量的影响
丙二醛(MDA)是膜脂过氧化作用的产物之一,可以作为鉴定植物遭受盐胁迫程度的生理指标。由表2可见,浇灌3次15%海水后对‘摩尔多瓦’葡萄造成了明显胁迫,显著增加了各器官MDA含量,根、茎、叶中MDA含量分别是清水对照的2.10、1.27、1.41倍,而根灌乙酸及葡萄酒均显著缓解了海水胁迫下葡萄根、茎、叶中MDA的含量,其中,浇灌乙酸比海水胁迫分别降低了29.3%、20.6%、15.8%;浇灌葡萄酒比海水胁迫分别降低了29.4%、20.2%、25.2%。
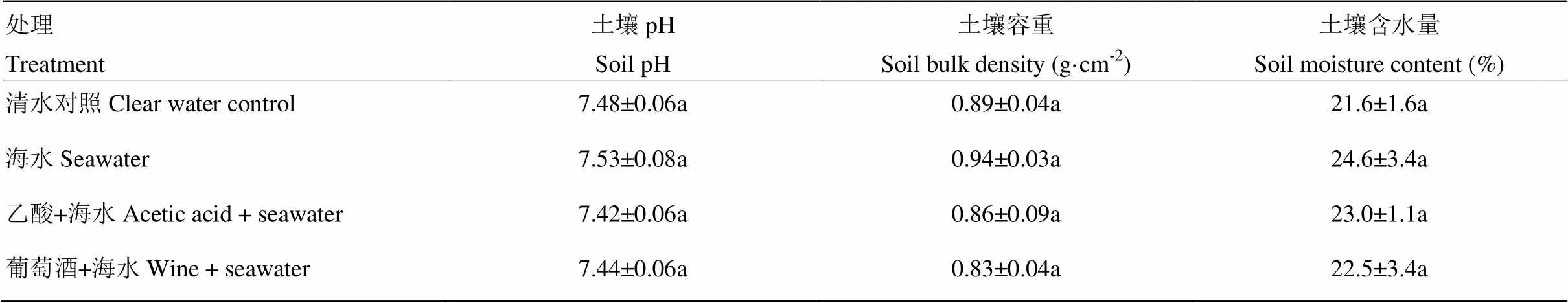
表1 浇灌乙酸及葡萄酒对海水胁迫下土壤理化性状的影响
同列数据后不同小写字母表示处理间差异显著(<0.05)。下同
Different lowercases in the same column indicate significant difference among treatments (<0.05). The same as below
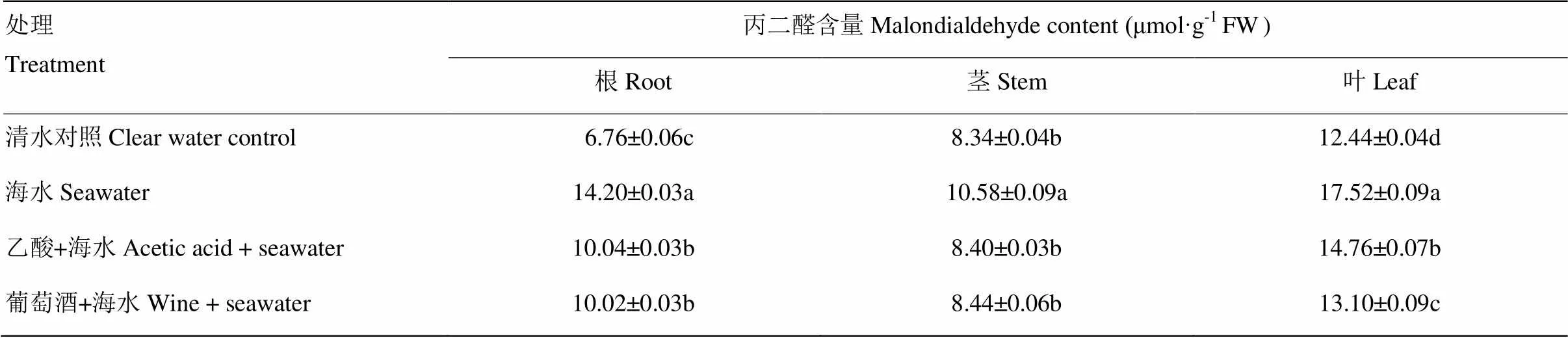
表2 浇灌乙酸及葡萄酒对海水胁迫下葡萄根、茎、叶中丙二醛含量的影响
2.3 浇灌乙酸及葡萄酒对海水胁迫下葡萄根系活力的影响
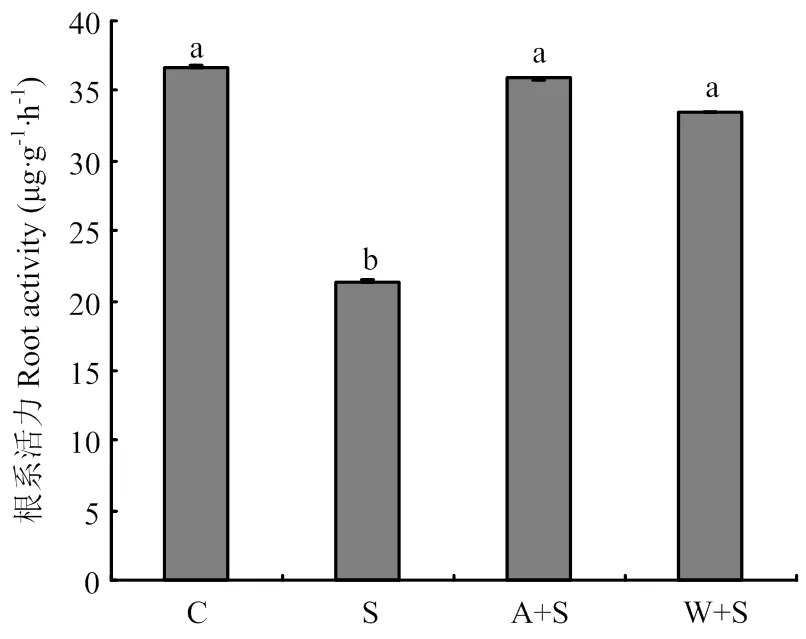
由图1可以看出,与清水对照相比,浇灌3次15%海水显著降低了葡萄的根系活力,比对照降低了41.9%。根灌30 mmol·L-1乙酸及2%葡萄酒浇灌均显著升高了根系活力,与15%海水处理相比,分别提高了68.4%、56.9%。
2.4 浇灌乙酸及葡萄酒对海水胁迫下葡萄叶片叶绿素含量的影响
叶绿素含量是反映植物光合能力的重要指标[15]。图2表明,15%海水胁迫显著降低了葡萄叶片中叶绿素含量,与对照相比降低了18.5%,而根灌乙酸和葡萄酒后叶绿素含量与海水处理相比分别提高了18.8%、 20.3%,与清水对照相比没有显著差异。
2.5 浇灌乙酸及葡萄酒对海水胁迫下葡萄叶片叶绿素荧光的影响
叶绿素荧光是反映植物光合性能的有效探针,最大光化学效率(v/m)反映了光系统Ⅱ(photosystem II,PSII)反应中心受损情况,P700最大量子产额(m)可以反映PSI活性[16]。如图3所示,15%海水处理下m、v/m呈现下降趋势。其中第3、6、9天的m分别比对照下降了5.0%、9.6%、13.0%;v/m在海水胁迫后第3天出现明显下降,根灌乙酸和葡萄酒处理均缓解了v/m和m的降低幅度,表明根灌乙酸和葡萄酒可以有效缓解15%海水胁迫下葡萄叶片的光抑制程度,其中以葡萄酒处理效果较明显。
C:清水对照Clear water control;S:海水Seawater;A+S:乙酸+海水Acetic acid + seawater;W+S:葡萄酒+海水Wine + seawater。下同The same as below
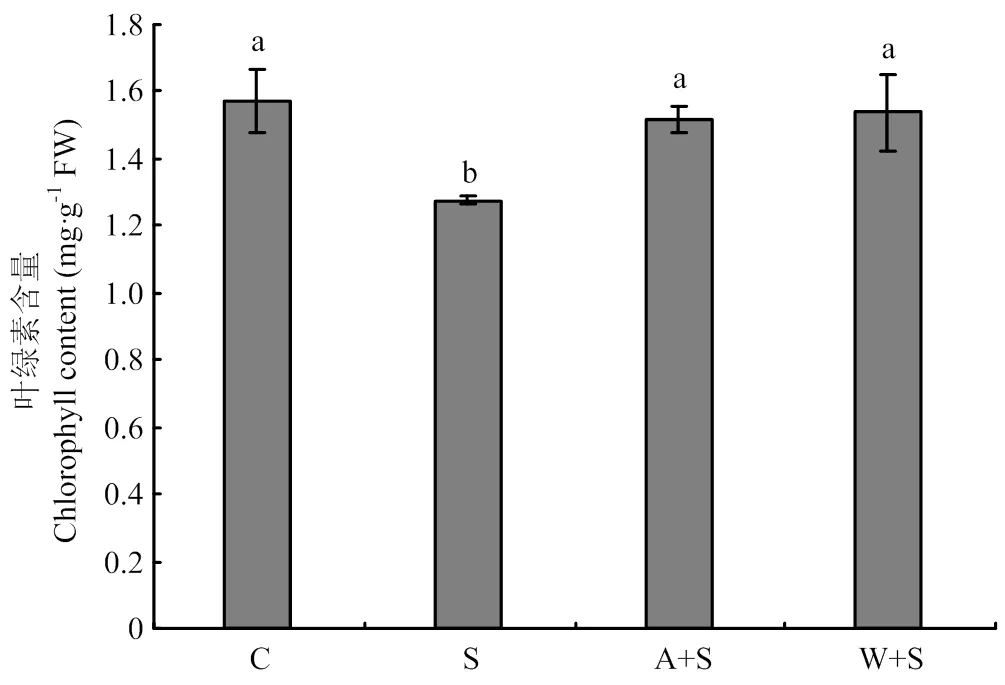
图2 浇灌乙酸及葡萄酒处理对海水胁迫下葡萄叶片叶绿素含量的影响
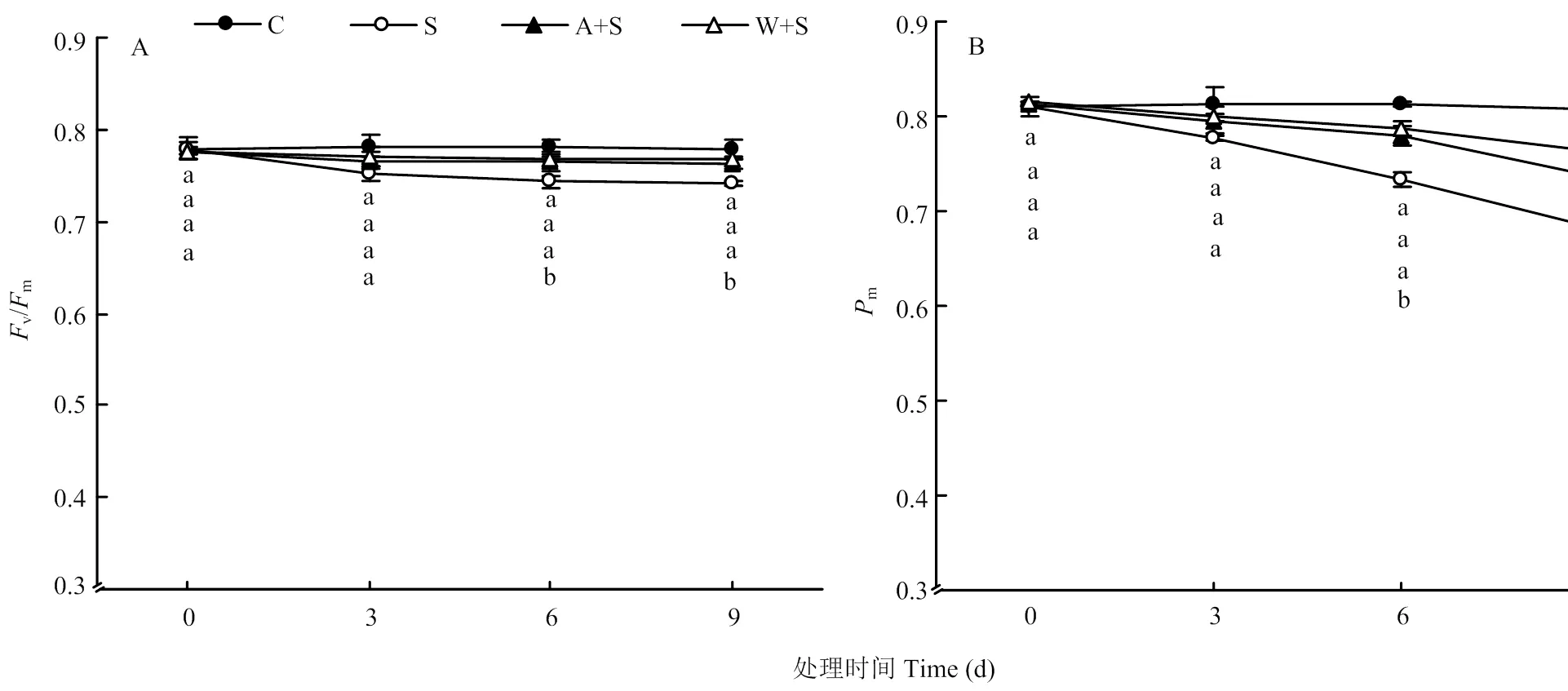
图3 浇灌乙酸及葡萄酒处理对海水胁迫下葡萄叶片Fv/Fm、Pm的影响
2.6 浇灌乙酸及葡萄酒处理对海水胁迫下葡萄叶片能量分配的影响
如图4所示,15%海水胁迫明显降低了葡萄叶片光下最大光化学效率(v′/m′)(图4-A),并且光化学淬灭系数(qP)(图4-B)逐渐降低,表明PSII反应中心在海水胁迫环境中处于关闭状态,从而导致用于光化学途径的能量减少,光合作用受到抑制。根灌乙酸及葡萄酒的v′/m′、qP与海水胁迫相比均明显提高,说明乙酸及葡萄酒可以提高PSII的光化学效率。15%海水灌溉也改变了葡萄叶片PSII和PSI的能量分配,与对照相比,15%海水灌溉下PSII实际光化学效率[Y(II)](图4-C)明显下降,但可调节性能量耗散[Y(NPQ)](图4-G)及非调节性耗散[Y(NO)](图4-E)均明显升高;PSI供体端侧抑制程度[Y(ND)](图4-H)发生明显变化,但PSI受体侧抑制程度[Y(NA)](图4-F)逐渐升高,导致PSI的实际光化学效率[Y(I)](图4-D)降低。根灌乙酸和葡萄酒明显提高了15%海水灌溉下葡萄叶片的Y(II)及Y(I),降低了Y(NPQ)及Y(NO)水平。进一步表明乙酸及葡萄酒可以缓解15%海水胁迫下葡萄叶片光抑制程度,从而改善能量的分配比例。
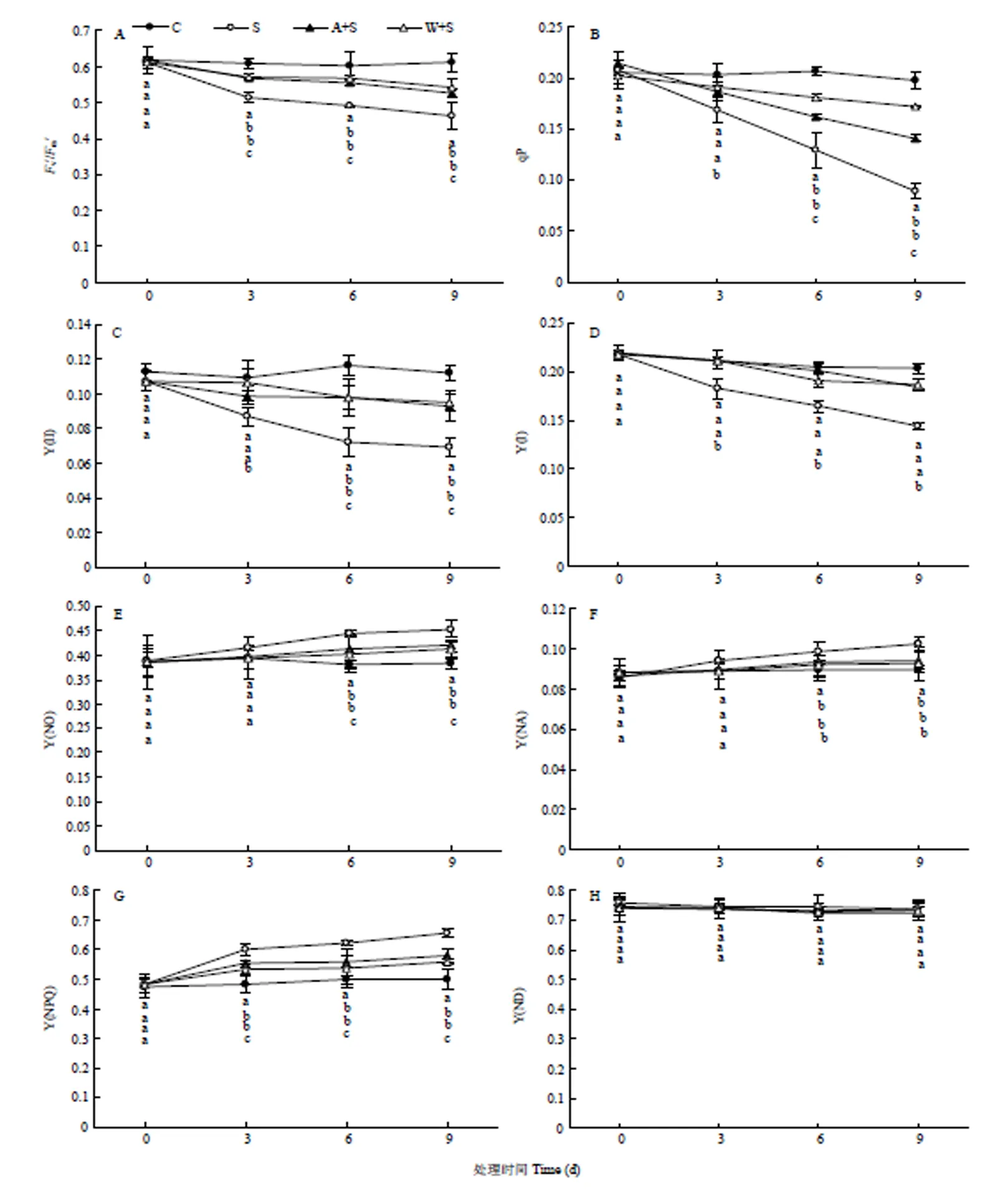
图4 浇灌乙酸及葡萄酒处理对海水胁迫下葡萄叶片能量分配的影响
3 讨论
众所周知,乙酸是一种简单的基本生物化合物,由于含有的羧基具有较强的络合作用,对土壤理化性质、矿物溶解和成土作用、根际营养、重金属毒害等方面产生着深刻的影响,同时也在植物化感作用方面起着重要的作用[17]。有研究发现,土壤中也存在乙酸[18],且在一定浓度范围内土壤中乙酸浓度越高植物体内累积的钠、钾离子浓度越低[19],而作为协调植物生存能力的初始因子,将植物的基本代谢、表观遗传调控和激素信号传导联系起来,最终赋予植物抗逆性,Kim等[12]在拟南芥及水稻上的研究表明,外源乙酸对逆境胁迫的缓解作用与茉莉酸(JA)信号途径有关,通过促进JA合成和组蛋白H4乙酰化的富集,从而影响JA信号通路对植物耐逆境性能的启动。这种新颖的醋酸功能在进化上是保守的,是植物作为抵抗环境变化的生存策略。
由于乙酸同时具有的酸性特征,最直接的考虑是其在盐渍化土壤上的应用,前期调查发现,黄河三角洲盐渍化土壤上栽培的葡萄春季发芽晚,生长慢,叶色浅淡。前人研究表明,土壤盐胁迫抑制根系的生长发育,会引起细胞膜质过氧化[20],增大膜通透性,破坏膜结构的完整性,降低根系活力。本研究发现海水胁迫导致葡萄根茎叶中的丙二醛含量均显著升高,根系活力下降,而浇灌乙酸和葡萄酒显著降低了各器官的膜脂过氧化,维持了较高的根系活力,与15%海水处理相比,分别提高了68.4%、56.9%。
盐胁迫对植物光系统活性产生抑制,严重的会发生光破坏,导致植物整株死亡[21]。叶绿素是光合作用的物质基础,其含量的高低在一定程度上反应了光合作用的强弱[22],盐胁迫对叶绿素含量的影响因植物种类、盐类型、处理浓度等因素的不同而异,其分别表现为下降[23]、升高[24]或先升高后降低的变化趋势[25-26]。本研究表明,15%海水胁迫显著降低了叶绿素的含量,可能因为海水胁迫下植物细胞色素系统遭到破坏,叶绿素酶活性提高,从而加速了叶绿素的降解[27-28]。当植物吸收的光能超过所能利用的光能时,过剩光能便会导致光合结构的光抑制,甚至光破坏[29]。本研究中,15%海水处理导致了叶片最大光化学效率(v/m)及P700量子产额(m)的显著降低,而浇灌乙酸及葡萄酒均明显提高了海水胁迫下叶片的荧光参数,缓解了PSII及PSI的光抑制程度[30]。与清水对照相比,15%海水胁迫明显改变了葡萄光系统的光能分配,叶片可调节的能量耗散[Y(NPQ)]显著升高,一方面表明其接受的光强过剩,另一方面说明叶片启动了与高能态淬灭有关的叶黄素循环过程[31-33],但是Y(NPQ)在海水胁迫条件下自身调节能力有限,并不能耗散掉所有的光能,随着盐胁迫时间的延长,过剩光能的比例越来越高,非调节性能量耗散[Y(NO)]逐渐升高,叶片发生严重光抑制[34]。而浇灌乙酸及葡萄酒后,葡萄叶片Y(NPQ)与Y(NO)均维持在降低水平,从而提高了PSII的实际光化学效率[Y(II)]。
不同浓度海水浇灌会引起土壤中及植物体内离子的失衡[35],合理的海水灌溉浓度不会引起土壤理化性状的显著改变[36]。本研究中浇灌15%海水胁迫及用30 mmol·L-1乙酸和2%葡萄酒缓解均未导致土壤pH、土壤容重和土壤含水量的显著变化,表明15%海水处理可能通过离子毒害进行伤害。
本研究中,浇灌乙酸及葡萄酒均显著降低了15%海水胁迫下葡萄各器官的膜脂过氧化程度,缓解了葡萄叶片的光抑制,促进了叶片吸收的光能用于光化学反应的比例,对以后用盐渍化土壤果园残次果酿造的葡萄酒、果酒或者果醋等的综合利用提供了理论支撑。
4 结论
15%海水灌溉导致葡萄根系活力及叶绿素含量下降,加剧膜脂过氧化程度,光系统活性受到了抑制。根灌乙酸及葡萄酒可以显著提高15%海水胁迫下根系活力及叶绿素含量,改善光系统能量分配并提高光能利用效率,从而缓解光抑制程度,提高了葡萄对盐环境的适应性。
[1] 王佳丽, 黄贤金, 钟太洋, 陈志刚. 盐碱地可持续利用研究综述. 地理学报, 2011, 66(5): 673-684.
Wang J L, Huang X J, Zhong T Y, Chen Z G. Review on sustainable utilization of salt-affected land., 2011, 66(5): 673-684. (in Chinese)
[2] 刘建新, 王金成, 王瑞娟, 贾海燕. 盐、碱胁迫对燕麦幼苗光合作用的影响. 干旱地区农业研究, 2015, 33(6): 155-160.
Liu J X, Wang J C, Wang R J, Jia H Y. Effects of salt and alkali stress on photosynthesis inseedlings., 2015, 33(6): 155-160. (in Chinese)
[3] 吴运荣, 林宏伟, 莫肖蓉. 植物抗盐分子机制及作物遗传改良耐盐性的研究进展. 植物生理学报, 2014, 50(11): 1621-1629.
Wu Y R, Lin H W, Mo X R. Research progress in the mechanism of plant salt tolerance and genetic engineering of salt resistant crops., 2014, 50(11): 1621-1629. (in Chinese)
[4] 牛东玲, 王启基.盐碱地治理研究进展. 土壤通报, 2002, 33(6): 449-455.
Niu D L, Wang Q J. Research progress on saline-alkali field control., 2002, 33(6): 449-455. (in Chinese)
[5] Ahmad P, Sharma S. Physio-biochemical attributes in two cultivars of mulberry (L.) under NaHCO3stress., 2010, 4(2): 79-86.
[6] 刘卫国, 丁俊祥, 邹杰, 林喆, 唐立松. NaCl对齿肋赤藓叶肉细胞超微结构的影响. 生态学报, 2016, 36(12): 3556-3563.
Liu W G, Ding J X, Zou J, LIN Z, Tang L s. Ultrastructural responses ofto a gradient of NaCl stress., 2016, 36(12): 3556-3563. (in Chinese)
[7] Kalaji H M, Jajoo A, Oukarroum A, Brestic M, Zivcak M, Samborska I A, Cetner M D, Łukasik I, Goltsev V, Ladle R J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions., 2016, 38: 102.
[8] Cheng T, Chen J, Zhang J, Shi S, Zhou Y, Lu L, Wang P, Jiang Z, Yang J, Zhang S, Shi J. Physiological and proteomic analyses of leaves from the halophytereveals diverse response pathways critical for high salinity tolerance., 2015, 6: 30.
[9] 郭艳超, 王文成, 李克晔, 吴新海, 董文琦, 吴菲. NaCl胁迫对八棱海棠幼苗生长及其生理指标的影响. 中国农学通报, 2011, 27(28): 130-134.
Guo Y C, Wang W C, Li K Y, Wu X H, Dong W Q, Wu F. Effects of NaCl stress on the growth and some physiological indexes ofrehd., 2011, 27(28): 130-134. (in Chinese)
[10] Ding H, Lai J, Wu Q, Zhang S, Chen L, Dai Y S, Wang C, Du J, Xiao S, Yang C. Jasmonate complements the function oflipoxygenase3 in salinity stress response., 2016, 244: 1-7.
[11] Jiang C Q, Cui Q R, Feng K, Xu D F, Li C F, Zheng Q S. Melatonin improves antioxidant capacity and ion homeostasis and enhances salt tolerance in maize seedlings., 2016, 38: 82.
[12] Kim J M, To T K, Matsui A, Tanoi K, Kobayashi N I, Matsuda F, Habu Y, Ogawa D, Sakamoto T, Matsunaga S, Bashir K, Rasheed S, Ando M, Takeda H, Kawaura K, Kusano M, Fukushima A, Endo T A, Kuromori T, Ishida J, Morosawa T, Tanaka M, Torii C, Takebayashi Y, Sakakibara H, Ogihara Y, Saito K, Shinozaki K, Devoto A, Seki M. Acetate-mediated novel survival strategy against drought in plants., 2017, 3: Article number 17097.
[13] 赵世杰, 史国安, 董新纯. 植物生理学实验指导. 北京: 中国农业科学技术出版社, 2015: 55-57, 142-143.
Zhao S J, Shi G A, Dong X C.. Beijing: China Agricultural Science and Technology Press, 2015: 55-57, 142-143. (in Chinese)
[14] Pfündel E, Klughammer C, Schreiber U. Monitoring the effects of reduced PS II antenna size on quantum yields of photosystems I and II using the Dual-PAM-100 measuring system., 2008, 1: 21-24.
[15] 蒋金豹, 陈云浩, 黄文江. 用高光谱微分指数估测条锈病胁迫下小麦冠层叶绿素密度. 光谱学与光谱分析, 2010, 30(8): 2243-2247.
Jiang J B, Chen Y H, Huang W J. Using hyperspectral remote sensing to estimate canopy chlorophyll density of wheat under yellow rust stress., 2010, 30(8): 2243-2247. (in Chinese)
[16] Huang W, Fu P L, Jiang Y J, Zhang J L, Zhang S B, Hu H, Cao K F. Differences in the responses of photosystem I and photosystem II of three tree species,andexposed to a prolonged drought in a tropical limestone forest., 2013, 33(2): 211-220.
[17] 赵鹏志, 陈祥伟, 杨小燕, 齐思明, 王恩姮. 低分子有机酸对东北黑土酶活性与养分关系的影响. 南京林业大学学报(自然科学版), 2018, 42(1): 105-112.
Zhao P Z, Chen X W, YANG X Y, Qi S M, Wang E H. Relationship between enzyme activities and nutrients of black soil subjected to low molecular organic acid., 2018, 42(1): 105-112. (in Chinese)
[18] 丁永祯, 李志安, 邹碧. 土壤低分子量有机酸及其生态功能. 土壤, 2005, 37(3): 243-250.
Ding Y Z, Li Z A, Zou B. Low-molecular-weight organic acids and their ecological roles in soil.,2005, 37(3): 243-250. (in Chinese)
[19] 吴雪, 杨晓婷, 王冰, 王林, 疏伟慧, 张丽丽, 韩玉林. 外源乙酸和EDTA对铜尾矿矿砂中芦苇幼苗生长及部分金属元素积累的影响. 植物资源与环境学报, 2011, 20(4): 29-34.
Wu X, Yang X T, Wang B, Wang L, Shu W H, Zhang L L, Han Y L. Effects of exogenous acetic acid and EDTA on growth and accumulation of some metal elements ofseedling in copper tailing ore., 2011, 20(4): 29-34. (in Chinese)
[20] 刘建新, 王鑫, 王瑞娟, 李东波. 碱胁迫对黑麦草幼苗根系活性氧代谢和渗透溶质积累的影响. 植物研究, 2011, 31(6): 674-679.
Liu J X, Wang X, Wang R J, LI D B. Effects of alkaline stress on the metabolism of reactive oxygen species and osmotic accumulation inryegrass seedling roots., 2011, 31(6): 674-679. (in Chinese)
[21] 王振兴, 吕海燕, 秦红艳, 赵滢, 刘迎雪, 艾军, 曹建冉, 杨义明, 沈育杰. 盐碱胁迫对山葡萄光合特性及生长发育的影响. 西北植物学报, 2017, 37(2): 339-345.
Wang Z X, Lü H Y, Qin H Y, Zhao Y, Liu Y X, Ai J, Cao J R, YAng Y M, Shen Y J. Photosynthetic characteristics and growth development of Amur grape (Rupr.) under saline- alkali stress., 2017, 37(2): 339-345. (in Chinese)
[22] 王伟华, 张希明, 闫海龙, 梁少民, 杨小林. 盐处理对多枝怪柳光合作用和渗调物质的影响. 干旱区研究, 2009, 26(4): 561-568.
Wang w h, Zhang x m, Yan h l, Liang S m, Yang X l. Effects of salt stress on photosynthesis and osmoregulation substance ofLedeb., 2009, 26(4): 561-568. (in Chinese)
[23] 王斌. NaCl和Na2SO4胁迫下沼泽小叶桦的生理响应. 南京林业大学学报 (自然科学版), 2013, 37(1): 132-136.
Wang B. Effects of NaCl and Na2SO4stress on physiological characteristics in., 2013, 37(1): 132-136. (in Chinese)
[24] 范希峰, 侯新村, 朱毅, 武菊英. 盐胁迫对柳枝稷苗期生长和生理特性的影响. 应用生态学报, 2012, 23(6): 1476-1480.
Fan x F, Hou x c, Zhu y, Wu J y. Impacts of salt stress on the growth and physiological characteristics ofseedlings., 2012, 23(6): 1476-1480. (in Chinese)
[25] 李宏, 邓江宇, 张振春, 杨森, 曹林. 盐胁迫对盐桦幼树光合特性的影响. 新疆农业科学, 2010, 47(2): 213-217.
Li h, Deng j y, Zhang z c, Yang S, Cao L. Influence of salt stress on physiological adaptability of youngtrees., 2010, 47(2): 213-217. (in Chinese)
[26] 徐静, 董宽虎, 高文俊, 谢开云. NaCl和Na2SO4胁迫下冰草幼苗的生长及生理响应. 中国草地学报, 2011, 33(1): 36-41.
Xu J, Dong K h, Gao W j, Xie K y. Growth and physiological responses ofseedlings under NaCl and Na2SO4stress., 2011, 33(1): 36-41. (in Chinese)
[27] Santos C V. Regulation of chlorophyll biosynthesis and degradation by salt stress in sunflower leaves.2004, 103(1): 93-99.
[28] 王斌, 巨波, 赵慧娟, 张群, 朱义, 崔心红. 不同盐梯度处理下沼泽小叶桦的生理特征及叶片结构研究. 林业科学, 2011, 47(10): 29-36.
Wang B, Ju B, Zhao H j, Zhang Q, ZHU Y, Cui X H. Photosynthetic performance and variation in leaf anatomic structure ofvar.under different saline conditions., 2011, 47(10): 29-36. (in Chinese)
[29] Reinbothe S, Reinbothe C. The regulating of enzymes involved in chlorophyll biosynthesis., 1996, 237(2): 323-343.
[30] Huang W, Yang Y J, Zhang J L, Hu H, Zhang S B. Superoxide generated in the chloroplast stroma causes photoinhibition of photosystem I in the shade-establishing tree species., 2017, 132(3): 293-303.
[31] Giimore A M. Mechanistic aspects of xanthophyll cycle-dependent photoprotection in higher plant chloroplasts and leaves., 1997, 99: 197-209.
[32] Niyogi K K, Grossman A R, Björkman O.mutants define a central role for the xanthophyll cycle in the regulation of photosynthetic energy conversion., 1998, 10(7): 1121-1134.
[33] Yamamoto H Y, Bugos R C, Hieber A D. Biochemistry and molecular biology of the xanthophyll cycle//.Springer. Dordrecht, 1999: 293-303.
[34] Horton P, Hague A. Studies on the induction of chlorophyll fluorescence in isolated barley protoplasts. IV. Resolution of non-photochemical quenching.-, 1988, 932: 107-115.
[35] 寇伟锋, 刘兆普, 郑宏伟. 海水胁迫对向日葵苗期生长及矿质营养吸收特性的影响. 生态学杂志, 2006, 25(5): 521-525.
Kou W f, Liu Z p, Zheng H w. Effects of sea water stress onL. seedlings growth and mineral nutrition., 2006, 25(5): 521-525. (in Chinese)
[36] 王杨. 长期海水灌溉对赤霞珠果实品质及土壤性质的影响[D]. 泰安: 山东农业大学, 2016.
Wang Y. The effect of long term seawater irrigation on the quality of Cabernet Sauvignon fruit and soil properties[D]. Taian: Shandong Agricultural University, 2016. (in Chinese)
Effect of root irrigationof acetic acid and wine on photoinhibition of grape under seawater stress
WANG Hui, GAO Yulu, YU Meng, DU Yuanpeng, SUN Yongjiang, ZHAI Heng
(College of Horticultural Science and Engineering, Shandong Agricultural University/State Key Laboratory of Crop Biology, Taian 271018, Shandong)
【Objective】In order to expand the effective utilization of salinized soil and improve the quality of grape growth and development in salinized soil, the effects of acetic acid and wine on leaf photoinhibition under 15% seawater stress were studied. The result will provide a theoretical basis and technical reference for the improvement of grape cultivation techniques in salinized soil. 【Method】One-year old potted ‘Moldova’ seedlings were grown in plastic pots (with a diameter of 20 cm, and a height of 18 cm). The ratio of soil to substrate in each pot was 1﹕1 and the pots were placed in greenhouse. the culture conditions of the greenhouse were as follows: the average daily temperature was about 25℃, the light transmittance was more than 50% of natural light intensity, the maximum light intensity was 800 μmol·m-2·s-1, the relative humidity was 40%-60%, and the normal fertilizer and water was managed until 8-10 pieces of fully expanded leaves, then the seawater and exogenous acetic acid were used. The plants were irrigated with 15% seawater, 30 mmol·L-1exogenous acetic acid and 2% wine at the same time, each treatment repeated 5 times, the controls were treated with clear water. each treatment was irrigated every 2 days and 3 times, the irrigation amount was 2 times of water holding capacity, and about 1/3 of the solution leaked out, chlorophyll fluorescence indicators were determined on the 2nd day of irrigation, after 10 days treatment, the effects of the two methods on malondialdehyde (MDA) content, chlorophyll content, and root activity under seawater stress were analyzed.【Result】15% seawater treatment significantly decreased the chlorophyll content of leaf and root activity, but increased MDA content of root, stem and leaf. Compared with clear water control, the chlorophyll content decreased by 18.5% and root activity decreased by 41.9%. MDA content of root, stem and leaf increased by 1.10, 0.27 and 0.41 times, respectively. However, 30 mmol·L-1acetic acid and 2% wine treatments significantly decreased the MDA content in grape. the MDA content in root, stem and leaf of grape treated with acetic acid decreased by 29.3%, 20.6% and 15.8%, respectively, compared with that of seawater stress, and the MDA content in root, stem and leaf of grape treated with wine decreased by 29.4%, 20.2% and 25.2%, respectively, compared with that of seawater stress. Irrigated with acetic acid and wine significantly increased the leaf chlorophyll content and root activity. Compared with seawater stress treatment, the root activity increased by 68.4% and 56.9%, and the chlorophyll content increased by 18.8% and 20.3%, respectively.The analysis of chlorophyll fluorescence showed that 15% seawater treatment decreased the levels of photosystem II (PSII) maximum photochemical efficiency (v/m) and the maximum photo-oxidizable P700 (m). Them on the 3rd, 6th, and 9th day decreased by 5.0%, 9.6% and 13.0% compared with the control, the levels of photochemical quenching coefficient (qP) and PSII actual photochemical efficiency (YII) decreased gradually, but the quantum yield of regulated energy dissipation Y (NPQ) and the quantum yield of non-regulated energy dissipation Y (NO) increased gradually. The nonphotochemical quantum yield due to PSI donor side limitation Y (ND) changed little, but the nonphotochemical quantum yield due to PSI acceptor side limitation Y (NA) increased gradually, resulting in the decrease of PSI actual photochemical efficiency Y (I). The irrigation of 30 mmol·L-1acetic acid and 2% wine significantly relieved the stress of 15% seawater. The actual photochemical efficiency of PSI and PSII increased significantly, meanwhile the energy dissipated through heat decreased, then enhanced the efficiency of light energy utilization of grape leaves, in which 2% wine treatment showed a more obvious alleviated effect.【Conclusion】Irrigation of acetic acid and wine significantly improved root activity and chlorophyll content, which alleviated the photoinhibition level and improved the adaptability of grape to saline environment. The results provided a theoretical basis for the use of extract of wine lees to improvement of coastal saline soil.
grape; seawater; photoinhibition; acetic acid; wine; chlorophyll fluorescence
10.3864/j.issn.0578-1752.2018.21.019
2018-05-07;
2018-08-01
国家现代农业产业技术体系建设专项(CARS-29-zp-2)、山东省重大科技创新工程(2018CXG0306)、山东省“双一流”建设奖补资金(SYL2017YSTD10)
王辉,E-mail:1924412287@qq.com。通信作者孙永江,Tel:0538-8241335;E-mail:sunhyongjiang12@163.com。通信作者翟衡,Tel:0538-8241335;E-mail:zhaih@sdau.edu.cn
(责任编辑 岳梅)

