应用FQ-PCR技术测定鼻咽癌患者血浆EB病毒DNA定量分析及其临床意义
李 冰 司勇锋 覃扬达 张 政
应用FQ-PCR技术测定鼻咽癌患者血浆EB病毒DNA定量分析及其临床意义
李 冰 司勇锋 覃扬达 张 政
目的 应用FQ-PCR技术测定鼻咽癌患者血浆中游离EB病毒DNA拷贝,探讨血浆EB病毒DNA定量测定的临床意义。方法 选取132例鼻咽癌患者,取其外周血样本,其中84例治疗前血样,48例治疗后血样(放疗或加化疗)。另收集60例健康血样作为正常对照。使用广州中山医科大学达安基因诊断中心提供(Cat.#DA-061)的EB病毒DNA-PCR试剂盒,测定鼻咽癌患者血浆中游离EB病毒DNA拷贝,阴性对照为空白PCR反应液,阳性对照为106、104、102拷贝/ul的阳性模板。结果 鼻咽癌组治疗前84例样本,阳性率为67.86%,鼻咽癌组治疗后48例样本阳性率为35.42%,正常对照者30例样本阳性率仅为8.33%,鼻咽癌组治疗前血浆游离EB病毒DNA的阳性率显著高于对照组,差异显著(χ2=11.497,P=0.001);鼻咽癌组治疗后与对照组血浆游离EB病毒DNA的阳性率比较,差异较显著(χ2=6.782,P=0.018);鼻咽癌组治疗前血浆中游离EB病毒-DNA阳性率约是治疗后的2倍,差异显著(χ2=6.271,P=0.023)。鼻咽癌组治疗前血浆游离EB病毒DNA拷贝中位数为522.0 copies/ml,治疗后中位数为0.0,对照组中位数为0.0,鼻咽癌组治疗前的血浆游离EB病毒DNA拷贝数显著高于治疗后,两者差异具有统计学意义(U=350.0,P=0.029),而且与正常对照组拷贝数比较,差异亦显著(U=274.0,P=0.001)。Ⅰ~Ⅱ期患者的血浆游离EB病毒DNA水平显著低于Ⅲ~Ⅳ期患者(U=141.0,P=0.039)。N0+N1期患者的血浆EB病毒DNA水平显著低于N2+N3期患者(U=147.0,P=0.031)。结论 FQ-PCR技术具有快速、精确和高灵敏性的特点,比其它传统检测手段更实用。血浆EB病毒DNA的定量PCR分析对鼻咽癌的筛选检查具有应用价值。
FQ-PCR技术;鼻咽癌;EB病毒;DNA定量分析
(ThePracticalJournalofCancer,2017,32:200~203)
自1970年Henle、Zur Hausen等人在鼻咽癌患者血液及肿瘤组织中检测到Epstein-Barr(EB)病毒,从此人们开始关注EB病毒感染与鼻咽癌发生之间的关系[1]。EB病毒相关抗原的抗体大量存在于鼻咽癌患者血液中,以病毒壳抗原-抗体(VCA-IgA)为主,是我国常用筛选检查鼻咽癌的方法之一[2],但其只能指示发生鼻咽癌的危险性,不能监测到鼻咽癌的进展[3]。Lo等人率先在97%的鼻咽癌患者血浆中,应用荧光定量FQ-PCR技术测得不同水平的EB病毒DNA拷贝[4]。荧光定量FQ-PCR技术具有快速、精确和高灵敏性的特点,所以本研究应用FQ-PCR技术测定鼻咽癌患者血浆中游离EB病毒DNA拷贝,探讨血浆EB病毒DNA定量测定的临床意义。
1 材料与方法
1.1 一般资料
选取2014年1月至2015年12月在我院确诊并诊治的132例鼻咽癌患者,其中治疗前血样84例,治疗后(放疗或加化疗)血样48例。另收集60例健康血样作为正常对照。鼻咽癌132例中,男性95例,女性37例,年龄18~70岁,中位年龄49.0岁。按2002年美国AJCC鼻咽癌TNM分期,Ⅰ期15例、Ⅱ期56例、Ⅲ期25例、Ⅳ期36例。60例健康血样中,男性44例,女性16例,年龄30~55岁,平均41岁;鼻咽癌患者淋巴结临床分级标准:N0,影像学及体检无淋巴结转移证据;N1,单侧及锁骨上窝淋巴结最大直径<6 cm;N2,双侧Ⅰb、Ⅱ、Ⅲ、Ⅴa区淋巴结转移,或直径>3 cm,或淋巴结包膜外侵犯;N3,Ⅳ、Ⅴb区淋巴结转移。
1.2 实验方法
定量PCR技术测定血浆EB病毒DNA拷贝:取外周血浆2 ml,在室温下溶解,取其在4 ℃的温度下以每分钟12 000转离心10 min后的沉淀物,然后加含0.5%SDS、100 μg/ml蛋白酶K的DNA提取液,煮沸10 min、随后冰浴10 min,最后取其在4 ℃的温度下以每分钟10 000转离心5 min后的上清液2 μl,行PCR定量扩增(美国PE公司Sequence Detector 7700)[5]。扩增程序为93 ℃ 2 min预变性;93 ℃ 45 s→55 ℃ 2 min,40个循环;33 ℃ 3 min延伸[6]。由广州中山医科大学达安基因诊断中心提供(Cat.#DA-061)的EB病毒DNA-PCR试剂盒:含DNA提取液、PCR反应管、定量阳模和稀释液[7]。引物序列为5’-TCTCTGCCTCCAGGCAAG-3’和5’-AGAGGGCCTGTCCACCGT-3’,双标记荧光探针的序列为5’-(FAM)CTGTCTGTAAAGTCCAGCCTCC-(ATMRA)-3’[8]。阴性对照为空白PCR反应液,阳性对照为106、104、102拷贝/μl的阳性模板。荧光检测波长为518 nm[9]。
阳性细胞:在胞膜周围着色较深,呈棕色的细胞,按阳性细胞的数量和着色的强度确定级别。阴性细胞:细胞呈浅棕色的背景颜色或不着色[10]。
1.3 统计学处理
应用统计学分析软件SPSS 20.0对实验数据进行处理。采用χ2检验两组间阳性率的比较,采用Mann-Whiney U非参数秩和检验两组间血浆EB病毒DNA拷贝数。P<0.05表示两者差异显著,具有统计学意义。
2 结果
2.1 血浆游离EB病毒DNA阳性率
经FQ-PCR测定鼻咽癌组治疗前84例样本,阳性率为67.86%;鼻咽癌组治疗后48例样本阳性率为35.42%;正常对照者30例样本阳性率仅为8.33%。可见,对照组血浆游离EB病毒DNA的阳性率明显比鼻咽癌组治疗前低,差异显著(χ2=11.497,P=0.001);鼻咽癌组治疗后与对照组血浆游离EB病毒DNA的阳性率比较,差异较显著(χ2=6.782,P=0.018);另外,鼻咽癌组治疗前血浆中游离EB病毒-DNA阳性率约是治疗后的2倍,差异显著(χ2=6.271,P=0.023)。见表1。
2.2 血浆游离EB病毒DNA拷贝数
鼻咽癌组治疗前血浆游离EB病毒DNA拷贝中位数为522.0 copies/ml,治疗后中位数为0.0,对照组中位数为0.0。可见,鼻咽癌组治疗前的血浆游离EB病毒DNA拷贝数显著高于治疗后,两者差异具有统计学意义(U=350.0,P=0.029),而且与正常对照组拷贝数比较,差异亦显著(U=274.0,P=0.001)。
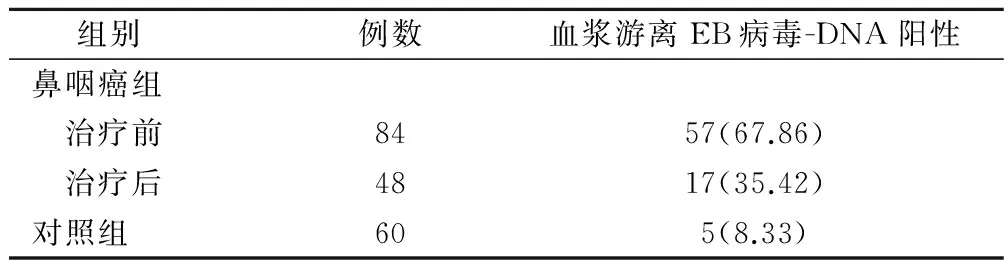
表1 各组患者血浆游离EB病毒-DNA阳性(例,%)
2.3 治疗前组早期与晚期血浆游离EB病毒DNA阳性率和拷贝数
鼻咽癌治疗前组,I~II期患者血浆游离EB病毒DNA阳性率为67.39%,与Ⅲ~Ⅳ期患者阳性率71.05%相近,两者比较无显著差异(χ2=1.352,P=0.793);Ⅰ~Ⅱ期患者血浆游离EB病毒DNA拷贝中位数为400.00 copies/ml,Ⅲ~Ⅳ期中位数为3130.00 copies/ml,四分位数差别亦很大,Ⅰ~Ⅱ期患者的血浆游离EB病毒DNA水平显著低于Ⅲ~Ⅳ期患者的(U=141.0,P=0.039),见表2。
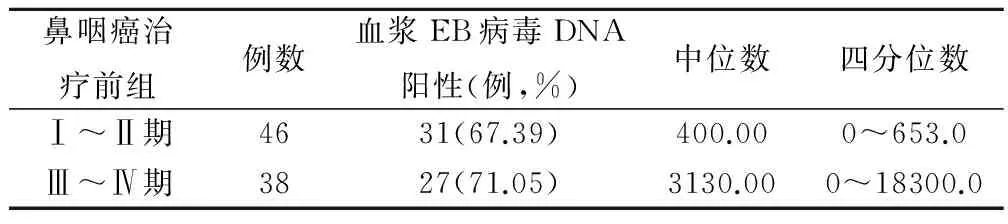
表2 治疗前组早期与晚期鼻咽癌患者血浆EB病毒DNA 阳性率和拷贝(copies/ml)
2.4 治疗前组不同N分级血浆游离EB病毒DNA阳性率和拷贝数
鼻咽癌治疗前组,N0、N1阳性检出率分别为50.00%、69.57%,与N2、N3阳性检出率80.00%、100.00%比较,总体无显著差异(χ2=2.326,P=0.611)。N0患者血浆游离EB病毒DNA拷贝数与N1患者比较无显著差异(U=56.0,P=1.032),N2患者血浆游离EB病毒DNA拷贝数与N3患者比较无显著差异(U=69.0,P=0.925);而N0+N1患者的血浆EB病毒DNA水平显著低于N2+N3患者(U=147.0,P=0.031),见表3。

表3 治疗前组不同N分级的鼻咽癌患者血浆EB病毒 DNA阳性率和拷贝(copies/ml)
3 讨论
1998年Mutirangura等率先应用PCR检侧鼻咽癌患者血清EB病毒DNA[11],次年Lo等率先在97%鼻咽癌患者血浆中,应用荧光定量FQ-PCR技术测得不同水平的EB病毒DNA拷贝[12]。从此,建立并逐渐完善实时定量测定鼻咽癌患者血浆EB病毒DNA的精确系统。FQ-PCR是在常规PCR基础上加入荧光素标记的探针,因此,它比常规PCR技术灵敏性更高,具有快速、精确的特点,更适宜应用在鼻咽癌的筛选检查上[13]。
EB病毒是1种疱疹病毒,对人类有致癌潜能[14]。人体内B淋巴细胞和复层鳞状上皮细胞是严格限定的EB病毒感染的靶细胞。EB病毒对B淋巴细胞的感染是非增殖性的,但对鳞状上皮细胞可发生增殖,严重可致癌。虽然EB病毒DNA广泛存在于鼻咽癌组织中,但EB病毒DNA从宿主细胞释放到血浆中的具体机制所知不多[15]。本研究结果显示,鼻咽癌组治疗前84例样本,阳性率为67.86%;鼻咽癌组治疗后48例样本阳性率为35.42%,鼻咽癌组治疗前血浆中游离EB病毒-DNA阳性率约是治疗后的2倍。鼻咽癌组治疗前血浆游离EB病毒DNA拷贝中位数为522.0copies/ml,治疗后中位数0.0;鼻咽癌组治疗前的血浆游离EB病毒DNA拷贝数显著高于治疗后,说明血浆游离EBV-DNA水平在一定程度上反映了鼻咽癌患者治疗前、后的肿瘤负荷[16]。
本研究结果显示,在鼻咽癌组治疗前中,Ⅰ~Ⅱ期患者的血浆游离EB病毒DNA阳性率67.39%,与Ⅲ~Ⅳ期患者阳性率71.05%相近,Ⅰ~Ⅱ期患者的血浆游离EB病毒DNA水平显著低于Ⅲ~Ⅳ期患者。可见,各阶段阳性检出率变化不大,血浆中的EB病毒DNA的阳性检出率不会随着病程的进展而提高[17]。N0组患者血浆游离EB病毒DNA拷贝数与N1组比较无显著差异,N2组患者血浆游离EB病毒DNA拷贝数与N3组比较无显著差异;说明EB病毒感染可能发生在肿瘤浸润性生长之前,而不是发生在鼻咽癌的进展期[18]。而N0+N1患者的血浆EB病毒DNA水平显著低于N2+N3患者。可见,随着肿瘤的进展,血浆中的EB病毒DNA拷贝数会逐渐升高,说明血浆EB病毒DNA水平可能与转移灶的肿瘤负荷有关[19]。
综上所述,FQ-PCR技术具有快速、精确和高灵敏性的特点,比其它传统检测手段更实用,应用FQ-PCR定量分析血浆EB病毒DNA对鼻咽癌的筛选检查具有重要意义,值得推广应用。
[1] Zheng X,Yan L,Nilsson B,et al.Epstem-Barn virus,salted fish and nasopharyngeal carcinoma〔J〕.Acta Oncol,1994,33(8):867-872.
[2] Deng H,Zeng Y,Lei Y,et al.Serological survey of nasopharyngeal carcinoma in 21 cities of south china〔J〕.Chin Med J(Engl),1995,108(8):300-303.
[3] 袁 晖.鼻咽癌患者血浆游离EB病毒DNA定量分析及其临床意义〔D〕.浙江大学医学院,2002.
[4] Chen XQ,Stroun M,Magnenat JL,et al.Microsatellite alterations in plasma DNA of small cell lung cancer patients〔J〕.Nat Med,1996,2(9):1033-1035.
[5] Nawroz H,Koch W,Anker P,et al.Microsatellite alterations in serum DNA of head and neck cancer patients〔J〕.Nat Med,2010,2(9):1035-1037.
[6] Vasioukhin V,Anker P,Mauric P,et al.Point mutations of the Nras gene in the blood plasma DNA of patients with myelodys plastic syndrome or acute myelogenous leukaemia〔J〕.Br J Haematol,2010,86(4):774-779.
[7] Shao JY,Li YH,Zhang Y,et al.Comparison of plasma Epstein-barr viruses DNA level and serum EBV VCA/Ig A antibody titers in nasopharyngeal carcinoma〔J〕.Cancer,2014,100(6):1162-1170.
[8] Lo YM,Chan AT,Chan LY,et al.Molecular prognostication of nasopharyngeal carcinoma by quantitative analysis of circulating Epstein-Barr virus DNA〔J〕.Cancer Res,2010,60(24):6878-6881.
[9] Lo YM,Chan LY,Chan AT,et al.Quantitative and temporal correlation between circulating cell-free Epstein-Barr virus DNA and tumor recurrence in nasopharyngeal carcinoma〔J〕.Cancer Res,2009,59(21):5452-5455.
[10] Baer R,Bankier AT,Biggin MD,et al.DNA sequence and expression of the B95-8 Epstein-Barr virus genome〔J〕.Nature,2012,310(4):207-211.
[11] Jons MD,Girfin BE.Clustered repeat sequences in the genome of stein-Barr virus〔J〕.Nucleic Acids Res,2013,11(12):3919-3937.
[12] Lo YM,Chan LY,Lo KW,et al.Quantitative analysis of cell-free Epstein-Barr virus DNA in plasma of patients with nasopharyngeal carcinoma〔J〕.Cancer Res,2009,59(6):1188-1191.
[13] Shotelersuk K,Khorprasert C,Sakdikul S,et al.Epstein-Barr virus DNA in serum/plasma as a tumor marker for nasopharyngeal carcinoma〔J〕.Clin Cancer Res,2010,6(3):1046-1051.
[14] Mutirangura A1,Pornthanakasem W,Theamboonlers A,et al.DNA in serum of patients with nasopharyngeal carcinoma Epstein-Barr viral〔J〕.Clin Cancer Res,2013,4:665-669.
[15] Gan YJ,Sullivan JL,Sixbey JW.Detection of cell-free Epstein-Barr virus DNA in serum during acute infectious mononucleosis〔J〕.J Infect Dis,1994,170(2):436-439.
[16] Zeng Y,Zhang LG,Li HY,et al.Serological mass survey for earlydet ection of Nasopharyngeal carcino main Wuzhou City China〔J〕.Int J Cancer,2012,29(2):139-141.
[17] Lo YM,Leung SF,Chan LY,et al.Kinetics of plasma Epstein-Barr virus DNA during radiation therapy for nasopharyngeal carcinoma〔J〕.Cancer Res,2000,60(9):2351-2355.
[18] Jin S,Inoue S,Weaver DT.Functions of the DNA dependent protein kinase〔J〕.Cancer Surv,2013,29(4):221-261.
[19] 叶新芊,李金高,龚晓昌,等.鼻咽癌血浆EB病毒DNA水平与肿瘤负荷的关系〔J〕.实用癌症杂志,2007,22(2):147.
(编辑:吴小红)
EB Virus DNA Quantitative Analysis of Plasma and Its Clinical Significance in Patients with Nasopharyngeal Application FQ-PCR Assay Technique
LIBing,SIYongfeng,QINYangda,etal.
ThePeople’sHospitalofGuangxi,Nanning,530021
Objective To study the FQ-PCR technique in patients with nasopharyngeal carcinoma plasma free EB virus DNA copies,and clinical significance of plasma EB virus DNA quantitative determination.Methods 132 patients with nasopharyngeal carcinoma were selected,whichever peripheral blood samples,including 84 cases of blood before treatment,after treatment,48 cases of blood(radiotherapy or chemotherapy).Another 60 healthy blood samples were collected as normal controls.Tat gene diagnosis center(Cat.# DA-061) of EB virus DNA-PCR kit plasma of patients with nasopharyngeal carcinoma of free copies of EB virus DNA negative control was blank PCR reaction solution,a positive control of 106,104,102 copies/ul positive template.Application of statistical analysis software SPSS20.0 experimental data processing.Results The treatment group 84 cases of nasopharyngeal carcinoma samples,the positive rate was 67.86%,after the treatment of 48 cases of nasopharyngeal carcinoma sample positive rate was 35.42%,30 cases of normal controls samples positive rate was 8.33%,nasopharyngeal carcinoma before treatment plasma free EB virus DNA positive rate was significantly higher,the difference was significant(χ2=11.497,P=0.001);compared with the control group,plasma free EB virus DNA positive rate of nasopharyngeal carcinoma after treatment,the 2 group was more significant(χ2=6.782,P=0.018);nasopharyngeal group therapy before bE viral-DNA positive rate of plasma free about 2 times after treatment,the difference was significant(χ2=6.271,P=0.023).Before treatment nasopharyngeal EB virus DNA in plasma free copy of median 522.0copies/ml,median was 0.0 after treatment,the median of the control group was 0.0,the copy number of free EB virus DNA in plasma of nasopharyngeal carcinoma group before treatment significantly higher than after treatment,the difference was statistically significant(μ=350.0,P=0.029),and compared with the control group,the number of copies,the difference was also significant(μ=274.0,P=0.001).Plasma free EB virus DNA level Ⅰ~Ⅱ patientswas significantly lower than the Ⅲ~Ⅳ patients(μ=141.0,P=0.039).N0+N1plasma EB virus DNA levels were significantly lower than patients with N2+N3(μ=147.0,P=0.031).Conclusion FQ-PCR technique is fast,accurate and highly sensitive,it is more practical than other traditional means of detection.Quantitative PCR analysis of EB virus DNA in plasma of nasopharyngeal cancer screening has value.
FQ-PCR technique;Nasopharyngeal carcinoma;EB virus;DNA quantitative analysis
530021 广西壮族自治区人民医院
10.3969/j.issn.1001-5930.2017.02.008
R739.63
A
1001-5930(2017)02-0200-04
2016-03-22
2016-10-13)

