积雪草酸预处理对脓毒症小鼠急性肾损伤的影响及机制
朱丽华,熊御云,夏琳,芮棵,阴晴
(江苏大学附属医院,江苏镇江212001)
积雪草酸预处理对脓毒症小鼠急性肾损伤的影响及机制
朱丽华,熊御云,夏琳,芮棵,阴晴
(江苏大学附属医院,江苏镇江212001)
目的 探讨积雪草酸(AA)预处理对脓毒症小鼠急性肾损伤的作用及机制。方法 选择雄性BALB/c小鼠24只,随机分为空白对照组、脂多糖(LPS)组、AA低剂量组、AA高剂量组各6只。AA低剂量组、AA高剂量组每天分别以AA 10、30 mg/kg灌胃预处理,空白对照组、LPS组每天以等量的0.5%羧甲基纤维素钠灌胃。第3天灌胃2 h后,空白对照组腹腔注射PBS,其余三组腹腔注射LPS 10 mg/kg建立小鼠脓毒症模型。各组于第3天腹腔注射4 h后,采用二乙酰肟比色法检测血清尿素氮(BUN)水平;取肾组织,行HE染色观察肾组织病理变化;采用real-time PCR法检测炎症因子(TNF-α、IL-1β、IL-6)及Notch信号通路相关基因(Notch1~4、Dll4 、Jag2)表达。结果 LPS组、AA低剂量组、AA高剂量组血清BUN水平均高于空白对照组,AA低剂量组、AA高剂量组血清BUN水平均低于LPS组(P均<0.05)。HE染色可见正常对照组小鼠肾组织正常;LPS组表现为肾小管坏死,内皮细胞肿胀以及炎性细胞浸润;AA低剂量组、AA高剂量组表现为内皮细胞肿胀减轻、炎症细胞浸润减少,病理损伤较LPS组明显减轻。LPS组TNF-α、IL-1β、IL-6 mRNA表达均高于空白对照组(P均<0.05),AA低剂量组、AA高剂量组TNF-α、IL-1β、IL-6 mRNA表达均低于LPS组(P均<0.05)。LPS组Notch信号相关基因Notch1、Notch2、Notch3、Notch4、Dll4及 Jag2 mRNA表达均低于空白对照组(P均<0.05),AA低剂量组、AA高剂量组Notch1、Notch2、Notch3、Notch4、Dll4及 Jag2 mRNA表达均高于LPS组(P均<0.05)。结论 AA预处理能够明显减轻脓毒症小鼠的肾损伤,其机制可能与调控Notch信号通路发挥抗炎作用有关。
积雪草酸;肾脏;脂多糖;Notch信号通路;炎症因子;脓毒症;急性肾损伤
严重脓毒症或脓毒性休克是导致严重肾损伤(AKI)的主要诱因,其发病率逐年上升,每年在全球范围内造成超过400万人死亡。因此,有效防治感染继发的肾功能障碍是降低全身性感染患者病死率的重要手段。Notch信号通路是一种高度保守的细胞内信号机制,在决定细胞生死、分化、肿瘤、神经退变病及炎症反应中具有重要意义[1,2]。细胞表面的Notch受体与配体结合后,可在蛋白水解的作用下释放胞内段,转而募集转录共激活物,活化下游靶基因的转录表达,参与细胞内的各种生理活动。积雪草酸(AA)是中草药积雪草提取物中五环三萜烯类组分的五环三萜类化合物,具有抗氧化、抗炎、神经保护及抗肿瘤等多种药理活性[3~6],但其对发生脓毒症时AKI的预防作用尚不清楚。2016年3~12月,我们通过腹腔注射脂多糖(LPS)建立小鼠脓毒症模型,观察AA对AKI的影响。
1 材料与方法
1.1 动物与试剂 雄性BALB/c小鼠24只,6~8周龄,体质量18~22 g,由扬州大学实验动物中心提供。AA购自Sigma公司;BUN试剂盒购自南京建成生物工程有限公司;反转录、PCR试剂盒购自Takara公司;PCR反应引物由南京金斯瑞有限公司提供;其余试剂均为国产分析纯。
1.2 分组与干预方法 小鼠适应性饲养3 d后,随机分为空白对照组、LPS组、AA低剂量组、AA高剂量组各6只。AA低剂量组、AA高剂量组每天分别灌胃AA 10、30 mg/kg,空白对照组、LPS组每天灌胃等量的0.5%羧甲基纤维素钠(CMC-Na)。第3天灌胃2 h后,LPS组、AA低剂量组、AA高剂量组腹腔注射LPS 10 mg/kg建立小鼠脓毒症模型,空白对照组腹腔注射PBS。
1.3 血清BUN检测 各组于第3天腹腔注射LPS 4 h后,眼眶后缘静脉取血,收集血清,采用二乙酰肟比色法检测血清BUN水平。
1.4 肾脏组织病理学观察 取血后麻醉大鼠,取出肾组织,肾组织标本经10%甲醛固定、脱水、石蜡包埋处理后,行HE染色观察肾组织病理变化。
1.5 肾脏组织中炎症因子及Notch信号通路相关基因检测 采用real-time PCR法检测炎症因子(TNF-α、IL-1β、IL-6)及Notch信号通路相关基因(Notch1~4、Dll4、Jag2)mRNA表达。小鼠处死后,剪下双肾组织,立即置于液氮中-180 ℃ 保存。肾组织总RNA的提取应用TRIzol试剂的方法进行。取1 μg RNA,应用Takara PrimeScriptTMRT-PCR试剂盒的操作步骤,进行逆转录反应合成cDNA。采用Takara公司SYBR Premix Ex TaqTM试剂盒进行real-time PCR操作。引物由南京金斯瑞有限公司合成,炎症因子、Notch信号通路相关基因及内参引物序列见表1。用2-ΔΔCt法计算目标基因与内标基因荧光强度比值,以其表示目标基因表达量。

表1 炎症因子、Notch信号通路相关基因引物序列
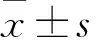
2 结果
2.1 各组血清BUN水平比较 空白对照组、LPS组、AA低剂量组、AA高剂量组血清BUN水平分别为(7.79±0.69)、(20.15±1.42)、(12.66±1.26)、(11.55±1.48)mmol/L。LPS组、AA低剂量组、AA高剂量组血清BUN水平均高于空白对照组(P均<0.05),AA低剂量组、AA高剂量组血清BUN水平均低于LPS组(P均<0.05)。
2.2 各组肾脏病理表现 HE染色可见正常对照组小鼠肾单位结构正常,无水肿和炎细胞浸润。LPS组则表现为肾小管坏死,内皮细胞肿胀以及炎性细胞浸润的增加。AA低剂量组、AA高剂量组表现为内皮细胞肿胀减轻、炎症细胞浸润减少,病理损伤较LPS组明显减轻。
2.3 各组肾脏组织炎症因子mRNA表达比较 LPS组TNF-α、IL-1β、IL-6 mRNA表达均高于空白对照组(P均<0.05),AA低剂量组、AA高剂量组TNF-α、IL-1β、IL-6 mRNA表达均低于LPS组(P均<0.05)。见表1。
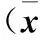
表1 各组肾脏组织炎症因子基因表达比较±s)
注:与空白对照组比较,*P<0.05;与LPS组比较,#P<0.05。
2.4 各组肾脏组织Notch信号通路相关基因表达比较 LPS组Notch1、Notch2、Notch3、Notch4、Dll 4及 Jag2 mRNA表达均低于空白对照组(P均<0.05),AA低剂量组、AA高剂量组Notch1、Notch2、Notch3、Notch4、Dll 4及 Jag2 mRNA表达均高于LPS组(P均<0.05)。见表2。
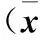
表2 各组肾脏组织Notch信号通路相关基因表达比较±s)
注:与空白对照组比较,*P<0.05;与LPS组比较,#P<0.05。
3 讨论
脓毒症时全身性的炎症会导致器官的受损,其中肾脏是首要的靶器官。脓毒症时过度的炎症反应可诱导AKI的发生,并导致肾功能的恶化,炎症因子在这一过程中发挥了重要作用[7]。因此,抑制炎症因子的产生成为脓毒症治疗中肾脏保护的重要策略[8]。AA是从常用中草药积雪草中提取的一种五环三萜类的化合物,具有抗炎作用[4]。但对于AA在脓毒症时肾损伤中的作用尚未见报道。
脓毒症是AKI产生的常见病因,能够明显提高AKI发病率,诱发慢性肾脏疾病,并使病死率提高6~8倍。脓毒症所致AKI的机制主要与微血管损伤、炎症及代谢异常引起的内皮细胞损伤有关[9],LPS可明显诱导炎症因子释放的增加,从而导致AKI[10]。大量的研究表明,炎症因子如TNF-α、IL-1β和IL-6等参与了LPS诱导的肾损伤,其原因可能是由于肾脏的高血流量使其易于受到循环中炎症因子的刺激[11]。本研究发现,LPS组血清BUN水平明显高于空白对照组,肾组织HE染色发现肾小管坏死、内皮细胞肿胀以及炎性细胞浸润的增加,肾组织中炎症因子TNF-α、IL-1β和IL-6 mRNA表达均显著增加,提示炎症因子在脓毒症后AKI的发病中具有重要意义。
已有文献报道,AA具有多种药理活性,如抗氧化、抗炎和抗肿瘤等[3,5,6]。AA的抗炎作用主要与其抑制炎症因子iNOS、COX-2、IL-6和IL-1β等有关,可以通过抑制线粒体介导的NLRP3炎症小体的激活,从而减轻糖酐酯钠诱导的小鼠肠炎[12]。同时在LPS诱导的小鼠急性肺损伤模型中也发现,AA可以对抗Toll样受体4(TLR4)/NF-κB通路介导的多种炎症因子的增加[4, 13]。本研究发现,AA低剂量组、AA高剂量组血清BUN水平均低于LPS组,肾组织内皮细胞肿胀减轻、炎性细胞浸润减少,病理损伤较LPS组明显减轻,肾组织TNF-α、IL-1β、IL-6 mRNA表达均低于LPS组,提示AA可以减轻LPS诱导的小鼠肾脏损伤,发挥肾脏保护作用,可能与抑制炎症因子表达有关。
近年来,关于Notch信号转导通路在炎症中作用的研究日益受到关注。哺乳动物表达4个Notch受体(Notch1~4)及5个Notch配体(Jag1、Jag2、Dll1、Dll3、Dll4)。细胞接受胞外信号后,Notch受体与配体结合,经水解酶切割后释放Notch胞内段。这个过程触发了γ 分泌酶依赖性的Notch胞内段的水解,诱导胞内段的核定位,导致了CSL家族复合物的形成。Notch胞内段替代了共抑制因子,招募共激活因子复合物,激活CSL依赖性的转录[14]。Notch信号通路参与了自身免疫和炎症反应过程,在类风湿关节炎、系统性红斑狼疮和细菌病毒感染等疾病中均具有重要意义[15~17]。研究[18]发现,LPS可以通过直接调控Notch的酪氨酸残基的硝化作用及抑制Notch胞内段的转录活性,从而抑制Notch信号通路。本研究发现,LPS组Notch信号相关基因Notch1、Notch2、Notch3、Notch4、Dll4及 Jag2 mRNA表达均低于空白对照组,提示Notch信号参与了LPS导致的肾损伤;AA低剂量组、AA高剂量组Notch1、Notch2、Notch3、Notch4、Dll4及 Jag2 mRNA表达均高于LPS组,提示AA可能通过激活Notch信号通路,提高Notch受体1~4及配体Dll4、Jag2转录水平的表达,从而发挥抗炎作用,进而发挥肾脏保护作用。
综上所述,AA预处理可通过激活Notch信号通路,抑制LPS引起的小鼠肾脏中促炎细胞因子的生成,从而减轻小鼠肾功能损伤的发生发展。我们的研究为AA抗炎作用的机制提供了新的方向及思路。
[1] Hurlbut GD, Kankel MW, Lake RJ, et al. Crossing paths with Notch in the hyper-network [J]. Curr Opin Cell Biol, 2007,19(2):166-175.
[2] Bray SJ. Notch signalling: a simple pathway becomes complex [J]. Nat Rev Mol Cell Biol, 2006,7(9):678-689.
[3] Lee MK, Kim SR, Sung SH, et al. Asiatic acid derivatives protect cultured cortical neurons from glutamate-induced excitotoxicity [J]. Res Commun Mol Pathol Pharmacol, 2000,108(1-2):75-86.
[4] Yun KJ, Kim JY, Kim JB, et al. Inhibition of LPS-induced NO and PGE(2) production by asiatic acid via NF-kappa B inactivation in RAW 264.7 macrophages: possible involvement of the IKK and MAPK pathways[J]. Int Immunopharmacol, 2008,8(3):431-441.
[5] Park BC, Bosire KO, Lee ES, et al. Asiatic acid induces apoptosis in SK-MEL-2 human melanoma cells[J]. Cancer Lett, 2005, 218(1): 81-90.
[6] Xiong Y, Ding H, Xu M, et al. Protective effects of asiatic acid on rotenone- or H2O2-induced injury in SH-SY5Y cells [J]. Neurochem Res, 2009,34(4):746-754.
[7] Sen V, Uluca U, Ece A, et al. Role of ankaferd on bacterial translocation and inflammatory response in an experimental rat model of intestinal obstruction[J]. Int J Clin Exp Med, 2014,7(9):2677-2686.
[8] Cohen J. The immunopathogenesis of sepsis [J]. Nature, 2002,420(6917):885-891.
[9] Gomez H, Kellum JA. Sepsis-induced acute kidney injury [J]. Curr Opin Crit Care, 2016,22(6):546-553.
[10] Doi K, Leelahavanichkul A, Yuen PS, et al. Animal models of sepsis and sepsis-induced kidney injury [J]. J Clin Invest, 2009, 119(10): 2868-2878.
[11] Zhang L, Sun DD, Bao Y, et al. Nerolidol protects against LPS-induced acute kidney injury via inhibiting TLR4/NF-kappa B signaling [J]. Phytother Res, 2017,31(3):459-465.
[12] Guo WJ, Liu W, Jin B, et al. Asiatic acid ameliorates dextran sulfate sodium-induced murine experimental colitis via suppressing mitochondria-mediated NLRP3 inflammasome activation [J]. Int Immunopharmacol, 2015,24(2):232-238.
[13] Li ZL, Xiao XZ, Yang MS. Asiatic acid inhibits lipopolysaccharide-induced acute lung injury in mice [J]. Inflammation, 2016, 39(5): 1642-1648.
[14] Mumm JS, Schroeter EH, Saxena MT, et al. A ligand-induced extracellular cleavage regulates gamma-secretase-like proteolytic activation of Notch1 [J]. Mol Cell, 2000,5(2):197-206.
[15] Park JS, Kim SH, Kim K, et al. Inhibition of Notch signalling ameliorates experimental inflammatory arthritis [J]. Ann Rheum Dis, 2015, 74(1):267-274.
[16] Zhang WJ, Xu W, Xiong SD. Blockade of Notch1 signaling alleviates murine lupus via blunting macrophage activation and M2b polarization [J]. J Immunol, 2010,184(11):6465-6478.
[17] Ito T, Allen RM, Carson WF, et al. The critical role of notch ligand delta-like 1 in the pathogenesis of influenza A virus (H1N1) infection [J]. PLoS Pathog, 2011, 7(11).
[18] Kim MY, Park JH, Mo JS, et al. Downregulation by lipopolysaccharide of Notch signaling, via nitric oxide [J]. J Cell Sci, 2008,121(9):1466-1476.
Effects of pretreatment of asiatic acid on acute renal injury of mice with sepsis
ZHULihua,XIONGYuyun,XIALin,RUIKe,YINQing,
(TheAffiliatedHospitalofJiangsuUniversity,Zhenjiang212001,China)
Objective To investigate the protective effects of pretreatment of asiatic acid (AA) on acute renal injury of mice with sepsis. Methods Twenty-four male BALB/c mice were randomly divided into the blank control group, lipopolysaccharide (LPS) group, low-dose AA group, and high-dose AA group with 6 mice in each. Mice in the low-dose AA group and high-dose AA group were administered 10 and 30 mg/kg AA for 3 consecutive days, meanwhile, mice in the blank control and LPS group were intragastrically administered equal volume of 0.5% sodium carboxyl methyl cellulose (CMC-Na). At 2 h after the last administration, mice in the blank control group were intraperitoneally injected with PBS, while mice in the other three groups were injected with 10 mg/kg LPS to make the sepsis models. After 4-hour LPS injection on day 3, the level of serum urea nitrogen (BUN) was measured by diacetylmonoxime colorimetric assay. The pathological change of kidney was investigated by HE staining. The mRNA expression levels of cytokines (TNF-α, IL-1β and IL-6) and Notch signaling pathway-related genes (Notch1-4, Dll4, and Jag2) were measured by real-time PCR. Results Compared with the blank control group, the level of BUN in the LPS, low-dose and high-dose AA groups was higher. Meanwhile, the level of BUN in the low-dose AA and high-dose AA groups was lower than that of the LPS group (allP<0.05). The histological examination showed LPS treatment resulted in neutrophil infiltration, tubular necrosis and endothelial cell swelling. Against these changes, the low-dose and high-dose AA groups showed significantly alleviated neutrophil infiltration, endothelial cell swelling and pathological injury as compared with that of the LPS group. The mRNA levels of TNF-α, IL-1β, and IL-6 in the LPS group were higher than those of the blank control group (allP<0.05), at the same time, the mRNA levels of TNF-α, IL-1β, and IL-6 in the low-dose and high-dose AA groups were lower than those of the LPS group (allP<0.05). The mRNA levels of Notch related genes including Notch1, Notch2, Notch3, Notch4, Dll 4 and Jag2 in the LPS group were lower than those of the blank control group (allP<0.05), meanwhile, the mRNA levels in the low-dose and high-dose AA groups were higher than those of the LPS group (allP<0.05). Conclusion AA pretreatment may significantly alleviate the acute renal injury of mice with sepsis by regulating Notch signaling pathway to develop anti-inflammatory effect.
asiatic acid; kidney; lipopolysaccharide; Notch signaling pathway; inflammation; sepsis; acute kidney injury
国家自然科学基金资助项目(81301657);江苏省自然科学基金资助项目(BK20130476)。
朱丽华(1989-),女,技师,研究方向为脓毒症急性肾损伤。E-mail: 18344711862@163.com。
熊御云(1983-),女,博士,主管技师,研究方向为多脏器功能衰竭的治疗。E-mail: 191853184@qq.com。
10.3969/j.issn.1002-266X.2017.30.003
R967
A
1002-266X(2017)30-0010-04
2017-02-19)

