Treatment of coronary in-stent restenosis: a systematic review
Leos Pleva, Pavel Kukla, Ota Hlinomaz
1Department of Cardiovascular Diseases, University Hospital Ostrava, Czech Republic
2Department of Cardioangiology, St. Anne's University Hospital and Faculty of Medicine, Masaryk University, Brno, CZ, Czech Republic
3International Clinical Research Center, St. Anne's University Hospital, Brno, CZ, Czech Republic
1 Introduction
Coronary stent implantation has significantly improved percutaneous coronary intervention (PCI) and enabled the management of early complications of plain balloon angioplasty (POBA). By preventing elastic recoil and constrictive remodeling, coronary stent implantation decreases the frequency of restenosis after PCI. However, a new complication has accompanied these improvements: in-stent restenosis (ISR) arising from neointimal hyperplasia. The clinical incidence of ISR after bare-metal stent (BMS) implantation is approximately 20%–35%. The use of drug-eluting stents(DES) has led to a further decrease in the occurrence of ISR to 5%–10%.[1,2]ISR after coronary angioplasty is currently one of the main limitations of this method, leading to the recurrence of exertional angina pectoris or acute coronary syndromes.[1,2]In this manuscript, we undertook a systematic review of the pathophysiology, diagnostics and treatment options for BMS- and DES-ISR.
Restenosis is defined as the repeated narrowing of the dilated segment of a coronary artery. Angiographic restenosis is defined as the ≥ 50% narrowing of the artery’s diameter during a subsequent coronary angiography. Restenosis resulting in the recurrence of clinical manifestations of ischemia is called “clinical restenosis”, which is usually associated with the necessity to repeat target lesion or vessel revascularisation (TLR/TVR). In-stent restenosis is a restenosis in an implanted coronary stent; if we evaluate not only the area of the stent, but the whole affected segment of the vessel, we refer to it as in-segment restenosis (+5 mm from the proximal and distal edges of the stent).[3]
The generally used Mehran’s angiographic classification divides ISR into four types: I-focal; II-diffuse; III-proliferative and IV-occlusive (Table 1).[3,4]
2 Drug-eluting stents restenosis
DES enable the local release of antiproliferative agents[paclitaxel or “limes” drug group (sirolimus, everolimus,zotarolimus, biolimus, etc)], which prevent excessive neointimal hyperplasia after stent implantation and lead to a reduction in the occurrence of ISR.[5]
While initial DES clinical trials reported near undetectable rates of ISR following DES implantation in short-termfollow-up, both long-term follow-up as well as “real-world”applications of DES in complex lesions have determined that the incidence of DES-ISR (‘DES failure’) is about 5%-10% (Table 2).[6–15]

Table 1. Mehran’s angiographic classification of ISR.
3 Patophysiology
Vascular injury sustained during PCI and stent implantation results in a complex inflammatory and reparative process. The acute vascular reaction is characterized by early deposition of platelets and fibrin and adhesion of circulating neutrophils and monocytes to the injured vessel surface.Over weeks, acute inflammatory cells are replaced by chronic inflammatory cells (macrophages and giant cells).In addition to this inflammatory response, platelet- and leukocyte-related growth factors drive further vascular smooth muscle cells (VSMCs) proliferation and migration from the media to the nascent neointima and subsequent extracellular matrix formation. Two weeks following stent implantation,a complete neointimal layer, composed of VSMCs and a proteoglycan-rich extracellular matrix, can be observed above stent struts. The extracellular matrix is then covered from the luminal side by endothelial cells.[1–3]In BMS-ISR,the neointimal layer consists predominantly of VMSC surrounded by smaller amounts of extracellular matrix and a diffuse pattern of ISR is typical.[2,3,16]Peak BMS-ISR is observed at 3–6 months and remains relatively stable beyond one year.[1,2]
DES implantation results in delayed vessel wall healing,characterised by the presence of chronic fibrin deposits,incomplete neoendothelization and long inflammatory changes.[5]While fibrin deposits are replaced early on by neointimal tissue after BMS implantation, resulting in complete neoendothelisation within 3–6 months, DES implantation is associated with persistent fibrin deposits, a chronic inflammatory process and incomplete neoendothelisation for upto 48 months, accompanied by an associated risk of late stent thrombosis. Late vascular response after DES implantation is influenced further by the biocompatibility of the individual components of the stent, particularly the polymeric coating, which serves as a carrier and permits the controlled release of the active substance. This coating may cause persistent chronic inflammatory response in the vascular wall,leading to delayed healing and neointimal formation. In some cases, a non-specific acute inflammatory response may switch into a specific hypersensitivity reaction to polymer through the activation of eosinophils and T-lymphocytes.[5]
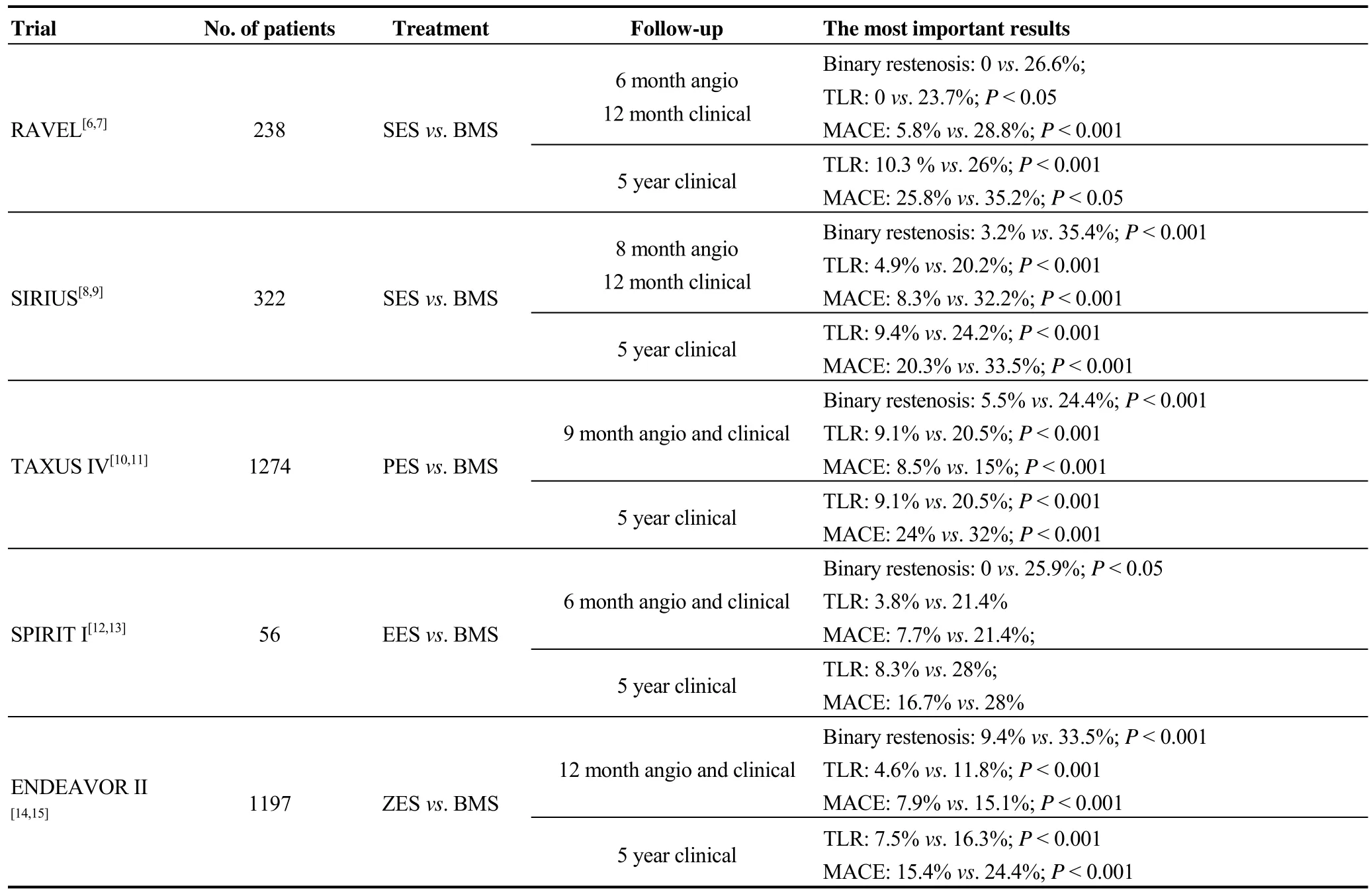
Table 2. Comparisons of BMS vs. DES trials in de novo lesions.
The stent polymer facilitates controlled elution of antiproliferative agents over a variable period of time. Importantly, the durable polymer (DP) serves no function once drug elution has been completed and, consequently, it may be associated with inflammation, delayed healing, incomplete endothelialization or accelerating neoatherosclerosis which may contribute to the risk of late device failure compared with BMS. Contrary, biodegradable polymers (BP)may facilitate stent healing, thus enhancing clinical safety.The common BPs with therapeutic uses include polylactic acid, poly (lactic-co-glycolic acid) and poly (D,L-lactide).However, according to a recently published meta-analysis the safety and efficacy of BP-DES was similar as that of second-generation DP-DES [cardiovascular (CV) death,myocardial infarction (MI), TVR or late stent thrombosis;P= NS for both].[17]
Compared to BMS-ISR, DES-ISR appears later; its presence can be felt from 6–9 months with a further increase up to the second year after implantation. The neointimal tissue consists mainly of an extracellular matrix with a minimum of VSMCs and the focal character of ISR lesions is typical, especially for sirolimus eluting stents restenosis(SES-ISR).[3,5,16]
4 Neoatherosclerosis
The neointimal layer on implanted stents may suffer from recurrent atherosclerotic changes, i.e., neoatherosclerosis. It appears that one of the predominant mechanisms involved in this process might be an incomplete regeneration of the endothelium leading to excessive uptake of circulating lipids and accelerated development of atherosclerotic plaques in the nascent neointima. Intimal thickening,intracellular lipid deposition with thin fibro-atheroma cap or the presence of necrotic tissue have been detected using histopathological or optical coherence tomography (OCT)evaluations.[18]
Nakazawa,et al.[18]found in their histopathological authopsy study that neoatherosclerosis as a result of persistent endothelial dysfunction and incomplete neoendothelisation occurs more frequently (31%vs. 16%;P< 0.001) and earlier (median: 420vs. 2160 days;P< 0.001) following DES than BMS implantation. Moreover, neoatherosclerosis in DES shows unstable characteristics (Thin-Cap Fibroatheromas or plaque rupture) earlier (about two years) after implantation, whereas similar features in BMS occur relatively later (about six years). Independent predictors of neoatherosclerosis included younger age, longer implant durations, sirolimus-eluting stent (SES) or paclitaxel-eluting stent(PES) implantation and underlying unstable plaques.[18]It is believed that neoatherosclerosis, along with the rupturing of the thin fibro-atheroma plaque, are among the predominant causes of late stent failure (i.e., delayed ISR and late or very late stent thrombosis) which could manifest as acute coronary syndromes.[18]
5 ISR risk factors
BMS-ISR risk factors can be divided into patient-, lesion- or peri-procedural risk factors (Table 3).[1,3,19]Kastrati,et al.[20]found lesion and periprocedural characteristics as predictive factors for ISR. Complex lesions (B2/C), restenosis, vessel size < 3 mm, stented segment > 15 mm and particular types of BMS are associated with an occurrence of ISR. The strongest risk factor seems to be a small vessel size, with a 79% increase in the risk for a vessel of 2.7 mm versus a vessel of 3.4 mm in diameter. Differences in the incidence of ISR between different types of BMS may vary between 20% and 50%,[3,20]with significantly higher incidence in stents with thick struts compared to the newer stents with a thinner strut.[21,22]
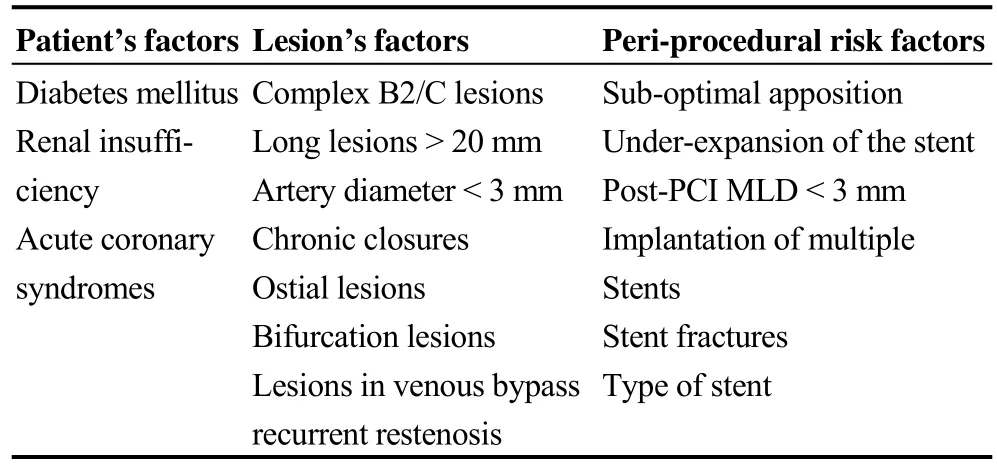
Table 3. ISR risk factors.
The most important ISR risk factors seem to be diabetes mellitus, the length of the lesion and small vessel diameter.In a multivariate regression analysis, the main BMS-ISR risk factors are diabetes mellitus (OR: 1.86), implantation of multiple stents (OR: 1.81) and a post-procedural minimum lumen diameter < 3 mm (OR: 1.81).[7]Diabetes itself increases the risk of BMS-ISR by 30%-50%.[23–25]Similarly,the risk of DES-ISR is increased in diabetic patients when compared to those without diabetes.[1,26]The length of the lesion and vessel diameter pose other ISR risk factors independent of the presence of diabetes,[1,23,26]i.e., an implanted stent length > 35 mm is associated with almost double the ISR risk compared to < 20 mm stents.[1,27]
The major risk factors for recurrent ISR are diabetes mellitus, previous ISR and type according to Mehran’s classification (with an increase from I to IV).[3,4]
6 Biochemical and genetic risk factors
In addition to these known ISR risk factors, more biochemical or genetic factors are searched for that might contribute to the development of ISR. Mainly higher plasma levels of matrix metalloproteinases (MMPs), the proteolytic enzymes that degrade the extracellular matrix (ECM) and facilitate the proliferation and migration of endothelial and VSMCs, seem to be associated with a higher risk of in-stent restenosis.[28–30]
Many genome-wide association studies have observed single-nucleotide polymorphisms (SNPs) in individual genes,which could affect the occurrence of ISR, albeit with only a limited reach to clinical practice. One relatively large study was the GENetic DEterminants of Restenosis (GENDER)project, which examined 3100 patients with over 300 ISR cases after BMS implantation.[31]The association between some SNPs inAGTR, GPX1, KAT2B, MMP12, FGBandVDRgenes and an increased risk of ISR was found.[32]
7 Diagnostics
7.1 Selective coronarography (SCG)
SCG is the standard diagnostic tool for the assessment of restenosis. Restenosis is arbitrarily defined as the repeated narrowing of the vessel lumen diameter with a cut-off value of ≥ 50%, known as “binary restenosis”.[3]However, neointimal hyperplasia, leading to re-narrowing of the lumen,represents a continuous process. Therefore, continuous variables are used to evaluate restenosis: the minimal lumen diameter (MLD), the percentage of diameter stenosis (DS%)and/or late lumen loss (LLL), representing the difference between post-procedural MLD and MLD at control coronary angiography. The use of these so-called “angiographic surrogate end-points” helps, reduce the required cohort size while maintaining adequate statistical force.[33,34]A correlation, especially between LLL and DS%, and the incidence of angiographic and clinical restenosis, is proven.[3,33]Mauri,et al.[35]found a correlation between the LLL and the incidence of binary restenosis; LLL from 0.2 to 0.4 mm was associated with a 3.1% incidence of binary restenosis, as opposed to LLL of 0.4 to 0.6 mm, where the occurrence of ISRs increased to 6.4%. Similarly, Pocock,et al.[33]showed that LLL and DS% are able to predict with high accuracy the subsequent (TLR) after BMS/DES implantation (correlation coefficient about 0.90), wherein the predictive value of DS% does not depend on the artery’s diameter.
7.2 Intravascular ultrasound (IVUS)
IVUS allows for a detailed display of the stented segment during an assessment of the cross-section of individual vessel wall layers. It is able to exclude possible mechanical causes of ISR (under-expansion, stent fracture, etc.) and provide detailed information on the extent of neointimal hyperplasia.[36]Although stent implantation under IVUS control is not routinely recommended, one of the IVUS risk factors of angiographic and clinical restenosis is the resulting minimal lumen area of the stented segment (minimal stent area, MSA). MSA increases by 1 mm2are associated with a 20% decrease in BMS-ISR.[3,37]Post-procedural MSA > 5 mm2with SES and > 6.5 mm2with BMS is a predictor of a satisfactory minimal lumen area (> 4 mm2) in the follow-up.[5,37,38]
7.3 Optical coherence tomography
OCT uses beam deflection with a frequency near to infrared light. This way, it achieves a significantly higher resolution compared to IVUS and allows for a more detailed assessment of stented segment.[3,5]Gonzalo,et al.[39]differentiate restenotic tissues according to their OCT images into the following subgroups: homogeneous, heterogeneous and stratified (layered) as well as low and high backscatter, whilst they also assess micro-vascularisation, lumen shape and the presence of intraluminal tissue. Diffuse ISR is characterised by a layered structure of neointimal tissue with high scattering, while focal lesions contain heterogeneous, low-scatter tissue.
7.4 Multi-slice CT (MS-CT) coronarography
64- and more-slice MS-CT have been shown to permit the detection of coronary artery stenoses in the native coronary arteries with sensitivities and specificities up to 99%,but the visualization of the lumen within coronary artery stents by MS-CT is more challenging.[40]A major issue with assessment of metallic stents involves “metal-blooming”artifact (blooming of stent struts)such that stents appear larger than they actually are or “beam-hardening” artifacts resulting in artificial luminal narrowing and decreased intraluminal attenuation values.[41,42]These artifacts are more significant in stents < 3.0 mm in diameter.[41,42]A method to determine coronary in-stent restenosis was based mainly on contrast attenuation inside the stent lumen. In-stent restenosis was considered if the vessel distal to the stent was not visualized (occlusion) or massive low-density area (or filling defects) inside the stent lumen was detected visually when compared with the reference vessel.[43]In meta-analyses, the sensitivity and specificity of MS-CT in detection of ISR achieved 90% and 91%, with positive and negative predictive value of 68% and 98%, respectively.[44,45]
8 Clinical presentation of ISR
Since ISR is based on gradually increasing neointimal hyperplasia, in-stent restenosis used to be considered a relatively benign process leading to recurrent exertional angina.However, there is evidence that 30%–60% of ISR can manifest as acute coronary syndromes, mostly as unstable angina pectoris or non-ST elevation acute myocardial infarction (UAP/NSTEMI).[3,5]
In the clinical database APPROACH, ISR manifested in 52.2% of cases as UAP/NSTEMI, in 18.5% as STEMI and only in 25.3% as stable AP.[46]It is believed that ISR manifestation as an acute coronary syndrome could be caused by neoatherosclerosis and thin fibro-atheroma plaque rupture;this condition as well as delayed neoendothelisation may cause late and very late DES stent thrombosis. The cause of stent occlusions may not be completely clear in the angiographic image. The behaviour of the lesion during the procedure (thromboaspiration, residual restenosis, etc) and imaging using IVUS or OCT may be helpful in differentiation between occluding neointimal hyperplasia and thrombotic occlusions due to neo-atherosclerotic changes or incomplete stent struts endothelisation.[3,5]
The Mehran’s morphological character of ISR is a predictor of clinical events, with the necessity of repeated TVR between groups I-IV in 19%, 35%, 50% and 83% of cases,respectively (P< 0.001).[3,4]
9 Treatment of ISR
9.1 Bare-metal stent restenosis (BMS)
Repeated POBA or BMS implantation was associated with a high (nearly 40%) recurrence of binary restenosis,[47]cutting balloon dilatation did not reveal any significant benefit[48]and rotational atherectomy even led to outcomes inferior to POBA.[49]Brachytherapy has also been abandoned.
Current treatment for in-stent restenosis is based on DES.A drug released locally from the stent prevents new neointimal hyperplasia.[1,19]This treatment was established in the SISR and TAXUS V ISR trials, which compared the implantation of DES to relatively complicated brachytherapy.
教育部《关于全面提高高等职业教育教学质量的若干意见》指出,高等职业院校要坚持育人为本,德育为先,把立德树人作为根本任务。为了让每名护理教师都热爱自己的职业,有高度的事业心和奉献精神,对学生有强烈的爱心,必须加强教师职业道德建设。
In the SISR trial, the use of SES led to a significantly better angiographic outcomes and a trend to lower occurrence of repeated binary restenosis.[50]
The TAXUS V ISR trial found a significant decrease in TVR, repeated binary restenosis rate and also better clinical outcomes [major adverse cardiac events (MACE): CV death,MI or TVR] with the use of PES.[51]
The ISAR-DESIRE and RIBS II trials compared BMSISR treatment with DES implantation versus POBA. In the ISAR-DESIRE trial, implantation of SES or PES led to a significant decrease in repeated binary restenosis and TVR compared to POBA, whereas the direct comparison of both types of DES revealed a trend toward better outcomes in favor of SES.[52]Similarly, the RIBS II study revealed a significant decrease in restenosis and TVR after SES implantation.[53]
In contrast to DES, DEB catheters allow short-term passage of the active substance into the vascular wall, preventing hyperproliferation of VSMCs. Due to the short duration of the effect, DEB do not affect endothelial progenitor cells and stent neoendothelialization so much.[54–56]
Paclitaxel is used in the clinical practice as an effective antiproliferative agent in the case of DEB. Clinical data on the use of zotarolimus are also available.[57]Paclitaxel is highly lipophilic and rapidly penetrates into the tissues. Its concentrations used have stabilized at 3 μg/mm2.[55,56]
The main factor influencing the efficacy of paclitaxel-eluting balloon catheters (PEB) is the method of paclitaxel binding on the surface of the balloon catheter. Paclitaxel can be freely applied directly to the roughened surface of the balloon catheter (first generation DIOR®; Eurocor, Bonn, Germany) or is bound through the carrier, which affects its solubility and penetration through the vessel wall.In the original concept of Scheller,et al.,[55]paclitaxel was bound via iopromide, a hydrophilic contrast agent, which increased its solubility and penetration of the vascular wall(Paccocath). This method of preparation is used in modified form in PEB Sequent®Please (B.Braun, Melsulgen, Germany). In preclinical studies, iopromide-coated PEB showed a significantly better angiographic outcomes than PEB without coating. In contrast, uncoated PEB failed to demonstrate a any benefit compared to POBA.[58]
Many other PEBs are currently used in clinical practice: DIOR®II (shellac-coated; Eurocor, Bonn, Germany),IN.PACT™ Falcon [urea-coated;Medtronic, Minneapolis,USA), Pantera™ Lux (BTHC-coated (butyryl-tri-hexyl citrate); Biotronik, Berlin, Germany] and others.
The use of PEB in the treatment of ISR brings some benefits: compared to DES, PEBs allow homogeneous distribution of anti-proliferative treatment into the vessel wall with rapid achievement of an effective concentration; absence of polymers reduces chronic inflammatory response and the risk of subsequent late thrombosis; faster neoendothelization allows shorter dual antiplatelet therapy and there is no risk of the occlusion of side branches with another layer of metallic struts.[59]
The second generation DES releasing derivatives of sirolimus (everolimus, etc.) has higher efficacy and safety in the treatment of de novo lesions.[63]However, the Xience V US registry revealed significantly more target vessel failure(TVF; CV death, MI or TVR) after everolimus-eluting stent(EES) implantation in patients with ISR compared to those with non-ISR lesions.[64]
In several registries and observational trials, EES have been demonstrated to have at least the same angiographic and clinical outcomes in the treatment of BMS ISR as the first generation DES (PES/SES).[65,66]
In the recently published RIBS V trial, patients with BMS ISR were treated with PEB or EES (cobalt-chrome metallic platform). EES group had significantly higher 9-month MLD and lower DS%, however, there were not found any significant difference in LLL, repeated binary restenosis or TVR.[67]
Contrary to RIBS V, our TIS trial comparing iopromide-coated PEB and EES with a platinum-chromium metallic platform in the treatment of BMS-ISR demonstrated significantly lower 12-month LLL in the PEB group. The between-group differences in the incidence of repeated binary restenosis and 12-month MACE were also not significant.[68]
9.2 Restenosis in DES
In comparison with BMS-ISR, the treatment of DES-ISR is associated with worse long-term outcomes.[19,69]There is no clear consensus on whether the use of a different DES(hetero-DES) or a DES with a similar active substance(homo-DES) would be more beneficial.[19]The ISAR-DESIRE 2 trial found no angiographic or clinical differences in the treatment of SES-ISR using another SES or switching to PES.[70]
The prospective RIBS III registry compared the recommended strategy (hetero-DES, 75% of patients) to the control group (homo-DES, POBA, BMS). The hetero-DES group achieved a significantly better angiographic (higher MLD and fewer repeated binary restenosis) and clinical outcomes (MACE) compared to the disparate control group;whereas the direct comparison of hetero-DES and homo-DES subgroups revealed better outcomes in favor of hetero-DES.[71]
Similarly, the usage of PEB in the DES-ISR treatment was studied. The PEPCAD-DES trial compared the treatment of SES/PES-ISR using iopromide-coated PEB with POBA,and the use of PEB was associated with significantly better angiographic (lower LLL and repeated binary restenosis)and clinical end-points (MACE + stent thrombosis).[72]
In the PEPCAD ISR China trial, iopromide-coated PEB proved to be at least as effective as PES in the treatment of DES-ISR.[73]Habara,et al.[74]demonstrated better angiographic (lower LLL and repeated binary restenosis) and clinical outcomes (TVF) in patients with BMS/DES-ISR treated with iopromide-coated PEB compared to POBA. In the PEB group, significantly better results were achieved in the case of BMS-ISR compared to DES-ISR.
In the ISAR-DESIRE III study, the use of PEB was non-inferior to PES and either PEB or PES were superior to POBA alone in the treatment of SES-ISR, regarding to the primary angiographic end-point (follow-up residual %DS).[75]In the SeQuent Please World Wide Registry, PEB has been used predominantly for the treatment of ISR. Better 9-month clinical oucomes (TLR and MACE) were reported in patients with BMS- than DES-ISR.[76]
In the Valentine prospective study, patients with BMS/DES-ISR were treated with shellac-coated PEB; after 6 to 9 months, the overall incidence of TVR and MACE achieved 8.6% and 11.1%. In a sub-analysis of patients treated for DES-ISR, a significantly better clinical outcomes (MACE and TVR) was recorded in patients with PES-ISR compared to patients with SES-ISR.[77,78]
Naganuma,et al.[79]did not find any significant clinical difference (TVR and MACE) between urea-coated PEB and EES groups in the treatment of bifurcation BMS/DES-ISR.
9.3 New DES and different PEBs
New DES composed of biodegradable polymers, polymer free or containing novel antiproliferative drugs (e.g.,zotarolimus, biolimus) promise better biocompatibility. They have been tested mostly in the treatment of de-novo lesions.[17,80]However, the randomised comparisons of biodegradable and durable polymer DES in the treatment of ISR are not available.
A recently published RESTENT-ISR study, comparing EES and zotarolimus-eluting stents (ZES) used for DES-ISR treatment, did not find any significant difference between both groups.[81]
The efficacy of individual PEBs is not identical, as it is markedly influenced by the applied coating. Nijhoff,et al.[82]found a significantly better angiographic, fractional flow reserve and OCT outcomes in the urea-coated PEB compared to shellac-coated PEB used for treatment of BMS/DES-ISR; however, only a trend towards lower TLR was observed.
In our registry, comparing different PEBs in the treatment of BMS-ISR, a seal-wing PEB was associated with significantly worse angiographic (higher MLD, lower LLL and repeated binary restenosis) and clinical outcomes (TVR and MACE) compared to iopromide-coated PEB.[83]
Only in the Düsseldorf DCB registry, patients threated for ISR, using BTHC-coated PEB, showed significantly better clinical outcomes compared to iopromide-coated PEB.[84]
In summary, both DES and DEB represent effective treatment of ISR. In comparison with BMS-ISR, DES-ISR treatment is associated with worse long-term outcomes.Among different PEBs, the best results are achieved with iopromide-coated ones.Resultsof the most important ISR trials are listed in Table 4.
9.4 Bioresorbable vascular scaffold restenosis (ScR)
One of the options employed to avoid the implantation of multiple metallic layers of stents into ISR (so called “onion skin”) could be the use of bioresorbable vascular scaffolds(BVS), which, compared with DEB, are able to achieve greater acute gain, prevent restenotic tissue prolapse and cover any edge dissection.
Jamshidi,et al.[85]proved the 100% peri-procedural success rate of everolimus-eluting BVS implantation in ISR lesions (96% in DES-ISR), which was associated with 12.2% repeated TVR at the 12-month follow-up.
In an Italian registry involving 127 patients with BMS/DES-ISR treated with BVS, 12-month target lesion failure(TLF) was 9.1% (6.4% in BMS-ISR and 10.9% in DESISR). Repeated TLR due to recurrence of restenosis in BVS(scaffold restenosis; ScR) was necessary in 6.3% of cases.[86]
In most studies with everolimus-BVS used in the treatment of de-novo lesions, the primary endpoint was TLF and the incidence of scafold restenosis was not specified; however, the main issue of BVS seems to be a late scaffold thrombosis.[87]In the GHOST-EU registry, 6-month TLF was 4.4%, 82% of which (3.6% of cases in total) arising due to ScR.[88]The optimal treatment of ScR is unknown. In this registry, ScR was treated using PEB (in 43% of cases), DES(36%), POBA with NC post-dilatation (14%), and on one occasion a further BVS was implanted (7%).[88]
10 Clinical implications
Despite the risk of ISR, revascularization may reduce the absolute and relative risk of cardiac death more than medical therapy in patients with moderate-large amounts of stress induced myocardial ischemia. The degree of stressinduced ischemia and left ventricle ejection fraction (LVEF)predict the effect of revascularization on outcomes in patients with coronary artery disease. In patients with poststress LVEF ≤ 45%, the survival benefit of revascularization was seen even in the absence of stress-induced ischemia.Contrary, in patients with post-stress LVEF > 45%, the survival benefit was depended on the presence of stress-induced ischemia.[89]
Despite the higher initial costs, the cost-effectiveness of the second generation DES implantations have been proved in recent meta analyses. The cost-reduction in the long term was primarily due to avoidance of secondary revascularisations and absence of myocardial infarction.[90]
11 Conclusions
Although the widespread usage of DES has reduced the incidence of ISR, this issue remains currently one of the main limitations of coronary interventions. Evidence resulting from controlled clinical trials suggests that DES and DEB provide the best angiographic and clinical outcomes in the treatment of ISR. However, new eluting balloons or stents(new antiproliferative drugs, biodegradable polymers or polymer-free etc) are rapidly evolving. Further studies are required to identify their potential benefit in the treatment of ISR.
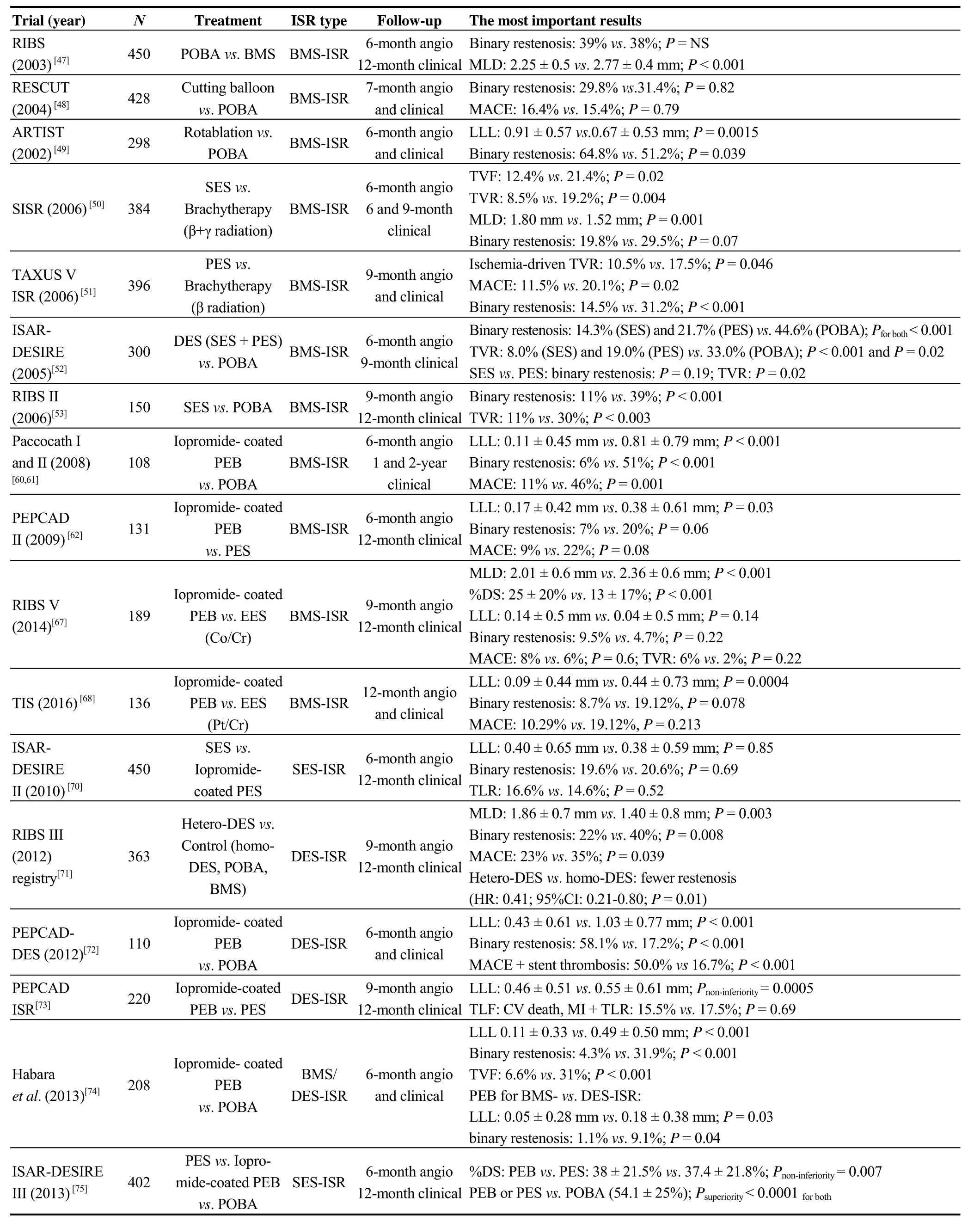
Table 4. Summary of the most important trials of DES/DEB treatment for BMS/DES-ISR.
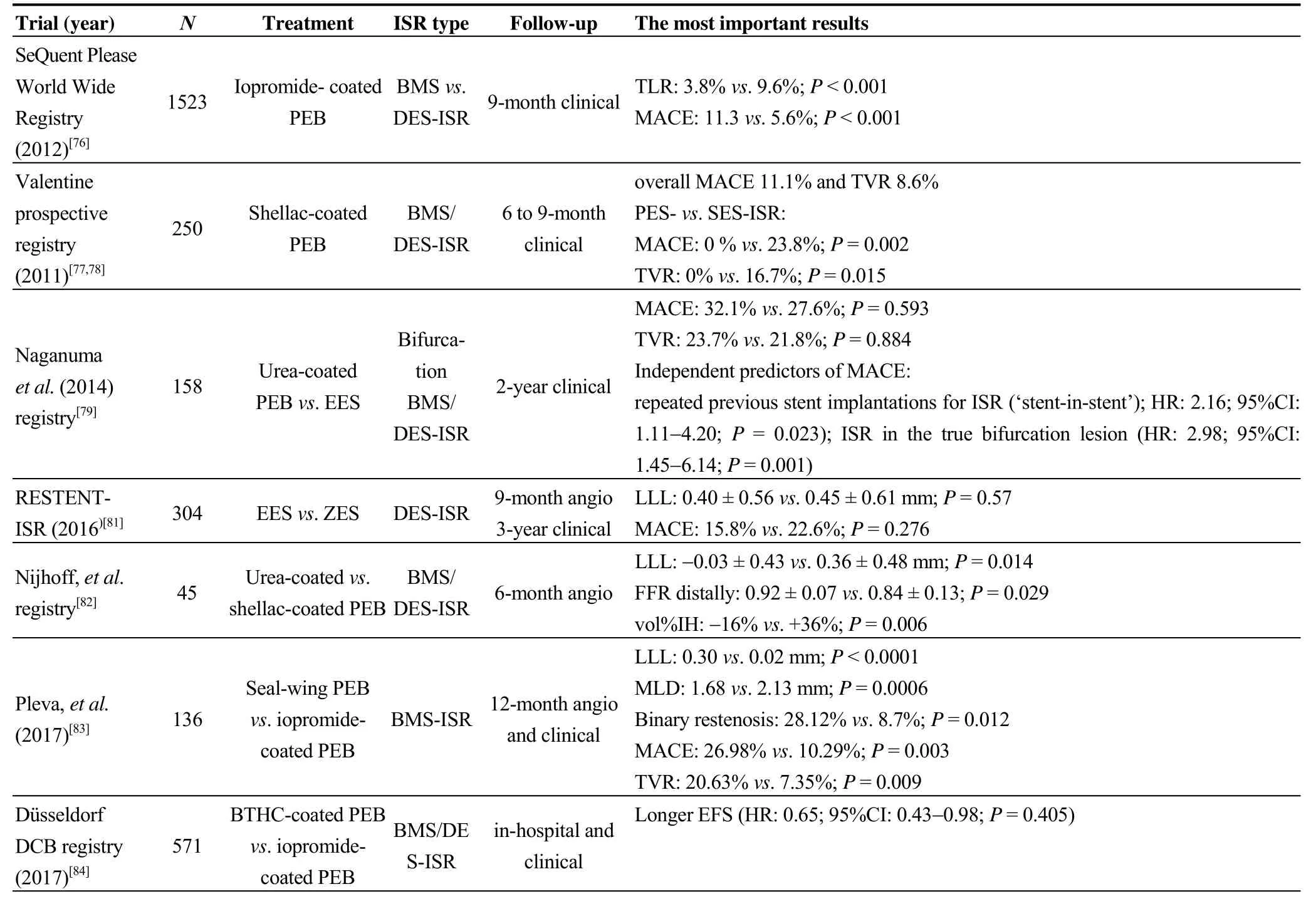
Table 4. Cont.
Acknowledgments
This article was supported by Ministry of Health, Czech Republic-conceptual development of research organizations―FNOs/2015.
References
1 Kim MS, Dean LS. In-stent restenosis.Cardiovasc Ther2011;29: 190–198.
2 Scott NA. Restenosis following implantation of bare metal coronary stents: Pathophysiology and pathways involved in the vascular response to injury.Adv Drug Deliv Rev2006; 58:358–376.
3 Byrne RA, Joner M, Massberg S,et al. Restenosis in bare metal and drug-eluting stents. InCoronary stenosis, imaging,structure and physiology, 1stEdition; Escaned J, Serruys PW,Eds.; Europa Edition: Toulouse, France, 2010; 475–496.
4 Mehran R, Dangas G, Abizaid AS,et al. Angiographic patterns of in-stent restenosis classification and implications for longterm outcome.Circulation1999; 100: 1872–1878.
5 Farooq V, Räber L, Gogas BD,et al. In-stent restenosis. InPercutaneous interventional cardiovascular medicine, the PCR-EAPCI textbook,1stEdition; Eeckhout E, Serruys PW,Wijns W,et al, Eds.; Europa Edition: Toulouse, France, 2012;785–826.
6 Morice MC, Serruys PW, Sousa JE,et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization.N Engl J Med2002; 346: 1773–1780.
7 Morice MC, Serruys PW, Barragan P,et al. Long-term clinical outcomes with sirolimus-eluting coronary stents: fiveyear results of the RAVEL trial.J Am Coll Cardiol2007; 50:1299–1304.
8 Holmes DR, MB, Moses JW,et al. Analysis of 1-year clinical outcomes in the SIRIUS trial a randomized trial of a sirolimus-eluting stent versus a standard stent in patients at high risk for coronary restenosis.Circulation2004; 109: 634–640.
9 Weisz G, Leon MB, Holmes DR,et al. Five-year follow-up after sirolimus-eluting stent implantation results of the SIRIUS (Sirolimus-Eluting Stent in De-Novo Native Coronary Lesions) Trial.J Am Coll Cardiol2009; 53: 1488–1497.
10 Stone GW, Ellis SG, Cox DA,et al. One-year clinical results with the slow-release, polymer-based, paclitaxel-eluting TAXUS stent the TAXUS-IV trial.Circulation2004; 109: 1942–1947.
11 Ellis SG, Stone GW, Cox DA,et al. Long-term safety and efficacy with paclitaxel-eluting stents 5-year final results of the TAXUS IV clinical trial.J Am Coll Cardiol Intv2009; 2:1248–1259.
12 Serruys PW, Ong ATL, Piek JJ,et al. A randomized comparison of a durable polymer everolimus-eluting stent with a bare metal coronary stent: the SPIRIT first trial.EuroInterv2005; 1: 58–65.
13 Beijk MA, Neumann FJ, Wiemer M,et al. Two-year results of a durable polymer everolimus-eluting stent in de novo coronary artery stenosis (The SPIRIT FIRST Trial).EuroInterv2007; 3: 206–212.
14 Fajadet J, Wijns W, Laarman GJ,et al. Randomized, doubleblind, multicenter study of the Endeavor zotarolimus-eluting phosphorylcholine-encapsulated stent for treatment of native coronary artery lesions: clinical and angiographic results of the ENDEAVOR II trial.Circulation2006; 114: 798–806.
15 Mauri L, Massaro JM, Jiang S,et al. Long-term clinical outcomes with zotarolimus-eluting versus bare-metal coronary stents.J Am Coll Cardiol Intv2010; 3: 1240–1249.
16 Cosgrave J, Melzi G, Biondi-Zoccai GGL,et al. Drug-eluting stent restenosis the pattern predicts the outcome.J Am Coll Cardiol2006; 47: 2399–2404.
17 El-Hayek G, Bangalore S, Casso Dominguez A,et al. Metaanalysis of randomized clinical trials comparing biodegradable polymer drug-eluting stent to second-generation durable polymer drug-eluting stents.JACC Cardiovasc Interv2017;10: 462–473.
18 Nakazawa G, Otsuka F, Nakano M,et al. The Pathology of neoatherosclerosis in human coronary implants bare-metal and drug-eluting stents.J Am Coll Cardiol2011; 57: 1314–1322.
19 Alfonso F, Byrne RA, Rivero F,et al. Current treatment of in-stent restenosis.JACC2014; 63: 2659–2673.
20 Kastrati A, Mehilli J, Dirschinger J,et al. Restenosis after coronary placement of various sent types.Am J Cardiol2001;87: 34–39.
21 Yoshitomi Y, Kojima S, Yano M,et al. Does stent design affect probability of restenosis? A randomized trial comparing multilink stents with GFX stents.Am Heart J2001; 142:445–451.
22 Pache J, Kastrati A, Mehilli J,et al. Intracoronary stenting and angiographic results: Strut thickness effect on restenosis outcome (ISAR-STEREO-2) trial.J Am Coll Cardiol2003; 41:1283–1288.
23 Kastrati A, Schomig A, Elezi S,et al. Predictive factors of restenosis after coronary stent placement.J Am Coll Cardiol1997; 30: 1428–1436.
24 Jukema JW, Verschuren JJ, Ahmed TA,et al. Restenosis after PCI. Part 1: pathophysiology and risk factors.Nat Rev Cardiol2012; 9: 53–62.
25 Elezi S, Kastrati A, Pache J,et al. Diabetes mellitus and the clinical and angiographic outcome after coronary stent placement.J Am Coll Cardiol1998; 32: 1866–1873.
26 Kastrati A, Dibra A, Mehilli J,et al. Predictive factors of restenosis after coronary implantation of sirolimus- or paclitaxel-eluting stents.Circulation2006; 113: 2293–2300.
27 Kobayashi Y, De Gregorio J, Kobayashi N,et al. Stented segment length as an independent predictor of restenosis.J Am Coll Cardiol1999; 34: 651–659.
28 Jones GT, Tarr GP, Phillips LV,et al. Active matrix metalloproteinases 3 and 9 are independently associated with coronary artery in-stent restenosis.Atherosclerosis2009; 207:603–607.
29 Tarr GP, Williams GT, Wilkins VHT,et al. Intra-individual changes of active matrix metalloproteinase-9 are associated with clinical in-stent restenosis of bare metal stents.Cardiology2013; 124: 28–35.
30 Katsaros KM, Kastl SP, Zorn G,et al. Increased restenosis rate after implantation of drug-eluting stents in patients with elevated serum activity of matrix metalloproteinase-2 and -9.J Am Coll Cardiol Intv2010; 3: 90–97.
31 Sampietro ML, Pons D, de Knijff P,et al. A genome wide association analysis in the GENDER study.Neth Heart J2009;17: 262–264.
32 Verschuren JJW, Trompet S, Postmus I,et al. Systematic testing of literature reported genetic variation associated with coronary restenosis: results of the GENDER Study.PLoS One2012; 7: e42401.
33 Pocock SJ, Lansky AJ, Mehran R,et al. Angiogaphic surrogate end points in drug-eluting stent trials.JACC2008; 51:23–32.
34 Cutlip DE, Windecker S, Mehran R,et al. Clinical end points in coronary stent trials. A case for standardized definitions.Circulation2007; 115: 2344–2351.
35 Mauri L, Orav EJ, Kuntz RE. Late loss in lumen diameter and binary restenosis for drug-eluting stent comparison.Circulation2005; 111: 3435–3442.
36 Garcia-Garcia HM, Shen Z, Piazza M. Study of restenosis in drug eluting stents: new insights from greyscale intravascular ultrasound and virtual histology.EuroInterv2009; 5(Suppl D):D84–D92.
37 Kasaoka S, Tobis JM, Akiyama T,et al. Angiographic and intravascular ultrasound predictors of in-stent restenosis.J Am Coll Cardiol1998; 32: 1630-1635.
38 Sonoda S, Morino Y, Ako J,et al. Impact of final stent dimensions on long-term results following sirolimus-eluting stent implantation: serial intravascular ultrasound analysis from the SIRIUS trial.J Am Coll Cardiol2004; 43: 1959–1963.
39 Gonzalo N, Serruys PW, Okamura T,et al. Optical coherence tomography patterns of stent restenosis.Am Heart J2009; 158:284–293.
40 Rixe J, Achenbach S, Ropers D,et al. Assessment of coronary artery stent restenosis by 64-slice multi-detector computed tomography.Eur Heart J2006; 27: 2567–2572.
41 Andreini D, Pontone G, Mushtaq S,et al. Coronary in-stent restenosis: assessment with CT coronary angiography.Radiology2012; 265: 410–417.
42 Mahnken AH. CT imaging of coronary stents: past, present,and future.ISRN Cardiol2012; 2012: 139823.
43 Sun Z, Davidson R, Lin CHS. Multi-detector row CT angiography in the assessment of coronary in-stent restenosis: A systematic review.Eur J Radiol2009; 69: 489–495.
44 Sun Z, Almutairi AMD. Diagnostic accuracy of 64 multislice CT angiography in the assessment of coronary in-stent restenosis: A meta-analysis.Eur J Radiol2010; 73: 266–273.
45 Kumbhani DJ, Ingelmo CP, Schoenhagen P,et al. Metaanalysis of diagnostic efficacy of 64-slice computed tomography in the evaluation of coronary in-stent restenosis.Am J Cardiol2009; 103: 1675–1681.
46 Bainey KR, Norris CM, Graham MM,et al. Clinical in-stent restenosis with bare metal stents: Is it truly a benign phenomenon?Int J Cardiol2008; 128: 378–382.
47 Alfonso F, Zueco J, Cequier Aet al. A randomized comparison of repeat stenting with balloon angioplasty in patients with in-stent restenosis.J Am Coll Cardiol2003; 42: 796–805.
48 Albiero R, Silber S, Di Mario C,et al. Cutting balloon versus conventional balloon angioplasty for the treatment of in-stent restenosis:Resultsof the restenosis cutting balloon evaluation trial (RESCUT).J Am Coll Cardiol2004; 43: 943–949.
49 vom Dahl J, Dietz U, Haager PKet al. Rotational atherectomy does not reduce recurrent in-stent restenosis results of the angioplasty versus rotational atherectomy for treatment of diffuse in-stent restenosis trial (ARTIST).Circulation2002; 105:583–588.
50 Holmes DR Jr, Teirstein P, Satler L,et al. Sirolimus-eluting stentsvsvascular brachytherapy for in-stent restenosis within bare-metal stents: the SISR randomized trial.JAMA2006; 295:1264–1273.
51 Stone GW, Ellis SG, O’Shaughnessy CD,et al. Paclitaxeleluting stentsvs. vascular brachytherapy for in-stent restenosis within bare-metal stents: The TAXUS V ISR Randomized Trial.JAMA2006; 295: 1253–1263.
52 Kastrati A, Mehilli J, von Beckerath N,et al. A. Sirolimuseluting stent or paclitaxel-eluting stentvsballoon angioplasty for prevention of recurrences in patients with coronary in-stent restenosis: A randomized controlled trial.JAMA2005; 293:165–171.
53 Alfonso F, Pérez-Vizcayno MJ, Hernandez R,et al. A Randomized comparison of sirolimus-eluting stent with balloon angioplasty in patients with in-stent restenosis results of the restenosis intrastent: balloon angioplasty versus elective sirolimus-eluting stenting (RIBS-II) trial.J Am Coll Cardiol2006; 47: 2152–2160.
54 Belkacemi A, Agostoni P, Voskuil M,et al. Drug-eluting balloons in coronary artery disease– current and future perspectives.ICR2011; 6: 157–160.
55 Scheller B, Speck U, Abramjuk C,et al. Paclitaxel balloon coating, a novel method for prevention and therapy of restenosis.Circulation2004; 110: 810–814.
56 Cortese B, Bertoletti A. Paclitaxel coated balloons for coronary artery interventions: A comprehensive review of preclinical and clinical data.Int J Cardiol2012; 161: 4–12.
57 Cremers B, Toner JL, Schwartz LB,et al. Inhibition of neointimal hyperplasia with a novel zotarolimus coated balloon catheter.Clin Res Cardiol2012; 101: 469–476.
58 Speck U, Scheller B, Abramjuk C,et al. Inhibition of restenosis in stented porcine coronary arteries: uptake of Paclitaxel from angiographic contrast media.Invest Radiol2004; 39: 182–186.
59 Chin K. In-stent restenosis: the gold standard has changed.EuroInterv2011; 7: K43–K46.
60 Scheller B, Hehrlein C, Bocksch W,et al. Treatment of coronary in-stent restenosis with a paclitaxel-coated ballon catheter.N Engl J Med2006; 355: 2113–2124.
61 Scheller B, Hehrlein C, Bocksch W,et al. Two year follow-up after treatment of coronary in-stent restenosis with a paclitaxel-coated balloon catheter.Clin Res Cardiol2008; 97:773–781.
62 Unverdorben M, Vallbracht C, Cremers B,et al. Paclitaxelcoated balloon catheter versus paclitaxel-coated stent for the treatment of coronary in-stent restenosis.Circulation2009;119: 2986–2994.
63 Alfonso F, Fernandez C. Second-generation drug-eluting stents. Moving the fi eld forward.J Am Coll Cardiol2011; 58:26–29.
64 Lee MS, Yang T, Mahmud E,et al. Clinical outcomes in the percutaneous coronary intervention of in-stent restenosis with everolimus-eluting stents.J Invasive Cardiol2014; 26:420–426.
65 Almalla M, Schröder JW, Pross V,et al. Everolimus-eluting versus paclitaxel-eluting stents for treatment of bare metal stent restenosis.Am J Cardiol2011; 108: 518–522.
66 Markovic S, Paliskyte R, Rottbauer W,et al. Everolimuseluting stents compared with paclitaxel-eluting stents for treatment of coronary in-stent restenoses.Cardiovasc Revasc Med2012; 13: 307–310.
67 Alfonso F, Pérez-Vizcayno MJ, Cárdenas A,et al. A randomized comparison of drug-eluting balloon versus everolimuseluting stent in patients with bare-metal stent in-stent restenosis.JACC2014; 63: 1378–1386.
68 Pleva L, Kukla P, Kusnierova P,et al. Comparison of the efficacy of paclitaxel-eluting balloon catheters and everolimus-eluting stents in the treatment of coronary in-stent restenosis. The treatment of in-stent restenosis study.Circ Cardiovasc Interv2016; 9: e003316.
69 Latib A, Mussardo M, Ielasi A,et al. Long-term outcomes after the percutaneous treatment of drug-eluting stent restenosis.J Am Coll Cardiol Intv2011; 4: 155–164.
70 Mehilli J, Byrne RA, Tiroch K,et al. Randomized trial of paclitaxel- versus sirolimus-eluting stents for treatment of coronary restenosis in sirolimus-eluting stents: the ISAR-DESIRE 2 (Intracoronary Stenting and AngiographicResults:Drug Eluting Stents for In-Stent Restenosis 2) study.J Am Coll Cardiol2010; 55: 2710–2716.
71 Alfonso F, Pérez-Vizcayno MJ, Dutary J,et al. Implantation of a drug-eluting stent with a different drug (switch strategy)in patients with drug-eluting stent restenosis.Resultsfrom a prospective multicenter study (RIBS III [Restenosis Intra-Stent: Balloon Angioplasty Versus Drug-Eluting Stent]).J Am Coll Cardiol Intv2012; 5: 728–737.
72 Rittger H, Brachmann J, Sinha AM,et al. A randomized,multicenter, single-blinded trial comparing paclitaxel-coated balloon angioplasty with plain balloon angioplasty in drugeluting stent restenosis: the PEPCAD-DES study.J Am Coll Cardiol2012; 59: 1377–1382.
73 Xu B, Gao R, Wang J,et al. A prospective, multicenter, randomized trial of paclitaxel-coated balloon versus paclitaxeleluting stent for the treatment of drugeluting stent in-stent restenosis: results from the PEPCAD China ISR trial.J Am Coll Cardiol Intv2014; 7: 204–211.
74 Habara S, Iwabuchi M, Inoue N,et al. A multicenter randomized comparison of paclitaxel-coated balloon catheter with conventional balloon angioplasty in patients with bare-metal stent restenosis and drug-eluting stent restenosis.Am Heart J2013; 166: 527–533.
75 Byrne RA, Neumann FJ, Mehilli J,et al. Paclitaxel-eluting balloons, paclitaxel-eluting stents, and balloon angioplasty in patients with restenosis after implantation of a drug-eluting stent (ISAR-DESIRE 3): a randomised, open-label trial.Lancet2013; 381: 461–467.
76 Wöhrle J, Zadura M, Möbius-Winkler S,et al. SeQuent Please World Wide Registry: Clinical results of SeQuent please paclitaxel-coated balloon angioplasty in a large-scale,prospective registry study.JACC2012; 60: 1733–1738.
77 Stella PR, Belkacemi A, Waksman R,et al. The Valentines Trial: results of the first one week worldwide multicentre enrolment trial, evaluating the real world usage of the second generation DIOR paclitaxel drug-eluting balloon for in-stent restenosis treatment.EuroInterv2011; 7: 705–710.
78 Loh JP, Pieter R. Stella PR,et al. Paclitaxel-coated balloon for the treatment of drug-eluting stent restenosis: subanalysis results from the Valentines I trial.Cardiovasc Revasc Med2014;15: 23–28.
79 Naganuma T, Latib A, Costopoulos C,et al. Drug-eluting balloon versus second-generation drug-eluting stent for the treatment of restenotic lesions involving coronary bifurcations.EuroInterv2016; 11: 989–995.
80 Kereiakes DJ, Meredith IT, Windecker S,et al. Efficacy and safety of a novel bioabsorbable polymer-coated, everolimuseluting coronary stent: the EVOLVE II randomized trial.Circ Cardiovasc Interv2015; 8: e002372.
81 Hong SJ, Ahn CM, Kim BK,et al. Prospective randomized comparison of clinical and angiographic outcomes between everolimus-elutingvs. zotarolimus-eluting stents for treatment of coronary restenosis in drug-eluting stents: intravascular ultrasound volumetric analysis (RESTENT-ISR trial).Eur Heart J2016; 37: 3409–3418.
82 Nijhoff F, Stella PR, Troost MS,et al. Comparative assessment of the antirestenotic efficacy of two paclitaxel drugeluting balloons with different coatings in the treatment of in-stent restenosis.Clin Res Cardiol2016; 105: 401–411
83 Pleva L, Kukla P, Zapletalova J,et al. Efficacy of a seal-wing paclitaxel-eluting balloon catheters in the treatment of bare metal stent restenosis.BMC Cardiovasc Disor2017; 17: 168.
84 Assadi-Schmidt A, Mohring A, Liebsch E,et al. SeQuent Pleasevs. Pantera Lux drug coated balloon angioplasty in real life:Resultsfrom the Düsseldorf DCB registry.Int J Cardiol2017; 231: 68–72.
85 Jamshidi P, Nyffenegger T, Sabti Z,et al. A novel approach to treat in-stent restenosis: 6- and 12-month results using the everolimus-eluting bioresorbable vascular scaffold.EuroInterv2016; 11: 1479–1486.
86 Moscarella E, Ielasi A, Granata F,et al. Long-term clinical outcomes after bioresorbable vascular scaffold implantation for the treatment of coronary in-stent restenosis a multicenter italian experience.Circ Cardiovasc Interv2016; 9: e003148.
87 Capodanno D, Gori T, Nef H,et al. Percutaneous coronary intervention with everolimus-eluting bioresorbable vascular scaffolds in routine clinical practice: Early and midterm outcomes from the european multicenter GHOST-EU registry.EuroInterv2015; 10: 1144–1153.
88 Longo G, Granata F, Capodanno D,et al. Anatomical features and management of bioresorbable vascular scaffolds failure: a case series from the GHOST registry.Catheter and Cardiovas Interv2015; 85: 1150–1161.
89 Petretta M, Acampa W, Daniele S,et al. Long-term survival benefit of coronary revascularization in patients undergoing stress myocardial perfusion imaging.Circ J2016; 80:485–493.
90 Baschet L, Bourguignon S, Marque S,et al. Cost-effectiveness of drug-eluting stents versus bare-metal stents in patients undergoing percutaneous coronary intervention.Open Heart2016; e000445.
 Journal of Geriatric Cardiology2018年2期
Journal of Geriatric Cardiology2018年2期
- Journal of Geriatric Cardiology的其它文章
- Thyrotoxicosis induced cardiogenic shock rescued by extracorporeal membrane oxygenation
- Intravascular ultrasound guided retrograde guidewire true lumen tracking technique for chronic total occlusion intervention
- Repetitive narrow QRS tachycardia in a 61-year-old female patient with recent palpitations
- Prediction of sudden death in elderly patients with heart failure
- Long term outcomes of drug-eluting stent versus coronary artery bypass grafting for left main coronary artery disease: a meta-analysis
- Adherence to pharmacological and non-pharmacological treatment of frail hypertensive patients
