低浓度乙醇抑制糖尿病大鼠心肌损伤和NF-κB炎症信号通路活化
李辉,康品方,陶敏,汤阳,唐碧,张恒,高琴,王洪巨*.
(1蚌埠医学院第一附属医院心血管病科,2蚌埠医学院生理学教研室,蚌埠 233004)
低浓度乙醇抑制糖尿病大鼠心肌损伤和NF-κB炎症信号通路活化
李辉1,康品方1,陶敏1,汤阳1,唐碧1,张恒1,高琴2,王洪巨1*.
(1蚌埠医学院第一附属医院心血管病科,2蚌埠医学院生理学教研室,蚌埠 233004)
目的 探讨低浓度乙醇对糖尿病心肌保护作用与NF-κB和TNF-α表达的关系。方法 选取8周、体重140~160g的健康雄性SD大鼠30只,随机分为对照组、糖尿病组、糖尿病+低浓度乙醇组(每组10只)。通过单次腹腔内注射链脲佐菌素(STZ)制备糖尿病大鼠模型。造模成功后1周给予糖尿病+低浓度乙醇组大鼠2.5%乙醇日常饮用,1周后改为5%的乙醇持续至造模成功后第8周,对照组和糖尿病组大鼠均日常饮水。8周后取大鼠腹主动脉血进行肌酸激酶(CK)及乳酸脱氢酶(LDH)检测,取大鼠离体心肌行HE染色观察心肌细胞的形态学改变,免疫组织化学检测心肌细胞NF-κB蛋白的免疫反应性表达,酶联免疫吸附法(ELⅠSA)检测大鼠心肌细胞中TNF-α含量。结果 与对照组相比,糖尿病组大鼠的血液中CK和LDH显著升高,心肌细胞排列紊乱,NF-κB和TNF-α水平增加;糖尿病+低浓度乙醇组CK及LDH水平的升高、心肌细胞排列紊乱及结构损伤程度、NF-κB及TNF-α增加幅度均不如糖尿病组明显。结论 低浓度乙醇可能通过调节糖尿病大鼠心肌细胞中NF-κB和TNF-α的表达发挥心脏保护作用。
低浓度乙醇;糖尿病心肌损伤;NF-κB;TNF-α
糖尿病(Diabetic Mellitus,DM)现已成为威胁人类生命及生活质量的重大疾病。其中糖尿病并发心脏损害是糖尿病患者致死的重要因素。同时,糖尿病也成为心血管疾病的一级预防因素,患有糖尿病的人群比未患人群心血管事件发生率提高2~5倍[1]。既往的一系列研究表明,炎性反应、氧化性应激、胰岛素抵抗、系统性免疫功能紊乱、肾素-血管紧张素系统(RAAS)的激活均参与糖尿病心脏损伤的病理过程,而炎性反应在其中起到主要作用[2]。NF-κB信号通路通过对不同基因的调控,参与氧化性应激、炎性反应、细胞凋亡等糖尿病心肌损伤的病理过程[3]。同时,NF-κB作为最经典的炎症信号通路,能反应组织细胞的炎症程度,诱导下游TNF-α、IL-1β等前炎性细胞及趋化因子,加速糖尿病心肌细胞的损伤。
既往的研究表明,激动乙醛脱氢酶2(aldehyde dehydrogenase 2, ALDH 2)具有不同程度的心肌细胞保护作用[4],但其生理机制尚未完全明确。本实验拟通过运用低浓度乙醇诱导激活ALDH2作用于糖尿病大鼠中,同时检测炎性反应、心肌损伤等相关指标以及NF-κB信号通路的激活程度,为明确低浓度乙醇对糖尿病心脏保护作用与NF-κB信号通路的相关性提供实验依据。
材料与方法
1 材料
健康雄性SD大鼠30只,8周龄,体重140~160g;链脲佐菌素(STZ),Sigma公司产品;HE染色试剂盒(上海威奥生物科技有限公司);兔抗鼠NF-κB p65磷酸化多克隆抗体(武汉艾美捷abcam科技有限公司)。
2 糖尿病大鼠模型复制
将大鼠于实验室适应性饲养1周后随机分成对照组、糖尿病组、糖尿病+低浓度乙醇组,每组各10只。在禁食12h后测定各自的血糖水平,其中糖尿病组(DM组)、糖尿病+低浓度乙醇组(DM+EtOH组)大鼠以55mg/kg剂量对其腹腔内注射STZ。72h后通过大鼠尾静脉血检测每组大鼠的血糖水平,以血糖值>16.7mmol/L为糖尿病模型。糖尿病组每日正常饮水,糖尿病+低浓度乙醇组给予2.5%的乙醇日常饮用,1周后改为5%的乙醇维持至造模成功后第8周。
3 血液中CK及LDH测定
大鼠麻醉成功后,取其腹主动脉血2ml,血液离心后取血浆,自动生化分析测定血浆中CK及LDH水平。
4 心肌组织的形态学观察
动物处死后,进行心肌组织取材、固定制片、HE染色,随后在光镜下观察心肌组织及细胞形态。
5 NF-κB免疫组织化学检测
取实验大鼠心肌组织分别置于甲醛溶液及透明液中浸泡,后于脱水剂中脱水、石蜡液中制成石蜡切片。切片于烘箱中常规脱蜡至水,3%H2O2溶液中孵育 10 min。将切片浸入0.01mol/L、pH6.0的枸橼酸缓冲液中,高压加热修复3min后按试剂盒说明书操作进行免疫组织化学检测心肌NF-κB免疫反应性;采用HPLAS-2000高清晰度彩色病理图文报告系统对免疫反应强度进行定量分析:每张切片随机选取10个高倍视野(40倍物镜下),测定阳性面积率(阳性反应总面积/单位面积中细胞总面积×100%。
6 ELISA法检测心肌细胞TNF-α的含量
取大鼠心肌组织加入适量生理盐水充分研磨后离心取上清液。测量时依据大鼠TNF-α ELISA检测试剂盒使用说明书操作;酶标仪在450nm 测定各样本OD值,应用Curve-Expert 1.3软件转换成浓度水平。
7 统计学处理
所有数据统计运用SPSS17.0软件处理。其中计量资料用均数±标准差(±s)表示;组间比较运用两样本均数t检验。计数资料以构成比描述,率的比较采用x2检验。P<0.05为差异有统计学意义。
结 果
1 低浓度乙醇抑制糖尿病大鼠血清CK及LDH水平升高
对血清CK及LDH水平检测显示,糖尿病(DM)大鼠血清CK及LDH水平较正常对照(NC)大鼠明显升高,而糖尿病+低浓度乙醇组(DM+EtOH)大鼠血清CK及LDH水平虽高于对照大鼠,但明显低于糖尿病组大鼠(表1)。
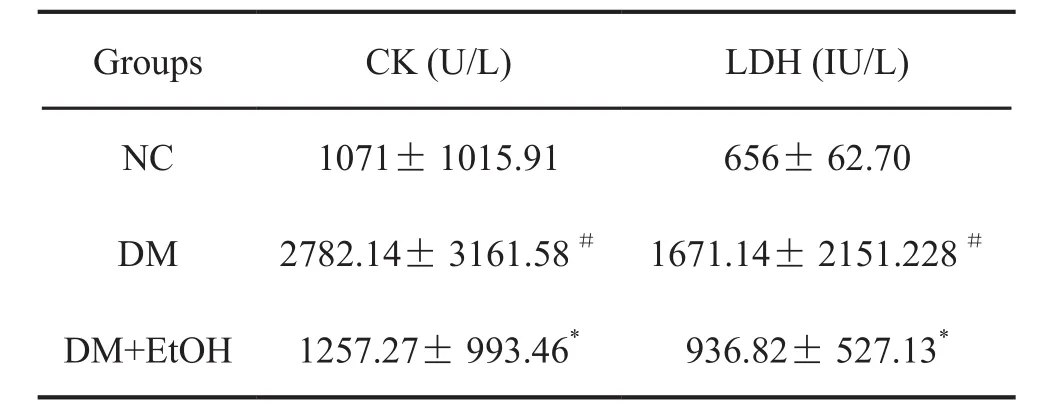
表1 低浓度乙醇抑制糖尿病大鼠血清CK及LDH水平升高Tab. 1 The effect of low concentration ethanol on the levels of serum CK and LDH in diabetic rats
2 低浓度乙醇减轻糖尿病大鼠心肌组织结构损害
对心肌组织进行HE染色观察显示,正常组大鼠心肌组织结构清晰,心肌细胞排列规则,心肌间隙正常(图1A);DM组大鼠心肌组织纤维及细胞排列杂乱无序,细胞核散乱,细胞质淡染呈水样变(图1B);DM+EtOH组大鼠心肌纤维紧凑规则、排列较有序,心肌细胞排列致密,较单纯糖尿病组组织细胞形态明显改善(图1C)。
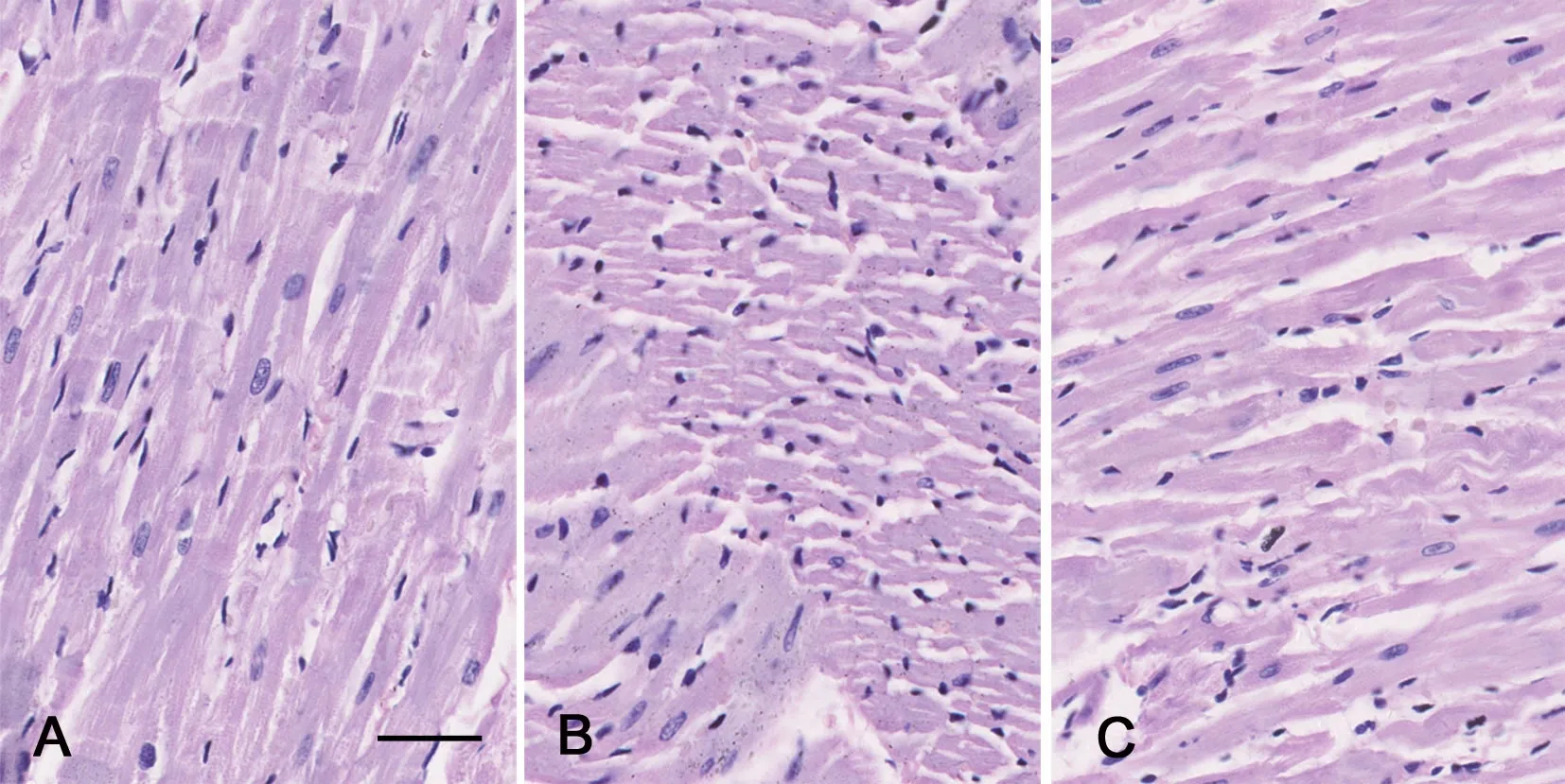
图1 低浓度乙醇对糖尿病大鼠心肌组织病理变化影响的HE染色观察。A,正常对照组大鼠心肌组织;B,DM组大鼠心肌组织;C,DM+E-tOH组大鼠心肌组织;比例尺,20μmFig. 1 Observation of the effect of low concentration ethanol on the morphology of myocardial tissue of diabetic rats by HE staining;A, myocardial tissue of the normal control rats; B, myocardial tissue of the DM rats; C, myocardial tissue of the DM+EtOH rats; scale bar, 20μm
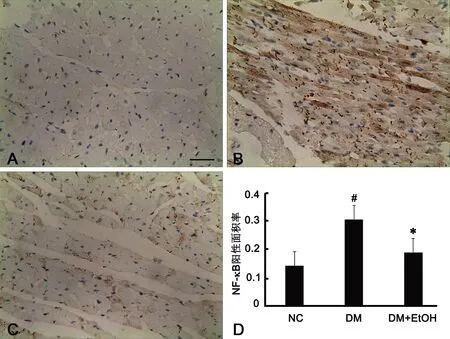
图2 低浓度乙醇对糖尿病大鼠心肌组织中NF-κB免疫反应性的影响。A,正常对照组大鼠心肌组织中NF-κB免疫反应性;B,DM组大鼠心肌组织中NF-κB免疫反应性;C,DM+EtOH组大鼠心肌组织中NF-κB免疫反应性;比例尺,20μm;D,低浓度乙醇对糖尿病大鼠心肌组织中NF-κB免疫反应性影响的统计学分析;#,与正常对照组比较,0.01<P<0.05;*,与DM组比较,0.01<P<0.05; n=10Fig. 2 The effect of low concentration ethanol on NF-κB immunoreactivity in the myocardial tissue of diabetic rats. A, NF-κB expression in the myocardial tissue of the NC group; B, NF-κB expression in the myocardial tissue of the DM group; C, NF-κB expression in the myocardial tissue of the DM+EtOH group; scale bar, 20μm; D, statistical analysis of the effect of low concentration ethanol on NF-κB immunoreactivity in the myocardial tissue of diabetic rats;#, 0.01<P<0.05, compared with NC; *, 0.01<P<0.05, compared with DM; n=10
3 低浓度乙醇抑制糖尿病大鼠心肌组织NF-κB 的活化
免疫组织化学检测显示,正常对照大鼠心肌细胞中NF-κB免疫反应性极弱,DM大鼠心肌细胞中NF-κB免疫反应性较正常对照组明显增强,DM+E-tOH大鼠心肌细胞中NF-κB免疫反应性较DM组明显减弱,但仍强于正常对照组。应用HPLAS-2000高清晰度彩色病理图文报告系统对免疫反应产物进行定量分析显示,DM大鼠心肌细胞中NF-κB免疫反应阳性面积率(0.307±0.085)明显高于正常对照组(0.142±0.035),DM+EtOH大鼠心肌细胞中NF-κB免疫反应阳性面积率(0.189±0.041)较DM大鼠降低,但仍高于正常对照组(图2)。
4 低浓度乙醇抑制糖尿病大鼠心肌组织中TNF-α含量的升高
ELⅠSA 检测显示,DM大鼠心肌组织中TNF-α的含量较正常对照大鼠明显升高,而DM+EtOH大鼠心肌组织内TNF-α的含量虽较正常对照大鼠升高,但明显低于DM大鼠。
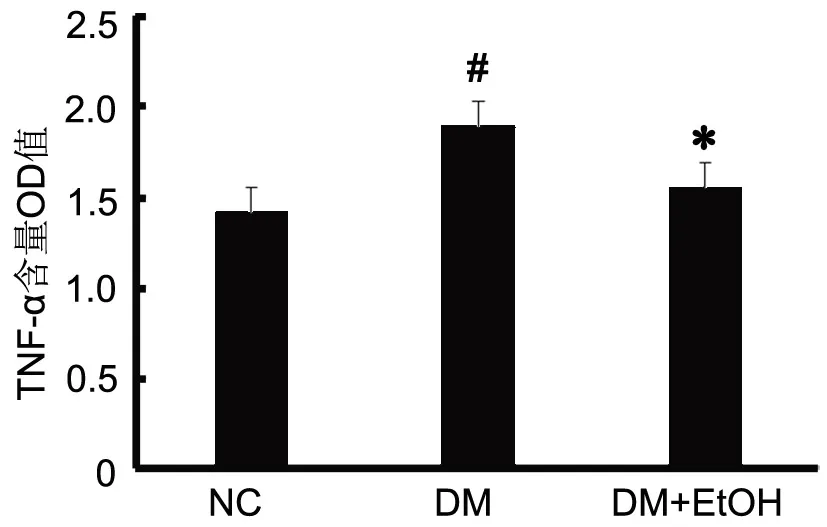
图3 低浓度乙醇对糖尿病大鼠心肌组织中TNF-α含量的浓度值的统计学分析。#,0.01<P<0.05;Compared with NC. *, 0.01<P<0.05;Compared with DM.Fig. 3 Statistical analysis of the effect of low concentration ethanol on the OD value of TNF-α in the myocardial tissue of diabetic rats. #, 0.01<P<0.05, compared with NC; *, 0.01<P<0.05, compared with DM
讨 论
糖尿病心肌病是指排除冠心病及高血压病后,在糖尿病的基础上并发了心肌细胞的肥大、纤维化及凋亡等病理改变,进而引起心脏结构及功能的紊乱[5]。糖尿病引起体内代谢物质的改变,而长期的高血糖和高血脂刺激血管内皮细胞增加血管活性肽(血管紧张素-2、内皮缩血管肽-1、去氧肾上腺素)和转化生长因子β(TGF-β)的释放,这些因素均直接或通过细胞因子介导激活NF-κB。随后NF-κB参与糖尿病心肌损伤中内皮细胞功能紊乱、炎性反应、细胞纤维化、细胞凋亡等一系列病理过程[6,7]。近期的研究也证实高血糖的心肌细胞中肿瘤坏死因子-α(TNF-α)、白介素-1β(IL-1β)等炎性因子、细胞粘附因子(VCAM-1)及趋化因子(MCP-1)明显升高,且可以通过抑制NF-κB信号通路介导的炎性病理过程,减轻糖尿病的心肌损害[8].。
本实验通过对大鼠单次腹腔注射STZ复制Ⅰ型糖尿病模型发现,糖尿病大鼠心肌细胞排列杂乱,且心肌组织结构损伤明显;血液心肌损伤标记物CK等指标明显上升,同时,反映氧化应激和炎性程度的LDH、NF-κB及TNF-α水平也较正常组升高,表明糖尿病大鼠心肌损伤明显加重,且主导炎性反应的NF-κB信号通路及TNF-α参与糖尿病心肌损伤的病理过程。在给予长期低浓度乙醇后发现,上述反映心肌损伤的标记物及炎性指标较单纯糖尿病有明显改善,心肌细胞形态和结构也有一定的恢复。这也证实了适量的乙醇可以通过抑制炎性反应发挥保护心肌损伤的作用。
乙醇通过在体内诱导其主要代谢酶乙醛脱氢酶2(ALDH2)起心肌保护作用。ALDH2是细胞线粒体中参与乙醇、乙醛及其他有毒醛类物质代谢、发挥解毒作用的关键酶。既往的研究表明ALDH2在心肌细胞缺血再灌注损伤、糖尿病心肌病中起到保护作用[9,10]。ALDH2可能通过激活细胞自噬发挥对心肌损伤的保护作用。Sanchez研究表明腺苷依赖性蛋白激酶(AMPK)是糖尿病心肌损伤细胞发生自噬作用的主要酶,其具体机制为AMPK通过促进下游信号分子UNC-51-样激酶 (ULK1)的磷酸化,同时抑制FOXO3a分子[11,12]。ALDH2通过调节细胞凋亡及维持细胞线粒体完整性发挥糖尿病心肌损伤的保护作用[13]。
现阶段,糖尿病及糖尿病并发心肌损伤人数在不断增加,特别是在亚洲人群中,其中约50%人群携带ALDH2基因突变体[14]。ALDH2基因多态性与糖尿病发生风险提升有密切关系,同时降低ALDH2的表达会加重糖尿病患者的氧化性应激和心脏损伤[15]。
低浓度乙醇对心肌的保护作用越来越受到人们的重视,但是其作用的分子机制及作用靶点还未完全清楚,本实验发现运用低浓度乙醇激活ALDH2发挥糖尿病心肌保护作用的同时,NF-κB蛋白的表达会随之降低,且与氧化应激、炎性反应有关的指标也下降,提示我们ALDH2可能通过抑制NF-κB蛋白的表达,减轻心肌损伤中的氧化应激、炎性反应等病理过程发挥心肌保护作用。
[1] Yang SH, Dou KF, Song WJ. Prevalence of diabetes among men and women in China. N Engl J Med, 2010, 362(25): 2425-2426.
[2] Mandavia CH, Aroor AR,Demarco VG, et al. Molecular and metabolic of cardiac dysfunction in diabetes. Life Sci, 2013, 92(11): 601-608.
[3] Lorenzo O, Picatoste B, Ares-Carrasco S, et al. Potential role of nuclear factor kappa in diabetic cardiomyopathy. Mediators Inflamm, 2011, 10: 1155-1159.
[4] 史晓俊,康品方,高琴,等.乙醛脱氢酶2在糖尿病大鼠致心肌损伤中的变化.中国临床药理学与治疗学,2014,19(12):1342-1346.
[5] Falcão-Pires Ⅰ, Leite-Moreira AF. Diabetic cardiomyopathy: understanding the molecular and cellular basis to progress in diagnosis and treatment. Heart Fail Rev, 2012, 17(3): 325–344.
[6] Min W, Bin ZW, Quan ZB, et al. The signal transduction pathway of PKC/NF-KB/c-fos may be involved in the influence of high glucose on the cardiomyocytes of neonatal rats. Cardiovascular. Diabetology, 2009, 8(1): 1-14.
[7] Fang ZY, Prins JB, Marwick TH. Diabetic cardiomyopathy: evidence, mechanisms, and therapeutic implications. Endocrine Reviews, 2004, 25(4): 543-567.
[8] Guo X, Xue M, Li CJ, et.al. Protective effects of triptolide on TLR4 mediated autoimmune and inflammatory response induced myocardial fibrosis in diabetic cardiomyopathy. J Ethnopharmacol, 2016, 193: 333-344.
[9] Ma H, Guo R, Yu L, et al. Aldehyde dehydrogenase 2 (ALDH2) rescues myocardial ischaemia/reperfusion injury: role of autophagy paradox and toxic aldehyde. Eur Heart J, 2011, 32(8): 1025-1038.
[10] Doser TA, Turdi S, Thomas DP, et al. Transgenic overexpression of aldehyde dehydrogenase-2 rescues chronic alcohol intake-induced myocardial hypertrophy and contractile dysfunction. Circulation, 2009, 119(14): 1941-1949.
[11] Sanchez AM, Csibi A, Raibon A, et al. AMPK promotes skeletal muscle autophagy through activation of forkhead FoxO3a and interaction with Ulk1. J Cell Biochem, 2012, 113(2): 695-710.
[12] Guo Y, Yu W, Sun D, et al, A novel protective mechanism for mitochondrial aldehyde dehydrogenase (ALDH2) in type Ⅰdiabetes-induced cardiac dysfunction: role of AMPK-regulated autophagy. Biochim Biophys Acta, 2015, 1852(2): 319-231
[13] Zhang Y, Babcock SA, Hu N, et al. Mitochondrial aldehyde dehydrogenase (ALDH2) protects against streptozotocin-induced diabetic cardiomyopathy: role of GSK3β and mitochondrial function. BMC Med, 2012, 10: 40.
[14] Zhang Y, Ren J. ALDH2 in alcoholic heart diseases: Molecular mechanism and clinical implications. Pharmacol Ther, 2011, 132(1): 86-95.
[15] Wang J, Wang H, Hao P, et al. Ⅰnhibition of aldehyde dehydrogenase 2 by oxidative stress is associated with cardiac dysfunction in diabetic rats. Mol Med, 2011, 17(3-4): 172-179.
Low concentration ethanol inhibits myocardial injury and activation of NF-κB inflammation signaling pathway in diabetic rats
Li Hui1, Kang Pinfang1, Tao Min1, Tang Yang1, Tang Bi1, Zhang Heng1, Gao Qin2,Wang Hongju1*
(1Department of Cardiology, the First Affiliated Hospital of Bengbu Medical College,2Department of Physiology, Bengbu Medical College, Bengbu, 233004, China)
Objective To study the relationship between the protective effect of low concentration ethanol on diabetic myocardium and the expression of NF-κB and TNF-α. Methods Thirty 8-week-old healthy male SD rats each weighing 140~160g were randomly divided into three groups: the control group, diabetes group, and diabetes with low concentration ethanol group (DM+ETOH),ten rats each group. The diabetic model was prepared by single intraperitoneal injection of streptozotocin (STZ), a week after successful modeling, the DM+ETOH group was fed with 2.5% ethanol daily, and another week later with 5% ethanol, continue to the eighth week the control and the diabetic groups were given water only. After eight weeks, the abdominal aortic blood was collected for creatine kinase (CK) and lactate dehydrogenase (LDH) detection, the morphological changes of myocardial cells were observed by HE staining, the expression of NF-κB protein in myocardial cells was determined by immunohistochemistry, and the content of tumor necrosis factor-α in myocardial cells was detected by ELISA. Results Compared with the control group, blood CK and LDH increased significantly in the diabetic rats, myocardial cells were irregularly arranged, and the levels of NF-κB and TNF-α also increased; these changes were less significant in the DM+EtOH group. Conclusion Low concentration ethanol protects the myocardium of diabetic rats possibly by inhibiting the expression of NF-κB and TNF-α in myocardial cells.
Low concentration ethanol; diabetic myocardial injury; NF-κB; TNF-α
R587.1
A DOⅠ:10.16705/ j. cnki. 1004-1850.04.006
2016-11-24
2017-05-02
国家自然科学基金资助项目(81550036);安徽省教育厅课题(KJ2016a484);安徽省自然科学基金资助项目(1508085MH169);蚌埠医学院研究生创新课题(Byycxz1503)
李辉,男(1989年),汉族,硕士,住院医师
*通讯作者(To whom correspondence should be addressed):1649134019@qq.com

