TCDD暴露对小鼠在位子宫内膜hMLH1表达的影响及其甲基化程度的研究
朱 芳,崔照领,宫心鹏,尚素霜,张香玲
(1.邯郸市第一医院,河北 邯郸 056000;2.石家庄市第六医院,河北 石家庄 050000)
TCDD暴露对小鼠在位子宫内膜hMLH1表达的影响及其甲基化程度的研究
朱 芳1,崔照领2,宫心鹏2,尚素霜1,张香玲1
(1.邯郸市第一医院,河北 邯郸 056000;2.石家庄市第六医院,河北 石家庄 050000)
目的探讨自胚胎期至成年期氯代二恶英(TCDD)暴露后小鼠子宫内膜组织、hMLH1的表达及错配修复基因1(hMLH-1)基因启动子区CpG岛甲基化程度。方法选60只C57BL/6雌性小鼠以2:1与30只C3H雄性小鼠随机合笼交配,见到阴道栓为受孕当日,60只已孕母鼠于受孕第8天(胎儿期)以鼻饲法暴露TCDD,且被随机分为对照组(TCDD:0μg/kg)及实验组(TCDD:3μg /kg),实验组所产雌性子鼠于出生后21天(青春期)再次分别暴露TCDD,每组均选12只雌性子鼠(体重6.40±0.20g),依据暴露剂量分为A组:0μg/kg,B组:3μg/kg,C组:10μg /kg。实验各组于出生后49天(成年期)再次暴露TCDD(3μg/kg),出生后70天再次给予TCDD 3μg/kg同时行子宫内膜移植术构建小鼠子宫内膜异位症模型,术后3、6、9周各组再次追加暴露TCDD(3μg/kg),术后12周脱臼处死雌性子鼠。免疫组化SP法检测在位内膜组织中hMLH1的表达。甲基化特异性PCR(MSP)方法检测各组hMLH1基因启动子区CpG岛的甲基化程度。结果hMLH1在对照组中的表达与A组相比无显著性差异 (t=0.561,P>0.05),但其他各组间两两比较有显著性差异,其中A组表达高于B组(t=3.056,P<0.05),B组表达高于C组(t=2.228,P<0.05)。各组hMLH1基因启动子区CpG岛甲基化程度比较:对照组与A组比较无显著性差异(χ2=1.339,P>0.05),其他各组间两两比较有显著性差异,其中A组低于B组(χ2=4.444,P<0.05),而 B组低于C组(χ2=6.316,P<0.05)。结论自胚胎期至成年期持续暴露TCDD有可能使hMLH1基因启动子区CpG岛发生超甲基化改变,导致hMLH1在组织中表达下降,从而影响成熟期子宫内膜的错配修复基因的功能,这些可能促进了成年期子宫内膜异位症的发生与发展。
氯代二恶英;在位内膜;错配修复基因1(hMLH1);超甲基化
子宫内膜异位症简称内异症(endometriosis,EMs)是临床常见的雌激素依赖性良性疾病,可导致女性痛经、月经失调、不孕、慢性盆腔痛等,同时也具有“侵袭、转移”以及恶变的可能。二恶英类是特殊的氯化三环芳烃类有机化合物, 毒性极强,包括多氯二苯并对二恶英(poly chloro dibenzo-para-dioxin,PCDD)和多氯二苯并呋喃(poly chlorodibenzo furan,PCDF)共210种。其中2,3,7,8-氯代二恶英(2,3,7,8 tetra chloro dibenzo-para-dioxin,2,3,7,8-TCDD或TCDD)为此类代表物,研究也较多[1]。TCDD暴露可以通过多种方式调节促进内异症的发生与发展[2-5]。表观遗传学是在基因序列不发生变化的情况下,调控基因的信息通过化学修饰或调节作用,影响和调节基因的功能和特性,并且可以通过细胞分裂和增殖周期遗传给后代,DNA甲基化调节是最常见的表观遗传学作用[6]。TCDD具有表观遗传学的甲基化调节作用[7],可使小鼠内异症模型在位内膜中PR亚型——PRB出现超甲基化的表现,可能促进了内异症的发生与发展[8],进一步阐明内异症为遗传表观学疾病[9]。目前研究表明,错配修复基因中hMLH1的超甲基化在众多疾病的发生、发展中起到关键作用。本研究旨在研究不同发育阶段的小鼠暴露TCDD后,其在位内膜hMLH1的表达及hMLH1基因启动子区CpG岛甲基化程度的情况,探讨TCDD对内异症发病的影响及其机制。
1材料与方法
1.1实验动物与试剂
选60只体重18~22g的成熟雌性无特殊病原体(specific pathogen free,SPF)级C57BL/6小鼠;30只体重20~24g成熟雄性SPF级C3H小鼠(购于北京维通利华实验动物技术有限公司)。2,3,7,8-TCDD为标准品(纯度>99%,ED-901-C,Cambridge Isotope Labe)。
1.2实验方法及内容
1.2.1 实验分组
60只C57BL/6雌性小鼠与30只C3H雄性小鼠以2:1随机合笼交配,以见到阴道栓为受孕日,60只已孕母鼠于受孕第8天(胎儿期)以鼻饲法暴露TCDD,且被随机分为对照组(TCDD:0μg/kg)及实验组(TCDD:3μg/kg),实验组所产雌性子鼠于出生后21天(青春期)再次分别暴露TCDD,每组均选12只雌性子鼠(体重6.40±0.20g),依据暴露剂量分为A组:0μg/kg,B组:3μg /kg,C组:10μg/kg。实验各组于出生后49天(成年期)再次暴露TCDD(3μg/kg),出生后70天再次给予TCDD(3μg/kg),同时行子宫内膜移植术构建小鼠子宫内膜异位症模型,术后3、6、9周各组再次追加暴露TCDD(3μg/kg),术后12周脱臼处死雌性子鼠。取其子宫角,中性福尔马林固定,石蜡包埋。在位内膜组织石蜡切片,厚度4μm,每例标本切片至少5张。
1.2.2动物建模术
采用自体子宫内膜移植法建立小鼠EMs模型。在无菌条件下,将一侧子宫角分成3块,分别缝合在小鼠卵巢,腹膜和腹部皮下,每块大小约2mm×2mm。
判断建模成功的标准为①肉眼观:移植的子宫内膜存活,移植物的体积增大,周围血循环丰富,可见内含清亮液体的小囊泡;②镜下观:病理检查见异位灶有子宫内膜腺体和间质细胞,其间可有炎性细胞浸润。
1.3异位病灶的形成和检测
于建模术后12周处死小鼠,根据大体外观和镜下病理组织形态学鉴定子宫内膜异位病灶的形成,将异位种植物及其周围粘连的组织取下,制成石蜡块,苏木素伊红(hematoxylin and eosin staining kit,HE)染色,用于病理组织形态学的鉴定和评估。
1.4指标检测
免疫组化SP检测在位内膜中hMLH1的表达及甲基化特异性PCR检测在位内膜组织中hMLH1基因启动子区CpG岛甲基化程度。
1.5实验步骤
1.5.1免疫组织化学染色法(SP法)
组织切片脱蜡、水化,微波炉间断加热抗原修复30个循环,3%过氧化氢甲醇溶液消除内源性过氧化物酶,PBS溶液清洗5min,重复3次。山羊血清封闭20min,甩干,勿洗。加一抗(鼠单克隆抗体hMLH1为1:50),37℃水浴箱孵育4h,PBS溶液清洗5min,重复3次。加二抗,室温15min,PBS溶液清洗5min,重复3次。HRP标记的链霉亲和素,室温10min,PBS溶液清洗5min,重复3次。DAB显色,显微镜下观察。苏木素复染2min,水洗,乙醇脱干,封片。以PBS液代替一抗染色,染色结果阴性为对照组。
免疫组化结果判定:高倍视野(400×)下随机选取切片中5个不重叠的区域,计算单位面积中阳性区域平均光密度( optical density,OD)来反映蛋白的相对含量。
1.5.2甲基化特异性PCR(methylation-specific PCR,MS-PCR)
酚-氯仿法提取石蜡组织中DNA并测定其DNA纯度用于甲基化特异性PCR。
PCR扩增引物设计参照有关文献[10]:
hMLH1 FM 5′-ACGTAGACGTTTTATTAGGGTCGC-3′
hMLH1 RM 5′-TCCGACCCGAATAAACCCAA-3′ 57℃
hMLH1 FUM 5′-TTTTGATGTAGATGTTTTATTAGGGTTGT-3′
hMLH1 RUM 5′-ACCACCTCATCATAACTACCCACA-3′ 58℃
F代表上游,R代表下游,U代表非甲基化,M代表甲基化。
引物由上海生工生物工程股份有限公司合成。
1.5.3 MSP步骤
1.5.3.1亚硫酸氢钠修饰提取DNA DNA 20μL加去离子水(dd·H2O)24.5μL,加3mol/L NaOH 5.5μL,37℃,15min。加入 3mol/L NaHSO3(pH 5.0) 280μL +新鲜配置的10mmol/L C6H6O215μL,55℃温浴18h(过夜)。
透析方法:500mmol/L NaAC,4℃,4h; 0.5mmol/L C6H6O24℃,4h;0.5mmol/L NaAc,4℃过夜;dd H2O 4℃,4h,3次,最后一次4℃过夜。透析完毕,沉淀DNA(NaAc 和乙醇),TE缓冲液20μL溶解DNA,置于-20℃保存。
1.5.3.2反应体系及条件 25μL,10μmol/L引物各1μL;模板2μL;10mmol/L dNTP 0.5μL;5U/μL Taq DNA聚合酶0.5μL;10×PCR反应缓冲液2.5μL;25mmol/L MgCl21.5μL;上样染料;优化剂;稳定剂;灭菌去离子水。震荡混匀后短暂离心,加少许矿物油于PCR仪扩增。扩增条件:95℃变性3min (95℃ 45s ,各自的退火温度50s,72℃ 60s) 35个循环;72℃ 延伸14min。
1.6统计学方法
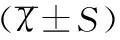
2结果
2.1子宫内膜移植术成功构建的小鼠内异症模型
研究发现随暴露剂量的增加异位灶外观由小囊泡发展为大囊泡,内有大量液体积聚,表面张力较大腹腔组织粘连严重(见图1、图2)。数据统计结果显示B组种植成功率显著高于对照组(χ2= 9.430,P<0.05),对照组中皮下、腹膜及卵巢异位种植成功率无显著差异(P>0.05)。A组中皮下和卵巢的异位种植成功率有显著差异(χ2=6.750,P<0.05),皮下和腹膜,腹膜和卵巢的异位种植成功率无显著差异(均P>0.05)。B组中卵巢部位种植成功率显著高于皮下种植成功率(χ2=6.316,P<0.05),皮下与腹膜,腹膜与卵巢种植成功率无显著差异(均P>0.05)。C组中卵巢部位的异位种植成功率显著高于皮下种植成功率(χ2=8.710,P<0.05),皮下与腹膜,腹膜与卵巢的异位种植成功率无显著差异(均P>0.05),见表1。
2.2各组别在位子宫内膜组织中hMLH1的表达
小鼠在胎儿期及生后不同的发育阶段暴露于TCDD并且造模成功后,采用免疫组织化学方法观察其在位子宫内膜组织中hMLH1的表达,免疫组化结果显示hMLH1主要在子宫内膜腺体细胞的胞核中表达,见图3。采用多个样本均数比较的方差分析,hMLH1在四组的表达具有显著性差异,经两两比较,hMLH1在对照组中的表达与A组相比无显著性差异 (t=0.561,P=0.071>0.05),但其他各组间两两比较有显著性差异,其中A组表达高于B组(t=3.056,P=0.000<0.05),B组表达高于C组(t=2.228,P=0.000<0.05),见表2。

图1子宫内膜移植术成功构建的小鼠内异症模型中囊泡及腹腔粘连
Fig.1 Bubbles and abdominal adhesion in mouse endometriosis model established successfully by endometrium transplatation
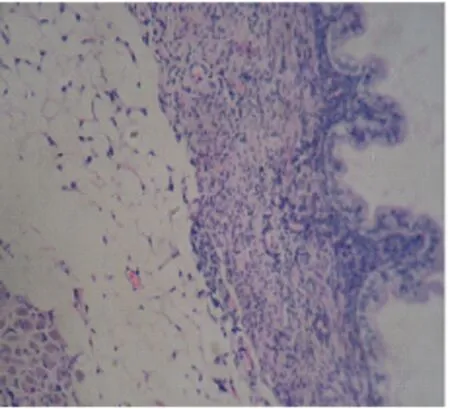
图2子宫内膜移植术成功构建的小鼠内异症模型中异位的内膜腺体(SP×400)
Fig.2 Ectopic endometrial glands in mouse endometriosis model established successfully by endometrium transplatation (SP×400)

表1 各组不同部位异位内膜种植成功率的比较[n(%)]
注:a为皮下与卵巢;b为皮下与腹膜;c为腹膜与卵巢;*为B组与对照组。

对照组A组B组C组
图3 hMLH1在各组中的表达(SP×400)
Fig.3 Expression of hMLH1 in different groups (SP×400)
2.3 hMLH1基因启动子区CpG岛甲基化程度
小鼠在胎儿期及生后不同的发育阶段暴露于TCDD并且造模成功后,采用甲基化特异PCR技术检测其在位子宫内膜组织中hMLH1的甲基化程度,见图4。采用多个样本率比较的卡方检验,各组hMLH1基因启动子区CpG岛甲基化程度比较:对照组与A组比较无显著性差异(χ2=1.339,P=1.000>0.05),其他各组间两两比较有显著性差异,其中A组低于B组(χ2=4.444,P=0.037<0.05),而 B组低于C组(χ2=6.316,P=0.012<0.05),见表3。
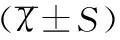


组别例数(n)hMLH1tP对照组120.5512±0.0031A组120.5350±0.00710.5610.071B组120.2562±0.00783.0560.000C组120.1212±0.00672.2280.000
表3各组hMLH1的甲基化程度[n(%)]
Table 3 Methylation of hMLH1 in different groups[n(%)]

组别例数(n)甲基化hMLH1非甲基化hMLH1χ2P对照组1211(91.67)1(8.33)A组122(16.67)10(83.33)1.3391.000B组127(58.33)5(41.67)4.4440.037C组1212(100.00)0(0.00)6.3160.012

图4 各组hMLH1的甲基化程度
3讨论
3.1 TCDD的甲基化调节作用与错配修复基因hMLH1的超甲基化
表观遗传学(epigenetics)是指DNA序列不发生改变的情况下,调控基因的信息通过化学修饰基因DNA和组蛋白, 蛋白质与蛋白质、RNA干扰、DNA与其它分子之间相互作用影响和调节基因的功能和特性, 并且具有可复性的遗传给后代。其重要方面就是DNA的启动子区中CpG岛的甲基化,一般是指S-腺苷蛋氨酸(SAM)作为甲基供体与基因启动子区CpG岛(二核苷酸岛)胞嘧啶环第5碳原子以共价键的形式结合,形成了5-甲基胞嘧啶。环境污染物可通过表观遗传学中甲基化调节作用导致人类肿瘤的发生[11]。已有研究证明了TCDD的甲基化调节作用[12]。TCDD并非ERα和ERβ的直接受体,但它却与ER活动相关的分子功能水平紧密相关,比如影响AhR受体与ER之间的作用[13]。另外也可能与PI3K/Akt、IGF-1、 PTEN信号旁路有关[14]。随着环境基因组学的应用和发展,有关其作用机制方面的具体研究正逐步深入。我们一系列研究表明自胚胎期至成年期持续增强TCDD暴露剂量,其在位子宫内膜DNMT-1的表达逐渐增强,且于PR-B基因启动子区CpG岛甲基化程度呈正相关,于PR-B表达呈负相关,从而表明了TCDD有可能通过DNMT-1所介导的甲基化作用使PR-B表达下降,导致局部子宫内膜的生物学功能以及其对激素的反应发生了改变,从而促进了内异症的发生与发展[9]。
错配修复基因(mismathe repair,MMR)是一组修复DNA碱基错配、增强DNA复制忠实性、维持基因组稳定性和降低自发性突变的具有高度保守功能的管家基因。目前已克隆出hMSH2、hMLH1、hPSM1和hPSM2四种MMR基因,一旦此基因发生突变失去其修复功能,则DNA复制时的错误就不能及时修复,形成微卫星不稳定性(MSI),促进疾病的发生、发展[15]。hMLH1定位于人类染色体3P21,含19个外显子,是错配修复基因中最为重要一种,其cDNA有2268bp的开放阅读框架,可编码756个氨基酸,在碱基错配修复过程中发挥着解旋及切开错配碱基的关键作用[16]。其缺失与多种恶性肿瘤有关,而其缺失的主要原因为基因启动子区超甲基化[17]。最近有研究表明,随着病情发展,在慢性胃炎、非典型增生及胃癌患者血清及组织中hMLH甲基化程度呈逐渐上升趋势,而hMLH蛋白表达呈逐渐下降趋势,这对胃癌的早期诊断及对疾病预后判断具有重要意义[18],另外在结直肠癌发病、尤其对Lynch综合征的预筛方面起到重要作用[19-20],而进一步有研究指出Lynch综合征相关的子宫内膜癌中K-ras 基因密码子12或13的热点突变与hMLH的超甲基化有关,这有望避免对散发子宫内膜癌患者进行Lynch综合征全基因测序的高额代价[21]。另有研究其在鼻咽癌等疾病中也起到了重要作用[22]。
3.2 hMLH1的超甲基化与妇科肿瘤及子宫内膜异位症的相关研究
目前国内外研究表明,多种妇科恶性肿瘤与hMLH1突变有关[23]。Kobayashi等[24]研究发现MMR的超甲基化与原发子宫内膜癌合并卵巢癌发病有关,尤其在Lynch综合征相关的子宫内膜癌中研究更为深入[25]。 子宫内膜异位症虽然为良性疾病,但却具有 “转移、侵袭” 等恶性肿瘤的行为特点。且子宫内膜属于不稳定细胞,生理状态下增生活跃,错配几率高。单等收集23例卵巢子宫内膜异位组织进行hMLH1启动子区的甲基化程度研究,其结果显示hMLH1启动子区的甲基化发生率明显高于正常子宫内膜组织,从而推测hMLH1启动子区的超甲基化有可能与子宫内膜异位症的发生与发展有关[26]。Ren等[27]对29例内异症恶变卵巢癌组织、20例内异症病灶及其对应在位内膜组织中hMLH1的表达及其启动子区的甲基化程度进行研究发现,内异症恶变卵巢癌组织hMLH1的表达低于内异症异位内膜及正常对照内膜组织,而其启动子区的甲基化程度却明显升高。其相对应的在位内膜也呈现相一致的变化 ,而内异症组织及正常内膜组织中hMLH1的表达及甲基化程度却无显著差异,从而推测hMLH1的异常甲基化有可能是内异症恶变的早期改变,其有望成为内异症恶变的早期分子诊断工具。Fuseya等[28]的研究也发现卵巢子宫内膜异位症的恶变与hMLH1的缺失相关。以上研究表明子宫内膜异位症的发病是一种与hMLH1突变相关的表观遗传学疾病[29]。
综上所述,通过本实验中TCDD暴露建立的小鼠子宫内膜异位症模型中,其胎儿期及青春期均未暴露过TCDD(对照组)与自胚胎期开始至成年期持续暴露TCDD(实验组)相比,其在位内膜中hMLH1的表达明显下降,而其甲基化程度却逐步提高,表明持续暴露于TCDD后,其在位内膜组织中hMLH1基因超甲基化导致其表达下降,这有可能是TCDD通过其甲基化调节作用诱导子宫内膜异位症发病的机制之一。其具体作用机制有待于基础研究的进一步深入。
[1]Huang T, Tian C G, Zhang K,etal. Gridded atmospheric emission inventory of 2,3,7,8-TCDD in China[J]. Atmos Environ, 2015, 108:41-48.
[2]Rier S E, Turner W E, Martin D C,etal. Serum levels of TCDD and dioxin-like chemicals in Rhesus monkeys chronically exposed to dioxin: correlation of increased serum PCB levels with endometriosis[J]. Toxicol Sci, 2001, 59(1):147-159.
[3]Rier S, Foster W G. Environmental dioxins and endometriosis[J]. Semin Reprod Med,2003,21(2):145-154.
[4]Martínez-Zamora M A, Mattioli L, Parera J,etal. Increased levels of dioxin-like substances in adipose tissue in patients with deep infiltrating endometriosis[J].Hum Reprod,2015, 30(5):1059-1068.
[5]Bruner-Tran K L, Gnecco J, Ding T,etal. Exposure to the environmental endocrine disruptor TCDD and human reproductive dysfunction: translating lessons from murine models[J]. Reprod Toxicol,2017,68:59-71.
[6]Perotti A, Rossi V, Mutti A,etal. Methy-sens Comet assay and DNMTs transcriptional analysis as a combined approach in epigenotoxicology[J]. Biomarkers,2015,20(1):64-70.
[7]Ma J, Chen X, Liu Y,etal. Ancestral TCDD exposure promotes epigenetic transgenerational inheritance of imprinted gene Igf2: Methylation status and DNMTs[J].Toxicol Appl Pharmacol,2015,289(2):193-202.
[8]朱芳,崔照领,黄向华.TCDD暴露对子宫内膜异位症模型小鼠在位内膜组织PR亚型和DNMT-1表达的影响[J].河北医药,2015,37(3):334-338.
[9]Borghese B, Zondervan K T, Abrao M S,etal. Recent insights on the genetics and epigenetics of endometriosis[J].Clin Genet, 2017,91(2):254-264.
[10]Veganzones S, Maestro M L, Rafael S,etal.Combined methylation of p16 and hMLH1 (CMETH2) discriminates a subpopulation with better prognosis in colorectal cancer patients with microsatellite instability tumors[J]. Tumour Biol,2015,36(5):3853-3861.
[11]Pogribny I P, Rusyn I. Environmental toxicants, epigenetics, and cancer[J].Adv Exp Med Biol,2013,754:215-232.
[12]Yamada T, Hirata A, Sasabe E,etal. TCDD disrupts posterior palatogenesis and causes cleft palate[J]. J Craniomaxillofac Surg,2014,42(1):1-6.
[13]Gao Z, Bu Y, Liu X,etal. TCDD promoted EMT of hFPECs via AhR, which involved the activation of EGFR/ERK signaling[J]. Toxicol Appl Pharmacol,2016,298:48-55.
[14]Zama A M, Uzumcu M. Targeted genome-wide methylation and gene expression analyses reveal signaling pathways involved in ovarian dysfunction after developmental EDC exposure in rats[J]. Biol Reprod,2013,88(2): 52.
[15]Prié D, Friedlander G.Genetic disorders of renal phosphate transport[J]. N Engl J Med,2010,362(25):2399-2409.
[16]Kunkel T A, Erie D A. DNA mismatch repair[J]. Ann Rev Biochem,2005,74:681-710.
[17]Markowitz S, Grady W. Methods and compositions for detecting cancers associated with methylation of hMLH1 promoter DNA:US8669060[P/OL].2014-02-11[2017-02-27].http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=HITOFF&p=1&u=%2Fnetahtml%2FPTO%2Fsearch-bool.html&r=1&f=G&l=50&co1=AND&d=PTXT&s1=%22Methods+compositions+detecting+cancers+associated+methylation+hMLH1+promoter+DNA%22&OS="Methods+compositions+detecting+cancers+associated+methylation+hMLH1+promoter+DNA"&RS="Methods+compositions+detecting+cancers+associated+methylation+hMLH1+promoter+DNA".
[18]Liu L, Yang X. Implication of Reprimo and hMLH1 gene methylation in early diagnosis of gastric carcinoma[J].Int J Clin Exp Pathol,2015, 8(11):14977-14982.
[19]Hodjat M, Rahmani S, Khan F,etal. Environmental toxicants, incidence of degenerative diseases, and therapies from the epigenetic point of view[J]. Arch Toxicol, 2017,91(7):2577-2597.
[20]Newton K, Jorgensen N M, Wallace A J,etal. Tumour MLH1 promoter region methylation testing is an effective prescreen for Lynch Syndrome (HNPCC)[J].J Med Genet,2014,51(12):789-796.
[21]Cohen J G, Wiedemeyer R, Karlan B Y,etal. A KRAS hot spot mutation correlates with MLH1 methylation in endometrial carcinomas with microsatellite instability:a potential triage tool for Lynch syndrome evaluation[J]. Gynecol Oncol,2014,133(Suppl 1):79-80.
[22]付蕾,盛剑秋,孙自勤,等.遗传性非息肉病性结直肠癌患者hMLH1和hMSH2基因的突变规律[J].中华医学杂志,2008,88(28):1983-1985.
[23]Lancaster J M, Powell C B, Chen L M,etal. Society of Gynecologic Oncology statement on risk assessment for inherited gynecologic cancer predispositions[J].Gynecol Oncol,2015,136(1):3-7.
[24]Kobayashi Y, Nakamura K, Nomura H,etal. Clinicopathologic analysis with immunohistochemistry for DNA mismatch repair protein expression in synchronous primary endometrial and ovarian cancers[J].Int J Gynecol Cancer,2015,25(3):440-446.
[25]Billingsley C C, Cohn D E, Mutch D G,etal.Clinical implications for MSI, MLH1 methylation analysis and IHC in Lynch screening for endometrial cancer patients: an analysis of 940 endometrioid endometrial cancer cases from the GOG 0210 study[J].Gynecol Oncol,2015,137(Suppl 1):4-5.
[26]单莉莉,岳瑛,肖天慧,等.子宫内膜异位症与hMLH1启动子的甲基化[J].实用妇产科杂志, 2010,26(6):454-456.
[27]Ren F, Wang D, Jiang Y,etal. Epigenetic inactivation of hMLH1 in the malignant transformation of ovarian endometriosis[J].Arch Gynecol Obstet,2012,285(1):215-221.
[28]Fuseya C, Horiuchi A, Hayashi A,etal. Involvement of pelvic inflammation-related mismatch repair abnormalities and microsatellite instability in the malignant transformation of ovarian endometriosis[J]. Hum Pathol,2012,43(11):1964-1972.
[29]Forte A, Cipollaro M, Galderisi U. Genetic, epigenetic and stem cell alterations in endometriosis: new insights and potential therapeutic perspectives[J].Clin Sci (Lond),2014,126(2):123-138.
[专业责任编辑:杨筱凤]
Influence of TCDD exposure on hMLH1 expression in eutopic endometrium and hypermethylation study in mice
ZHU Fang1, CUI Zhao-ling2, GONG Xin-peng2, SHANG Su-shuang1, ZHANG Xiang-ling1
(1.TheFirstHospitalofHandan,HebeiHandan056000,China;2.TheSixthHospitalofShijiazhuang,HebeiShijiazhuang050000,China)
ObjectiveTo explore the expression of hMLH1 in eutopic endometrium of mice exposed to TCDD from embryonic stage to adulthood and to study hypermethylation of CpG island in promoter region of hMLH-1 gene.MethodsSixty C57BL/6 female mice were mated with 30 C3H male mice at ratio 2:1 randomly. The day on which pessulum was seen was taken as gestational day. On the 8th gestational day (fetal stage), 60 pregnant mice were exposed to TCDD with nasal feeding method, and divided into control group (exposed in TCDD with 0μg/kg) and experimental group (exposed in TCDD with 3μg/kg). Female offsprings of mice in the experimental group were exposed to TCDD again on the 21st day after birth (puberty stage), and according to different exposure dose divided into group A with exposure dose of 0μg/kg, group B with dose of 3μg/kg and group C with dose of 10μg/kg with 12 female mices (6.40±0.20g of weight) in each group. Mices in the experimental group were exposed to TCDD at 3μg/kg on 49th day and on 70th day after birth, mouse endometriosis model was established through endometrium transplantation. Mices were exposed to TCDD again at 3, 6 and 9 weeks after transplantation and killed by cervical dislocation at 12 weeks after surgery. Expression of hMLH1 in endometrium was detected with SP immunohistochemical method and methylation of CpG island in promoter region of hMLH1 gene was detected using methylation-specific PCR (MS-PCR) in each group.ResultsDifference in expression of hMLH1 between the control group and group A was not significant (t=0.561,P>0.05). Pairwise comparison carried out in other groups showed significant difference. Expression level in group A was higher than that in group B (t=3.056,P<0.05), and that in group B was higher than that in group C (t=2.228,P<0.05). Comparison of methylation of CpG island in promoter region of hMLH1 gene among groups showed that there was no significant difference between the control group and group A (χ2=1.339,P>0.05), but pairwise comparison of methylation in other groups found significant difference and methylation in group A was lower than that in group B (χ2=4.444,P<0.05) and methylation in group B was lower than that in group C (χ2=6.316,P<0.05).ConclusionPersistent exposure to TCDD from embryonic period to adulthood perhaps triggers methylation of CpG islands in promoter region of hMLH1 gene, resulting in decrease of hMLH1expression and influencing function of mismatch repair gene in mature endometrium, which may promote occurrence and development of endometriosis in adulthood.
TCDD; eutopic endometrium; mismatch repair gene 1 hMLH1; hypermethylation
2017-02-27
朱 芳(1983—),女,主治医师,硕士,主要从事子宫内膜异位症及妇科肿瘤临床工作。
崔照领,副主任医师。
10.3969/j.issn.1673-5293.2017.09.008
R711.7
A
1673-5293(2017)09-1060-05

