褐飞虱海藻糖酶基因在表皮几丁质代谢中的调控作用
张露,朱世城,郑好,沈祺达,王世贵,唐斌
(杭州师范大学生命与环境科学学院,杭州 310036)
褐飞虱海藻糖酶基因在表皮几丁质代谢中的调控作用
张露,朱世城,郑好,沈祺达,王世贵,唐斌
(杭州师范大学生命与环境科学学院,杭州 310036)
【目的】昆虫海藻糖酶能够调控几丁质代谢并控制蜕皮过程。本研究通过 TRE表达被抑制后,检测褐飞虱(Nilaparvata lugens)蜕皮状况、几丁质含量及几丁质合成酶(chitin synthase,CHS)和几丁质酶(chitinase,Cht)基因表达情况,探究不同的海藻糖酶(trehalase,TRE)在褐飞虱表皮中对几丁质代谢的调控作用。【方法】采用RNAi技术,以实验室饲养种群褐飞虱为材料,通过向其体内注射双链RNA(dsRNA)分别抑制单个海藻糖酶基因或同时抑制多个海藻糖酶基因,注射48 h后通过Trizol法提取褐飞虱总RNA,反转录试剂盒合成第一链DNA后采用实时荧光定量PCR(qRT-PCR)技术检测该基因的表达情况,确定RNAi效果。氢氧化钾法测定48 h褐飞虱整体几丁质含量变化并对蜕皮困难虫体进行拍照;最后采用qRT-PCR检测褐飞虱CHS和Cht在mRNA水平上的相对表达量变化,分析TRE在调控几丁质代谢中的作用。【结果】与注射dsGFP相比较,其余各注射组褐飞虱整体几丁质含量显著下降,其中dsTRE1混合注射组与Validamycin注射组呈极显著下降,同时褐飞虱出现蜕皮困难等现象。qRT-PCR检测结果显示单个TRE的dsRNA注射后该基因的表达被抑制,但是部分TRE的表达有互补性上升。其中TRE1-2和TRE2在各注射组处理下表达均下降,dsTRE1s对TRE2的表达也有抑制效果,整体上dsTRE1混合注射组和海藻糖酶抑制剂Validamycin抑制效果明显;dsTRE注射组抑制CHS表达效果不明显,Validamycin能够显著降低CHS1和CHS1a在表皮中的表达,且2种dsTRE1注射后CHS1表达在上升,dsTRE1-2注射后表皮中的CHS1a的表达上升;Cht1和Cht8在dsTRE各注射组及Validamycin处理中表达下降或显著下降,dsTRE1-1注射后Cht2和Cht5表达显著上升;dsTRE1-2注射后Cht1、Cht6和Cht8表达下降,Cht2和Cht4表达显著上升;dsTRE2处理组中Cht1、Cht8和Cht10表达下降而Cht9表达显著上升;dsTRE1s注射后,Cht1和Cht5表达显著下降,而Cht9表达显著上升;Validamycin注射组中10个几丁质酶基因表达都显著或者极显著下降。【结论】TRE能够通过调控褐飞虱几丁质代谢途径来控制几丁质的合成,结果可为开展和筛选有效的海藻糖酶抑制剂控制褐飞虱等害虫提供理论依据。
褐飞虱;RNAi;海藻糖酶;表皮;几丁质代谢;实时荧光定量PCR
0 引言
【研究意义】水稻(Oryza sativa)是中国首要的粮食作物,保障水稻的稳产、高产对粮食安全生产具有重要意义,其生产和储存阶段经常受到各种害虫的威胁,据报道害虫总数达到800种以上[1]。2000—2010年期间,中国水稻因虫害导致损失达291.35万吨[2]。其中褐飞虱(Nilaparvata lugens)为水稻上危害最为严重的害虫,其为水稻的单食性害虫,具有繁殖速度快、生命周期短、内禀增长率高、环境适应性强等特点[3-6]。更为关键的是,褐飞虱为一种迁飞性的害虫,每年都从东南亚地区迁飞到中国危害,其危害范围广,防治难度大[7]。【前人研究进展】昆虫的外骨骼均由几丁质构成,不仅昆虫的表皮由几丁质组成,而且气管及中肠围食膜等结构的重要组成部分也由几丁质构成[8],其幼虫每生长到一定的阶段都需要蜕掉旧的表皮,形成新的表皮,而这个过程由几丁质合成通路及几丁质降解途径共同完成[9-10]。几丁质合成酶(chitin synthase,CHS)、几丁质酶(chitinase,Cht)和β-N-乙酰氨基葡萄糖苷酶(β-N-acetylglucosaminidases, NAGs)分别是合成和降解几丁质的关键酶类[11-12],它们的表达在蜕皮激素和保幼激素的调控作用下使昆虫体内的几丁质处于一个动态平衡。现已发现昆虫几丁质酶存在于体壁(蜕皮液)、中肠、脂肪体和毒腺等组织中,参与昆虫的蜕皮、围食膜降解和防御等重要生命活动。几丁质酶基因是一个多基因家族,并且在表达过程中有组织特异性,其包含几丁质酶和类似几丁质酶(chitinase-like),可以分为5个不同的家族[5]。而海藻糖酶(trehalase,TRE)是昆虫体内几丁质合成通路的第一个酶[13-14],其包含可溶性海藻糖酶(soluble trehalase,TRE1)和膜结合型海藻糖酶(membranebound trehalase,TRE2)两种类型,并且已在昆虫中克隆和发现多条不同的由TRE1编码的基因,这些基因间含有较高的序列相似性[13],在几丁质合成通路中有着重要的调控功能[15-17],前期研究发现褐飞虱中拥有两条可溶性海藻糖酶,即TRE1-1和TRE1-2[9,17]。海藻糖是昆虫的“血糖”[18],当海藻糖代谢的平衡被打破,昆虫的蜕皮与发育过程均会受到影响,而且相关研究结果表明海藻糖的合成和降解都能通过控制几丁质合成通路从而影响昆虫发育[13,15-16]。在甜菜夜蛾(Spodoptera exigua)等众多昆虫体内均发现两种几丁质合成酶,即CHS1(或CHSA)和CHS2(或CHSB),且CHS1包含了可变外显子两种可变剪接体[19]。CHS1和CHS2在昆虫不同组织中的表达情况各异,控制不同组织内的几丁质合成[9-10]。【本研究切入点】随着近几年基因组测序的快速发展,褐飞虱基因组测序完成,3个TRE在褐飞虱体内被发现,分别为TRE1-1、TRE1-2和TRE2。TRE1主要影响或者调节CHS1的表达来控制表皮中几丁质的合成,TRE2则影响CHS2的表达来控制中肠中几丁质的表达。几丁质合成受阻使得昆虫不能顺利完成蜕皮过程,最终导致昆虫因蜕皮困难和发育受阻而死亡。有关TRE调控不同几丁质酶的表达研究较少,褐飞虱仅存在CHS1,并无CHS2存在[20],但是其拥有10个几丁质酶[21]。对于不同的TRE如何调控褐飞虱的几丁质合成与降解鲜见报道[17]。【拟解决的关键问题】研究TRE调控褐飞虱表皮中几丁质合成和降解通路中CHS和Cht等基因的功能,评估TRE作为害虫控制的靶标的潜力,为控制水稻褐飞虱等害虫的生物农药的开发提供理论依据。
1 材料与方法
试验于2016年在杭州师范大学完成。
1.1 试验虫源
褐飞虱采集于中国水稻研究所,为杭州师范大学动物适应与进化重点实验室饲养种群,水稻品种全部采用感虫水稻TN1。水稻和褐飞虱均放在人工气候箱或者人工气候室中培育或者饲养,温度(25±1)℃、相对湿度(70±5)%、光周期 14L﹕10D。用于RNAi显微注射的褐飞虱为TN1水稻上饲养的种群,待褐飞虱长到5龄若虫后,用于后期注射试验。
1.2 仪器与试剂
1.2.1 主要仪器 -80℃冰箱(艾本德 U410-86)、常规冰箱(西门子 KK25V61TI)、高压蒸汽灭菌锅(ZEALWAY GR60DA)、恒温水浴锅(上海精宏DK-8D)、台式低温离心机(Eppendorf)、台式高速离心机(Eppendorf和Sigma1-14)、实时荧光定量PCR仪(Bio-RAD CFX96)、无菌超净工作台(ESCO)、电子分析天平(梅特勒-托利多AL204)和微量测定分光光度计(NanoDropTM2000)。
1.2.2 主要试剂 DNA Marker DL2000等、6×Loading buffer、pMD18-T、AMV反转录试剂盒及实时荧光定量PCR试剂等购自大连TaKaRa公司;总RNA提取采用 Trizol试剂盒购自 Lifetech Scientific Corporation;PCR引物由上海英潍捷基有限公司合成;dsRNA合成试剂盒为T7 RiboMax Express RNAi System(Promega,Madison,USA);实时荧光定量PCR八连管等耗材购自美国Bio-RAD公司;氯仿、异丙醇、无水乙醇、EDTA等常规试剂均购自国药集团化学试剂有限公司;琼脂糖购自西班牙Elisa公司。
1.3 试验方法
1.3.1 总RNA的抽提及cDNA的合成 褐飞虱虫体及组织的总RNA抽提采用Trizol试剂盒并按照产品说明进行提取。提取后用1%的琼脂糖检测总RNA的质量,然后用NanoDrop 2000分光光度计测定提取RNA的浓度及纯度。使用Prime Script®RT reagent Kit With gDNA Eraser试剂盒配置体系,进行第一链cDNA的合成。
1.3.2 dsRNA的合成 设计适合3个海藻糖酶基因dsRNA特异性片段并合成的特异性引物进行 PCR扩增,产物进行T克隆,具体引物序列见表1。随后用带T7启动子的引物进行交叉PCR反应,根据T7RiboMAXTMExpress RNAi System试剂盒的说明进行dsRNA合成,待dsRNA合成后,采用NanoDropTM2000测定合成dsRNA的浓度,同时以GFP作为对照组,采用同样方法合成GFP的dsRNA[17]。用于显微注射的材料分为dsTRE1-1(451 bp)、dsTRE1-2(321 bp)、dsTRE2(440 bp)和dsTRE1s(dsTRE1-1与dsTRE1-2混合注射组)以及作为对照的dsGFP(688 bp)和海藻糖酶抑制剂(Validamycin:10 μg·μL-1)。

表1 褐飞虱3个海藻糖酶及GFP dsRNA合成引物序列Table 1 The primers of 3 trehalases in N. lugens and GFP for dsRNA synthesis
1.3.3 褐飞虱的显微注射 在注射前用标准毛细管定量显微注射器每次泵出dsRNA的体积,并根据需要注射的量,通过调整氮气压来调整dsRNA的体积使其泵出体积符合注射量。将5龄褐飞虱用CO2麻醉后放置于制备有琼脂胶板的一次性培养皿中,使虫体腹部朝上,注射第一对足中间偏下较软部位,注射量均为200 ng/头(海藻糖酶抑制剂也注射200 ng/头),注射后将其放置装有新鲜水稻的试管中,分别于注射后48 h解剖表皮组织用于定量分析,整体的褐飞虱用于几丁质含量测定试验。
1.3.4 RNAi后褐飞虱组织中海藻糖酶及几丁质代谢途径关键基因表达量测定 褐飞虱显微注射后48 h解剖组织材料用于荧光定量PCR的检测,PCR引物见表2。每次取样时取褐飞虱表皮材料分装于3个平行管中,即每个样品得到3管平行cDNA,放置于-80℃保存待用。试验时,每管cDNA做3个定量复孔,确保数据重复性好,3管平行cDNA可以得到9个数据,即每个样品为平均值±标准误,保证数据的可靠性。配置以下体系进行反应:Mix 10 μL、primer F(10 pmol)1 μL、primer R(10 pmol)1 μL、模板DNA 1 μL、RNase Free ddH2O 7 μL。反应程序为95℃预变性3 min;95℃变性5 s,55—62.5℃退火延伸25 s(循环40次);最后绘制65—95℃熔解曲线。

表2 褐飞虱海藻糖酶、几丁质酶及几丁质合成酶实时荧光定量PCR检测引物序列Table 2 The primers of trehalase, chitinase and chitin synthase in N. lugens for qRT-PCR detection
1.3.5 几丁质含量的测定 (1)自制消化管:取试管一根,配以橡皮塞,在橡皮塞上钻上5 mm孔,插上玻璃管,橡皮塞外端的玻璃管上再连接一根橡皮管,长30—50 cm,橡皮管的另一端再接一根玻璃管,并将开口的玻璃管通入水槽内,以防加热时碱液溅出;(2)将30头褐飞虱用清水冲洗干净,置于滤纸上,50℃烘箱内烘干,约4 d;(3)将烘干的褐飞虱研磨成粉末,在电子分析天平上称重,记为W1;(4)将已知质量的褐飞虱粉末倒入消化管中,加入5 mL饱和氢氧化钾,160℃甘油浴15—20 min;(5)用滤纸过滤残渣,然后用缓慢的流水冲洗干净;(6)将残渣置于50℃烘箱中烘1 h,在分析天平称重,计为W2;(7)按照如下公式计算几丁质的相对含量。几丁质的相对含量。式中[22-23],W1为消化前褐飞虱质量(mg);W2为消化后褐飞虱质量(mg);1.26为转换系数,转换系数=乙酰胺基葡萄糖相对分子质量/胺基葡萄糖相对分子质量。
1.3.6 数据统计分析 通过定量 PCR测定出 6个基因的CT值,每管样品的3个重复孔取平均值用于计算(数值相差在0.5以内可用,3个数值中有2个或3个接近,数值可用,否则重做)。每次样品有3组数值,即最后得到的数据为平均值±标准误。再通过2-△△CT法进行计算,对照组为褐飞虱dsGFP注射组的 CT值。最后将换算出的值再进行具体分析。2-△△CT计算公式[24]:
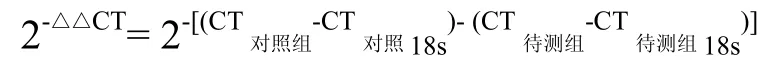
采用SPSS和One-Way ANOVA数据分析软件进行差异显著性分析。
2 结果
2.1 RNAi后海藻糖酶基因在表皮组织中的表达
RNAi抑制3个海藻糖酶基因表达后,结果显示单个海藻糖酶基因的 dsRNA注射后都能够有效地抑制该基因在表皮中的表达。TRE1-1、TRE1-2和TRE2分别在dsTRE1-1、dsTRE1-2和dsTRE2注射后48 h显著下降;TRE1-2和TRE2在所有的处理组,包括3种单个dsTRE注射、dsTRE1s及海藻糖酶抑制剂Validamycin注射后,其mRNA水平相对于dsGFP注射组都在下降;dsTRE1s不仅能够显著降低TRE1-1和TRE1-2的表达,也能够降低TRE2的表达;Validamycin能够显著降低 TRE1-1和TRE1-2的表达,而不能显著降低TRE2在表皮中的表达(图1)。
2.2 RNAi抑制海藻糖酶基因表达后几丁质含量及蜕皮情况变化
与对照组相比较,3个 TRE单基因的 dsRNA注射后 48 h几丁质含量显著下降(P<0.05),而dsTRE1s与Validamycin注射后48 h几丁质含量极显著下降(P<0.01,图2-A)。几丁质含量下降的幅度达到1/3—1/2。同时,与对照注射dsGFP组相比,这5种处理中部分褐飞虱都出现蜕皮困难等现象(图2-B)。
2.3 TRE基因RNAi后几丁质合成酶及可变转录子表达变化
在mRNA水平上对褐飞虱几丁质合成酶CHS1及其两个不同转录子CHS1a和CHS1b的表达进行了检测,结果显示Validamycin注射后能够有效地降低CHS1和CHS1a的表达(图3-A、3-B),单个dsTRE及dsTRE1s注射后48 h,褐飞虱表皮中CHS1及其两个不同转录子CHS1a和CHS1b的表达与dsGFP组相比较无显著差异(图3)。且两种dsTRE1注射后CHS1表达在上升,dsTRE1-2注射后表皮中CHS1a的表达上升。

图1 褐飞虱3个海藻糖酶基因在RNAi后mRNA相对表达水平Fig. 1 The relative expression levels of 3 trehalase genes of N. lugens after RNAi
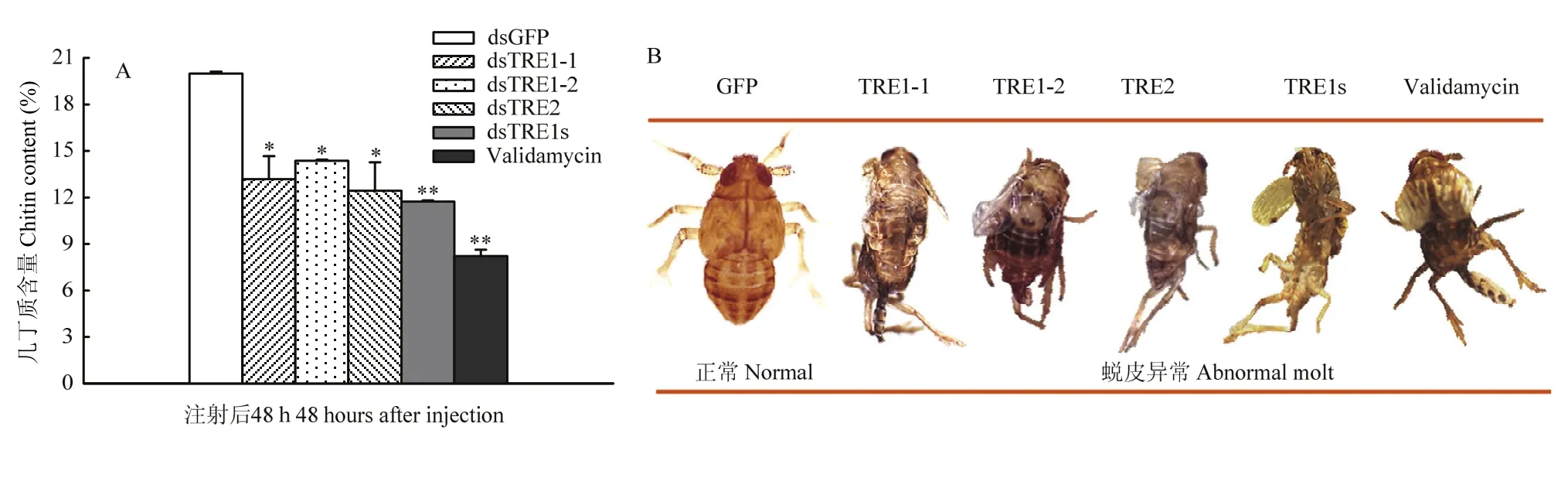
图2 褐飞虱3个海藻糖酶基因表达被抑制后几丁质含量检测及蜕皮情况Fig. 2 The chitin content detected and moulting changes after 3 trehalase genes expression inhibited by the way of RNAi

图3 褐飞虱3个海藻糖酶基因RNAi后几丁质合成酶及可变转录子在mRNA相对表达水平Fig. 3 The relative expression level of chitin synthase and its splicing variants of N. lugens after 3 trehalase genes RNAi
2.4 TRE基因RNAi后几丁质酶基因表达变化
dsTRE1-1注射后48 h,Cht1、Cht3、Cht8和Cht10表达下降,其中与注射dsGFP组相比较仅Cht8表达显著下降,其余6个Cht表达上升,且Cht2和Cht5显著上升;dsTRE1-2注射组中,Cht1、Cht6和Cht8表达下降,Cht2和Cht4表达显著上升;dsTRE2处理组中,Cht1、Cht8和Cht10表达下降,而Cht9表达显著上升;dsTRE1s注射后,Cht1和Cht5表达显著下降,而 Cht9表达显著上升;海藻糖酶抑制剂Validamycin注射组中所有的10个几丁质酶基因表达都显著或极显著下降。进一步分析发现在4个TRE的dsRNA注射组中,Cht1和Cht8的表达下降,但并不是所有的处理组都显著下降(图4)。
3 讨论
RNAi是基因功能研究的有效工具,主要通过注射dsRNA或siRNA抑制基因的表达[25]。目前,RNAi技术已经广泛用于探索和研究昆虫的相关基因功能,相关研究结果显示当昆虫发育的关键基因被沉默后,经常会在不同的组织表型上显示出来[5-6,26-29]。前期研究中,注射单个基因的dsTRE后,48 h和72 h褐飞虱整体中该基因的表达都能有效地被抑制[17]。本研究中,单个和混合TRE基因RNAi后,表皮中该基因的表达也都下降(图1),这与TRE基因RNAi后整体中本基因的表达类似。同时,在褐飞虱几丁质合成通路、海藻糖合成酶和海藻糖酶等相关的基因功能研究中,褐飞虱出现蜕皮困难等现象[5-6,15-16],本试验结果显示单个TRE的dsRNA或者注射混合TRE1的dsRNA均出现了蜕皮困难现象,与几丁质合成或降解受到阻碍的结果一致(图2-B),与前期研究也一致[17]。在前期研究中发现TRE表达被抑制后,褐飞虱不但蜕皮困难,翅发育也会受阻,这表明几丁质合成或降解受到严重影响,本试验进一步检测TRE基因RNAi后几丁质含量情况,结果显示与对照组相比较,褐飞虱的几丁质含量显著下降(图 2-A),这与采用Validamycin抑制褐飞虱海藻糖酶酶活性后的结果吻合[22]。
昆虫拥有可溶性和膜结合型两种类型海藻糖酶,其在多数昆虫组织中都有表达[13]。近年来,借助于转录组等研究发现昆虫TRE1不止一条编码基因存在,如褐飞虱包含TRE1-1和TRE1-2两条[17],赤拟谷盗(Tribolium castaneum)拥有4条可溶性海藻糖酶基因[30],异色瓢虫(Harmonia axyridis)中至少存在 3条或3条以上的TRE1[31-32]。不同可溶性海藻糖酶可能存在着功能差异。TRE1主要在中肠中表达,负责内源性海藻糖的分解,在血淋巴、中肠和马氏管中高表达[33];而TRE2主要负责外源性海藻糖酶的吸收和同化,在脂肪体、中肠和马氏管中高表达[9,15,34-37]。表皮作为昆虫几丁质最主要的部分,几丁质含量比例高,本研究发现与dsGFP注射组几丁质含量(21%)相比较,Validamycin注射组几丁质含量显著降低至9%的水平,而4个dsTRE处理组中几丁质含量在12%—14%,表明TRE1和TRE2都能够控制褐飞虱几丁质的合成。但是,采用海藻糖酶抑制 Validamycin能够非常有效降低两类TRE酶活[22],从而控制褐飞虱几丁质的代谢,降低几丁质含量,提高褐飞虱的死亡率和畸形率。
近年来,对赤拟谷盗、甜菜夜蛾、褐飞虱等昆虫海藻糖酶-几丁质调控通路的研究较多,结果均表明 TRE对几丁质的合成与分解存在一定的调控作用[15-16,38-39],而飞蝗(Locusta migratoria)海藻糖酶基因表达干扰后,测定几丁质合成关键基因——UDP-N-乙酰氨基葡萄糖焦磷酸化酶1 基因(LmUAP1)和几丁质合成酶1基因(LmCHS1)的表达,发现并未对表达量产生影响,5龄若虫可正常蜕皮至成虫[14]。因此,不同生物体内TRE功能存在一些差别,但大多数研究表明TRE1与TRE2影响不同部位的几丁质合成[9]。研究表明褐飞虱体中可能缺乏CHS2,分别向褐飞虱注射dsCHS1、dsCHS1a和dsCHS1b,发现CHS1和CHS1a的基因干扰会导致褐飞虱翅畸形、细腰、表皮皱缩并最终死亡[20]。本试验中,注射单个dsTRE或者混合的dsTRE1s后48 h,表皮中CHS1、CHS1a和CHS1b表达无显著差异(图3),而在前期试验中发现当TRE1和TRE2的表达被抑制后,褐飞虱整体中的CHS1a和CHS1b表达都会降低或显著降低[17],表明2个TRE1基因在褐飞虱不同组织的几丁质合成酶中的功能不同,TRE1可能在调控褐飞虱其他组织CHS表达上更为明显。
同样,采用RNAi方法抑制褐飞虱任何一个TRE的表达后,Cht3和Cht10在48 h的表达都显著下降,而有些Cht的表达会显著上升,但是在dsTRE注射后72 h大多数的Cht表达都显著下降或极显著下降[17]。对dsTRE注射后48 h Cht的表达情况进行研究,发现dsTRE1-1注射后表皮中 Cht8的表达显著下降,dsTRE1s注射后Cht1和Cht5表达显著下降,而多数情况下不同Cht的表达无显著差异或者上升(图4)。推测当2个TRE1同时表达抑制后主要通过降低Cht5的表达来调控几丁质合成,且Cht5本身在昆虫表皮合成中具有重要作用[5]。更为重要的是当TRE的表达被抑制后,褐飞虱几丁质代谢平衡被打破,昆虫出现发育畸形、体重减轻、几丁质合成减少、生长受阻、飞行减少甚至死亡的现象[39]。干扰TRE后出现了蜕皮困难等现象,这与几丁质合成酶[19-20,40-41]、几丁质酶[5]、几丁质脱乙酰酶[21,42]、β-N-乙酰乙糖胺酶[6]等几丁质合成和降解通路中相关酶被 RNA干扰后的现象一致。综上所述,无论是抑制了TRE还是几丁质代谢路径中的其他酶,几丁质含量减少是导致蜕皮困难最重要的原因。
4 结论
dsTRE注射到褐飞虱体内,能够有效降低表皮中靶标基因的表达;褐飞虱体内海藻糖代谢被打破后,可导致昆虫蜕皮困难,翅畸形等表型;海藻糖酶基因表达被抑制后,可导致褐飞虱体内几丁质代谢的紊乱,进一步导致死亡。
[1] BARRION A T, LITSINGER J A. Taxonomy of rice insect pests and their arthropod parasites and predators//HEINRICHS E A. Biology and Management of Rice Insects. Wiley Eastern Ltd., India and IRRI, Manila, Philippines, 1994.
[2] 赵梦, 欧阳芳, 张永生, 李魏, 曹婧, 戈峰. 2000-2010年我国水稻病虫害发生为害特征分析. 生物灾害科学, 2014, 37(4): 275-280.
ZHAO M, OUYANG F, ZHANG Y S, LI W, CAO J, GE F. Characteristics of occurrence and damage from diseases and insect pests in rice production in China during 2000-2010. Biology Disaster Science, 2014, 37(4): 275-280. (in Chinese)
[3] 赵颖, 黄凤宽, 童晓立, 庞雄飞. 水稻品种对褐飞虱不同生物型抗性的HPLC分析. 华南农业大学学报, 2005, 26(2): 52-55.
ZHAO Y, HUANG F K, DONG X L, PANG X F. HPLC analysis of rice variety resistance to different biotypes of Nilaparvata lugens. Journal of South China Agricultural University, 2005, 26(2): 52-55. (in Chinese)
[4] WANG Y C, TANG M, HAO P Y, YANG Z F, ZHU L L, HE G C. Penetration into rice tissues by brown plant hopper and fine structure of the salivary sheaths. Entomologia Experimentalis et Applicata, 2008, 129(3): 295-307.
[5] XI Y, PAN P L, YE Y X, YU B, XU H J, ZHANG C X. Chitinase-like gene family in the brown planthopper, Nilaparvata lugens. Insect Molecular Biology, 2015, 24(1): 29-40.
[6] XI Y, PAN P L, ZHANG C X. The β-N-acetylhexosaminidase gene family in the brown planthopper, Nilaparvata lugens. Insect Molecular Biology, 2015, 24(6): 601-610.
[7] GHAFFAR M B, PRITCHARD H, FORD L B. Brown planthopper (N. lugens Stål) feeding behavior on rice germplasm as an indicator of resistance. PLoS ONE, 2011, 6(7): e22137.
[8] 张建珍. 昆虫几丁质代谢与植物保护. 中国农业科学, 2014, 47(7): 1301-1302.
ZHANG J Z. Insect chitin metabolism and plant protection. Scientia Agricultura Sinica, 2014, 47(7): 1301-1302. (in Chinese)
[9] 张文庆, 陈晓菲, 唐斌, 田宏刚, 陈洁, 姚琼. 昆虫几丁质合成及其调控研究前沿. 应用昆虫学报, 2011, 48(3): 475-479.
ZHANG W Q, CHEN X F, TANG B, TIAN H G, CHEN J, YAO Q. Insect chitin biosynthesis and its regulation. Chinese Journal of Applied Entomology, 2011, 48(3): 475-479. (in Chinese)
[10] ZHU K Y, MERZENDORFER H, ZHANG W Q, ZHANG J Z, MUTHUKRISHNAN S. Biosynthesis, turnover, and functions of chitin in insects. Annual Review of Entomology, 2016, 61: 177-196.
[11] 屈明博, 刘田, 陈磊, 陈琦, 杨青. 昆虫糖基水解酶20家族β-N-乙酰己糖胺酶研究进展. 中国农业科学, 2014, 47(7): 1303-1312.
QU M B, LIU T, CHEN L, CHEN Q, YANG Q. Research progresses in insect glycosyl hydrolyase family 20 β-N-acetylhexosamindase. Scientia Agricultura Sinica, 2014, 47(7): 1303-1312. (in Chinese)
[12] 宋慧芳, 李应龙, 马恩波, 张建珍. 飞蝗 β-N-乙酰氨基葡萄糖苷酶基因的表达及酶学特性分析. 中国农业科学, 2016, 49(21): 4140-4148.
SONG H F, LI Y L, MA E B, ZHANG J Z. The heterogenous expression and enzymatic characteristics of β-N-acetylglucosaminidase from Locusta migratoria. Scientia Agricultura Sinica, 2016, 49(21): 4140-4148. (in Chinese)
[13] 唐斌, 魏苹, 陈洁, 王世贵, 张文庆. 昆虫海藻糖酶的基因特性及功能研究进展. 昆虫学报, 2012, 55(11): 1315-1321.
TANG B, WEI P, CHEN J, WANG S G, ZHANG W Q. Progress in gene features and functions of insect trehalases. Acta Entomologica Sinica, 2012, 55(11): 1315-1321. (in Chinese)
[14] 刘晓健, 孙亚文, 崔淼, 马恩波, 张建珍. 飞蝗海藻糖酶基因的分子特性及功能. 中国农业科学, 2016, 49(22): 4375-4386.
LIU X J, SUN Y W, CUI M, MA E B, ZHANG J Z. Molecular characteristics and functional analysis of trehalase genes in Locusta migratoria. Scientia Agricultura Sinica, 2016, 49(22): 4375-4386. (in Chinese)
[15] CHEN J, TANG B, CHEN H X, YAO Q, HUANG X F, CHEN J, ZHANG D W, ZHANG W Q. Different functions of the insect soluble and membrane-bound trehalase genes in chitin biosynthesis revealed by RNA Interference. PLoS ONE, 2010, 5(4): e10133.
[16] YANG M M, ZHAO L N, SHEN Q D, XIE G Q, WANG S G, TANG B. Knockdown of two trehalose-6-phosphate synthases severely affects chitin metabolism gene expression in the rice brown planthopper Nilaparvata lugens. Pest Management Science, 2017, 73(1): 206-216.
[17] ZHAO L N, YANG M M, SHEN Q D, SHI Z K, WANG S G, TANG B. Knockdown of three trehalases regulating trehalose and chitin metabolism in the rice brown planthopper Nilaparvata lugens. Science Reports, 2016, 6: 27841.
[18] 于彩虹, 卢丹, 林荣华, 王晓军, 姜辉, 赵飞. 海藻糖——昆虫的血糖. 昆虫知识, 2008, 45(5): 832-837.
YU C H, LU D, LIN R H, WANG X J, JIANG H, ZHAO F. Trehalose—the blood sugar in insects. Chinese Bulletin of Entomology, 2008, 45(5): 832-837. (in Chinese)
[19] CHEN X F, TIAN H G, ZOU L Z, TANG B, HU J, ZHANG W Q. Disruption of Spodoptera exigua larval development by silencing chitin synthase gene A with RNA interference. Bulletin of Entomological Research, 2008, 98(6): 613-619.
[20] WANG Y, FAN H W, HUANG H J, XUE J, WU W J, BAO Y Y, XU H J, ZHU Z R, CHENG J A, ZHANG C X. Chitin synthase 1 gene and its two alternative splicing variants from two sap-sucking insects, Nilaparvata lugens and Laodelphax striatellus (Hemiptera: Delphacidae). Insect Biochemistry and Molecular Biology, 2012, 42(9): 637-646.
[21] 席羽. 褐飞虱几丁质降解酶系基因家族分析[D]. 杭州: 浙江大学, 2014.
XI Y. Analyses of chitinolytic enzyme gene families in Nilaparata lugens[D]. Hangzhou: Zhejiang University, 2014. (in Chinese)
[22] TANG B, YANG M M, SHEN Q D, XU Y X, WANG H J, WANG S G. Suppressing the activity of trehalase with Validamycin disrupts the trehalose and chitin biosynthesis pathways in rice brown planthopper, Nilaparvata lugens. Pesticide Biochemistry and Physiology, 2016, doi:10.1016/j.pestbp.2016.10.003.
[23] 曹传旺, 高彩球. 昆虫生化与分子生物学实验技术. 哈尔滨: 东北林业大学出版社, 2009: 24-26.
CAO C W, GAO C Q. Experimental Technology of Insect Biochemistry and Molecular Biology. Harbin: Northeast Forestry University Press, 2009: 24-26. (in Chinese)
[24] LIVAKA K J, SCHMITTGENB T D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-CT△△method. Methods, 2001, 25(4): 402-408.
[25] FIRE A, XU S, MONTGOMERY M K, KOSTAS S A, DRIVER S E, MELLO C C. Potent and specific genetic interference by doublestranded RNA in Caenorhabditis elegans. Nature, 1998, 391(6669): 806-811.
[26] ZHU Q S, ARAKANE Y, BEEMAN R W, KRAMER K J, MUTHUKRISHNAN S. Functional specialization among insect chitinase family genes revealed by RNA interference. Proceedings of the National Academy of Sciences of the United States of America, 2008, 105(18): 6650-6655.
[27] BELLES X. Beyond Drosophila: RNAi in vivo and functional genomics in insects. Annual Review of Entomology, 2010, 55: 111-128.
[28] SCOTT J G, MICHEL K, BARTHOLOMAY L C, SIEGFRIED B D, HUNTER W B, SMAGGHE G, ZHU K Y, DOUGLAS A E. Towards the elements of successful insect RNAi. Journal of Insect Physiology, 2013, 59(12): 1212-1221.
[29] FU K Y, LI Q, ZHOU L T, MENG Q W, LÜ F G, GUO W C, LI G Q. Knockdown of juvenile hormone acid methyl transferase severely affects the performance of Leptinotarsa decemlineata (Say) larvae and adults. Pest Management Science, 2016, 72(6): 1231-1241.
[30] TANG B, WEI P, ZHAO L N, GUO H S, WANG S G. Knockdown of five trehalase genes using RNA interference regulates the gene expression of the chitin biosynthesis pathways in Tribolium castaneum. BMC Biotechnology, 2016, 16(1): 67.
[31] TANG B, QIN Z, SHI Z K, WANG S, GUO X J, WANG S G,ZHANG F. Trehalase in Harmonia axyridis (Coleoptera: Coccinellidae): effects on beetle locomotory activity and the correlation with trehalose metabolism under starvation conditions. Applied Entomology and Zoology, 2014, 49(2): 255-264.
[32] SHI Z K, LIU X J, XU Q Y, QIN Z, WANG S, ZHANG F, WANG S G, TANG B. Two novel soluble trehalase genes cloned from Harmonia axyridis and regulation of the enzyme in a rapid changing temperature. Comparative Biochemistry and Physiology Part B, Biochemistry & Molecular Biology, 2016, 198: 10-18.
[33] TAKIGUCHI M, NIIMI T, SU Z H, YAGINUMA T. Trehalase from male accessory gland of an insect, Tenebrio molitor. cDNA sequencing and developmental profile of the gene expression. The Biochemical Journal, 1992, 288(1): 19-22.
[34] BECKER A, SCHLÖDER P, STEELE J E, WEGENER G. The regulation of trehalose metabolism in insects. Experientia, 1996, 52(5): 433-439.
[35] SILVA M C, TERRA W R, FERREIRA C. The role of carboxyl, guanidine and imidazole groups in catalysis by a midgut trehalase purified from an insect larvae. Insect Biochemistry and Molecular Biology, 2004, 34(10): 1089-1099.
[36] MITSUMASU K, AZUMA M, NIIMI T, YAMASHITA O, YAGINUMA T. Membrane-penetrating trehalase from silkworm Bombyx mori. Molecular cloning and localization in larval midgut. Insect Molecular Biology, 2005, 14(5): 501-508.
[37] TANG B, CHEN X F, LIU Y, TIAN H G, LIU J, HU J, XU W H, ZHANG W Q. Characterization and expression patterns of a membrane-bound trehalase from Spodoptera exigua. BMC Molecular Biology, 2008, 9: 51.
[38] 张倩, 鲁鼎浩, 蒲建, 吴敏, 韩召军. 灰飞虱海藻糖酶基因的克隆及RNA干扰效应. 昆虫学报, 2012, 55(8): 911-920.
ZHANG Q, LU D H, PU J, WU M, HAN Z J. Cloning and RNA interference effects of trehalase genes in Laodelphax striatellus (Homoptera: Delphacidae). Acta Entomologica Sinica, 2012, 55(8): 911-920. (in Chinese)
[39] SHUKLA E, THORAT L J, NATH B B, GAIKWAD S M. Insect trehalase: physiological significance and potential applications. Glycobiology, 2015, 25(4): 357-367.
[40] ARAKANE Y, SPECHT C A, KRAMER K J, MUTHUKRISHNAN S, BEEMAN R W. Chitin synthases are required for survival, fecundity and egg hatch in the red four beetle, Tribolium castaneum. Insect Biochemistry and Molecular Biology, 2008, 38(10): 959-962.
[41] TIAN H G, PENG H, YAO Q, CHEN H X, XIE Q, TANG B, ZHANG W Q. Developmental regulation of a lepidopteran pest Spodoptera exigua by ingestion of bacteria expressing dsRNA of a non-midgut gene. PLoS ONE, 2009, 4(7): e6225.
[42] ARAKANE Y, DIXIT R, BEGUM K, PARK Y, SPECHT C A, MERZENDORFER H, KRAMER K J, MUTHUKRISHNAN S, BEEMAN R W. Analysis of functions of the chitin deacetylase gene family in Tribolium castaneum. Insect Biochemistry and Molecular Biology, 2009, 39(5/6): 355-365.
(责任编辑 岳梅)
Regulatory Function of Trehalase Genes on Chitin Metabolism in the Cuticle of Nilaparvata lugens
ZHANG Lu, ZHU ShiCheng, ZHENG Hao, SHEN QiDa, WANG ShiGui, TANG Bin
(College of Life and Environmental Science, Hangzhou Normal University, Hangzhou 310036)
【Objective】The previous research results showed that insect trehalase (TRE) can regulate chitin metabolism and control the molting process. In this study, the brown planthopper (Nilaparvata lugens) molting process, the changes of the expression of chitin content and chitin synthase (CHS) and chtinase (Cht) genes were detected when TRE genes were knocked down by the way of RNAi, in order to explore the roles of different trehalase genes in the regulation of chitin metabolism in the epidermis.【Method】N. lugens fed in the lab was chosen as the experimental material, and RNAi technology was used to inhibit the single or two TRE genes’ expression by injection of double stranded RNA. The total RNA was extracted from the cuticle of N. lugens using the TRIzol®reagent as instructed by manufacturer. First-stand cDNA was synthesized using the PrimeScriptTMRT reagent Kit with gDNA Eraser following the manufacturer’s instructions. And the effect of RNAi was firstly determined after 48 h of injection by quantitative real-time PCR (qRT-PCR). Secondly, the chitin content of N. lugens whole body was determined at 48 h using potassium hydroxide method qRT-PCR, and photos of the insects with molting difficulties were taken in the same time. In the last, the relative expression levels of CHS and Cht of N. lugens were detected by qRT-PCR, and the regulatory function on chitin metabolism of TRE was analyzed at the same time. 【Result】Compared with the injection of dsGFP which was used as a control group, the results showed that in other groups injected with dsRNA the chitin content of N. lugens was decreased significantly, in which the dsTRE1 mixed injection group and the Validamycin injection group showed a significant decrease, meanwhile its molting problems appeared at the same time. qRT-PCR results showed that the gene expression of individual TRE was inhibited at 48 h after one TRE dsRNA injection, and the other TRE expression was increased and indicated it has a complementary function. The expressions of TRE1-2 and TRE2 were decreased in all groups, and dsTRE1s also inhibited the expression of TRE2. Secondly, the obvious effects could be found when mixed dsTRE1 trehalase inhibitor Validamycin injected into N. lugens and TRE genes were decreased significantly. The expression level of CHS and its splicing variants had no obvious effect when every TRE genes’ expression was knocked down, while CHS1 and CHS1a expressions were significantly decreased at 48 h after Validamycin injection. The expression of CHS1 in the cuticle increased after dsTRE1-2 injection and the expression of CHS1a increased after injection of dsTRE1-2. Thirdly, the expression levels of Cht1 and Cht8 decreased or decreased significantly after four dsTRE and Validamycin injection. The expression levels of Cht2 and Cht5 increased significantly when dsTRE1 was injected, as well as Cht2 and Cht4 increased significantly while Cht1, Cht6 and Cht8 decreased after dsTRE1-2 injection and Cht2 expressed increased significantly while the expression of Cht1, Cht8 and Cht10 decreased at 48 h when TRE2 knocked down. In the same time, the expressions of Cht1 and Cht5 decreased significantly while Cht9 increased significantly at 48 h after dsTRE1s injection. In the last, about all of 10 chitinase genes’ expression decreased significantly or extremely significantly after Validamycin injection.【Conclusion】TRE can control the synthesis of chitin through the regulation of chitin metabolic pathways in N. lugens. The results of this study will provide a theoretical basis for developing and screening effective trehalase inhibitors to control N. lugens.
Nilaparvata lugens; RNA interference; trehalase; cuticle; chitin metabolism; quantitative real-time PCR (qRT-PCR)
2016-12-13;接受日期:2017-01-16
国家自然科学基金(31371996,31672081)、杭州市科技局计划(20140432B01)
联系方式:张露,E-mail:zhanglu_1140@163.com。通信作者唐斌,Tel:0571-28865680;E-mail:tbzm611@163.com

