18F-FDG PET/CT代谢参数在局灶性自身免疫性胰腺炎和胰腺癌鉴别诊断中的应用
贾国荣 张建 程超 李翠翠 冯菲 邱爽 左长京
200433 上海,第二军医大学长海医院核医学科
·论著·
18F-FDG PET/CT代谢参数在局灶性自身免疫性胰腺炎和胰腺癌鉴别诊断中的应用
贾国荣张建程超李翠翠冯菲邱爽左长京
200433上海,第二军医大学长海医院核医学科
【摘要】目的探讨(18)F-FDG PET/CT代谢参数在局灶性自身免疫性胰腺炎(F-AIP)与胰腺癌(PC)鉴别诊断中的价值。方法收集2011年5月至2014年12月经病理或临床随访证实并行(18)F-FDG PET/CT检查的10例F-AIP患者和20例同期经病理学证实且性别、年龄相匹配的PC患者。采用50%最大标准摄取值(SUV(max))作为阈值勾画感兴趣区,测量胰腺病灶SUV(max)、平均标准摄取值(SUV(mean))、肿瘤代谢体积(MTV)、糖酵解总量(TLG)、靶本比(TBR),并计算延迟前后各代谢参数的滞留指数(RI),分析PET/CT的形态学表现。结果F-AIP和PC均表现为局灶性代谢增高灶,F-AIP病灶位于胰头6例、胰体尾4例,PC病灶位于胰头12例、胰体尾8例。F-AIP组中2例主胰管扩张,6例肝内外胆管扩张,8例纵隔淋巴结代谢增高,2例腹腔淋巴结肿大;PC组上述表现分别为8、5、5、14例。F-AIP组纵隔淋巴结肿大检出率显著高于PC组,腹腔淋巴结肿大检出率显著低于PC组,差异均有统计学意义(P值均<0.05)。两组胰管扩张率及肝内外胆管扩张率的差异均无统计学意义。F-AIP组早期显像胰腺病灶的SUV(max)、SUV(mean)、MTV值分别为5.37±0.88、3.48±0.66、21.79±15.60,延迟期显像分别为6.45±1.51、4.23±1.10、19.36±14.63;PC组早期为8.31±3.08、5.41±1.95、9.26±8.35,延迟期为9.75±3.86、6.36±2.56、9.09±10.71。F-AIP组SUV(max)、SUV(mean)显著低于PC组,MTV显著高于PC组,差异均有统计学意义(P值均<0.05)。SUV(max)、SUV(mean)、MTV的受试者工作特征曲线下面积(AUC)以早期SUV(mean)为最大,达到0.85,最佳诊断临界值为4.45,诊断AIP的灵敏度为65%,特异度为90%。两组患者TLG、TBR及各参数的RI差异均无统计学意义。结论(18)F-FDG PET/CT的SUV(max)、SUV(mean)、MTV等代谢参数在F-AIP与PC的鉴别诊断中有一定的参考价值。
【关键词】自身免疫疾病;胰腺炎;胰腺肿瘤;体层摄影术,发射型计算机;体层摄影术,X线计算机;脱氧葡萄糖;诊断,鉴别
自身免疫性胰腺炎(autoimmune pancreatitis, AIP)是一种特殊类型的慢性胰腺炎,以胰腺局部或弥漫性肿大伴有胰管不规则狭窄、类固醇激素疗效显著为特征[1-2]。AIP根据组织病理学特征可分为两型[3],Ⅰ型为淋巴浆细胞硬化型胰腺炎,Ⅱ型为导管中心型胰腺炎。Ⅰ型AIP常见于亚洲老年男性,与IgG4系统性疾病相关,常见胰腺外器官浸润[1,4]。根据病灶的分布特点AIP又可分为弥漫性和局灶性[2,5]。AIP与胰腺癌(pancreatic cancer, PC)的影像学表现类似,但预后不同,局灶型AIP(F-AIP)与PC的鉴别尤为困难,因此探讨两者的鉴别方法有重要临床价值[1]。18F-FDG PET/CT是一种全身功能学显像技术,常用于良恶性病灶的鉴别及全身情况观察[6]。本研究收集行该项检查的F-AIP患者资料,分析其胰腺病灶代谢参数和胰外病灶形态特点,以评价该检查在F-AIP与PC鉴别诊断中的价值。
方法与材料
一、研究对象
收集2011年5月至2014年12月间在长海医院行18F-FDG PET/CT检查的F-AIP患者10例,并收集同期性别、年龄相匹配的PC患者20例。纳入标准:(1)PET/CT检查前2周内未行相关抗炎、抗肿瘤治疗;(2)F-AIP是在PET/CT检查后经EUS-FNA发现IgG4阳性细胞或根据典型影像学表现或临床随访6个月以上确诊,PC经手术病理或细胞学检查确诊。排除标准:PET/CT检查前2周内行ERCP或FNA等有创检查者。10例F-AIP患者均为男性,年龄45~67岁;活检标本中发现IgG4阳性细胞确诊3例,根据影像及临床随访确诊7例。20例PC患者也均为男性,年龄45~66岁;EUS-FNA及细胞学确诊11例,手术病理确诊9例(8例导管腺癌,1例腺鳞癌)。
二、PET/CT检查
采用西门子Biograph truepoint高分辨PET/CT仪进行检查。18F-FDG由上海原子科兴药业有限公司提供,放化纯度>95%。受检者检查前禁食6 h以上,血糖值<11.1 mmol/L,按体重静脉注射18F-FDG 3.70~5.55 MBq/kg。静息45~60 min后行常规PET/CT扫描,6~7个床位,1个床位2.5 min,扫描范围从颅顶至股骨中段。常规扫描结束后约40 min进行延迟显像,扫描范围从膈顶到双侧肾脏以下平面,1个床位,2.5 min。扫描结束后数据传至Multimodality后处理工作站TureD系统进行图像重建、融合,形成冠状面、横断面、矢状面断层图像及PET三维投影图像。
三、图像分析
采用后处理工作站The New 3D Freeform Isocontour Tool中的Contrast method,以50%最大标准摄取值(SUVmax)作为阈值勾画PET/CT检查感兴趣区(ROI),测量SUVmax、平均标准摄取值(SUVmean)、肿瘤代谢体积(MTV)、糖酵解总量(TLG,TLG=SUVmean×MTV)、靶本比(TBR,TBR=胰腺病灶SUVmax/肝脏SUVmax),并计算SUVmax、SUVmean、MTV、TLG和TBR的滞留指数[RI,RI=(延迟后病灶值-延迟前病灶值)/延迟前病灶值×100%]。PET/CT诊断胰腺摄取增高的标准为胰腺内结节状或肿块状放射性浓聚,代谢高于肝脏或明显高于周围胰腺组织;纵隔及双侧肺门淋巴结摄取增高的标准为淋巴结代谢高于纵隔血池;腹腔内淋巴结增大的标准为淋巴结短径≥5 mm;肝内胆管扩张的标准为一级胆管分支直径≥5 mm,肝外胆管扩张为≥8 mm;胰管扩张标准为主胰管直径>3.0 mm。由2名经验丰富的核医学科医师分别对每例融合图像进行独立分析,测量数据取两者平均值,诊断不一致时以讨论一致后的结果为准。另外也统计两组患者的CA19-9值。
四、统计学处理

结果
一、一般情况
F-AIP组平均年龄为(59±8)岁,PC组为(54±7)岁。F-AIP患者就诊原因为腹痛不适4例,腹痛合并黄疸1例,黄疸4例,B超体检发现胰腺占位1例;PC患者就诊原因为腹痛不适15例,黄疸4例,体检发现血清肿瘤标记物异常1例。10例F-AIP患者中血浆IgG4水平升高3例,20例PC患者均无升高。F-AIP患者血CA19-9水平为(49.03±44.14)U/ml,其中4例升高,为37~200 U/ml(正常值<37 U/ml);PC患者CA19-9水平为(617.18±481.41)U/ml,其中16例升高,12例>200 U/ml。PC患者的血CA19-9水平显著高于F-AIP患者,差异有统计学意义(t=-3.295,P=0.004)。10例F-AIP中6例病灶位于胰头,2例位于胰体,2例位于胰尾;20例PC患者中12例病灶位于胰头,3例位于胰体,5例位于胰尾。
二、影像学表现
F-AIP组中2例主胰管扩张,6例肝内外胆管扩张;PC组中8例主胰管扩张,5例肝内外胆管扩张,两组间差异均无统计学意义。F-AIP组中8例纵隔淋巴结代谢增高,PC组中5例纵隔淋巴结肿大,F-AIP组显著高于PC组,差异有统计学意义(K=0.512,P=0.006)。F-AIP组2例腹腔淋巴结肿大,PC组14例腹腔淋巴结肿大,F-AIP组显著低于PC组,差异有统计学意义(K=0.455,P=0.016;图1、2)。
三、胰腺病灶代谢参数
无论早期或延迟期显像,两组患者SUVmax、SUVmean、MTV的差异均有统计学意义,而TLG、TBR的差异均无统计学意义(表1)。F-AIP组SUVmax、SUVmean、MTV、TLG、TBR的RI分别为(18.99±12.01)%、(20.32±11.48)%、(-11.20±14.64)%、(6.32±17.38)%、(32.50±17.40)%,PC组分别为(16.04±37.76)%、(21.56±27.28)%、(-4.79±50.38)%、(10.30±47.62)%、(32.70±26.40)%,两组差异均无统计学意义(t值分别为0.239、-0.136、-0.391、-0.254、0.086,P值均>0.05)。早期显像中各项参数AUC大于延迟期显像各参数AUC,以早期SUVmean的AUC最大,达到0.85。SUVmax最佳诊断临界值为6.35,对应的灵敏度为70%,特异度为90%;SUVmean最佳诊断临界值为4.45,诊断AIP的灵敏度为65%,特异度为90%;MTV最佳诊断临界值为16.39,对应的灵敏度为60%,特异度为90%。
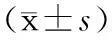
表1 F-AIP组和PC组双时相18F-FDG PET/CT显像的胰腺病灶代谢参数
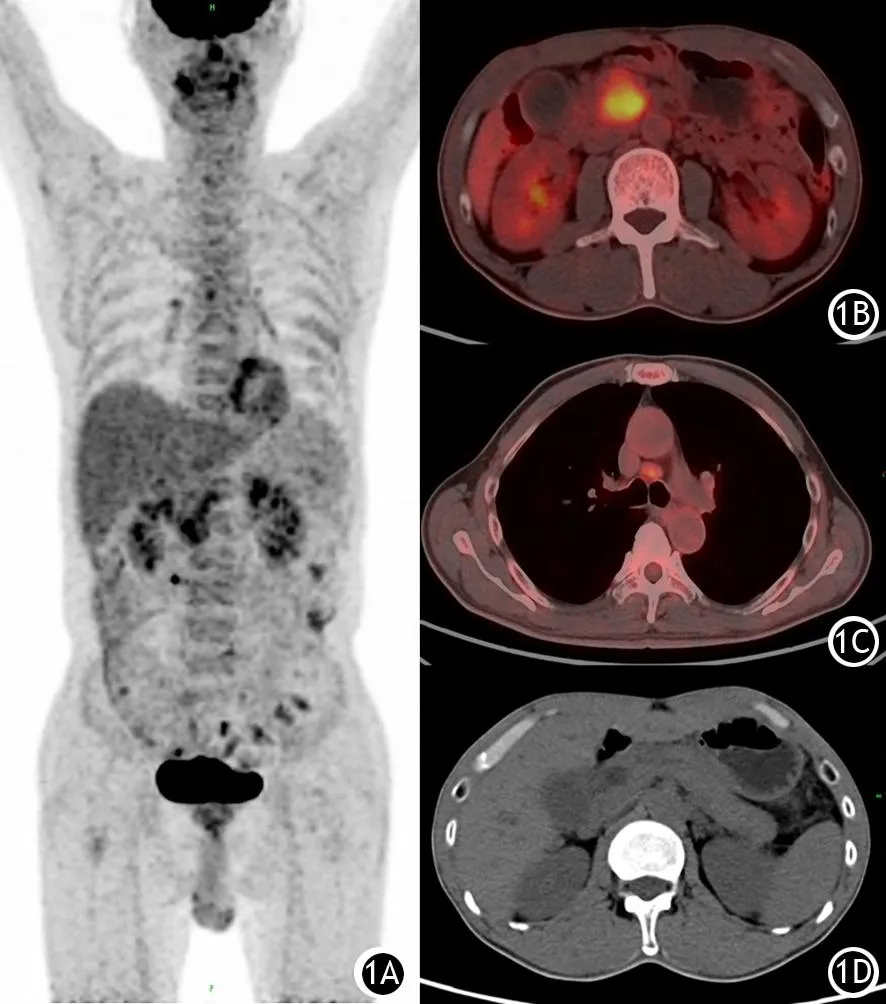
图1 F-AIP患者18F-FDG PET/CT显像图。1A.全身代谢图;1B.胰腺病灶;1C.纵膈淋巴结代谢增高;1D.主胰管轻度扩张
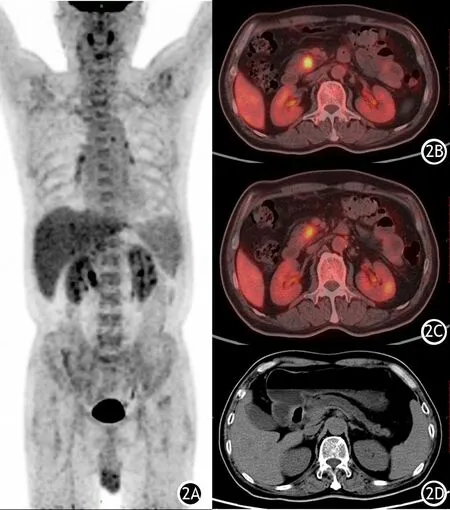
图2 PC患者18F-FDG PET/CT显像图。2A.全身代谢图;2B.胰腺病灶;2C.后腹膜淋巴结代谢轻度增高;2D.主胰管明显扩张
讨论
目前影像学诊断AIP的常用方法为增强CT[7],其特征表现为胰腺腊肠样肿胀,密度减低,动脉期强化程度低于正常胰腺,持续强化,延迟期强化程度接近胰腺实质[8-9]。其他征象包括延迟期轻度强化的胰腺周围包膜样环状影、肝内外胆管扩张等。AIP患者胰腺病灶的常规MR图像表现为T1等低信号,T2等高信号,强化特点同增强CT类似[10]。MRCP在显示胰管不规则狭窄方面较CT有优势,但在显示病灶代谢情况和胰腺外器官侵犯方面,PET/CT作为一种全身功能学显像技术与CT和MRI相比较有独特的优势。
根据AIP病灶分布特点,目前将AIP分为弥漫性、局灶性[2,5]。弥漫性AIP在PET/CT上表现为全胰腺FDG代谢增高,而PC常表现为局灶性摄取增高灶,二者易于鉴别[11-12]。但有两种情况AIP与PC表现类似,需注意鉴别。其一,当胰腺癌阻塞主胰管引起弥漫性胰腺炎时,弥漫性AIP与PC难以区分[7]。本研究有2例PC伴有阻塞性胰腺炎,胰腺癌病灶和阻塞性胰腺炎病灶均表现为不同程度的FDG浓聚,但胰腺癌病灶的FDG浓聚程度高于阻塞性胰腺炎病灶,通过病灶FDG浓聚程度的差异可以辅助与弥漫性AIP的鉴别。其二,当F-AIP发生于胰头时[12],仅通过局部病灶的FDG浓聚特征难以与PC鉴别[7]。因此本研究探讨胰腺病灶的代谢参数和胰外病灶形态特点,以提高两者的鉴别能力。
PET/CT检查的SUV是一种最常用来评价糖酵解代谢情况的参数,用于鉴别病变良恶性。Metser等[13]报道,超过半数的良性FDG代谢增高病灶表现为中度到显著高代谢,炎症是最常见的原因,但与肿瘤难以鉴别。Ozaki等[14]对15例AIP和26例PC的FDG代谢情况进行了半定量比较,结果显示两组病例SUV值差异无统计学意义。因此,由于肿瘤异质性的特点,SUVmax不能全面概括肿瘤的代谢情况, MTV、TLG能更好反映肿瘤整体代谢情况[15]。本研究结果显示,PC组与F-AIP组胰腺病灶的SUVmax、SUVmean、MTV的差异均有统计学意义,而TLG的差异无统计学意义。本结果与Ozaki等的研究结果不同,可能因本研究对象为F-AIP,而Ozaki等的研究对象为各型AIP所致。本研究中F-AIP组的MTV较PC组大,可能对鉴别F-AIP与PC有一定的参考意义。TLG作为MTV值与SUV值的乘积,F-AIP较高的MTV值和较低的SUV值相乘最终导致TLG值与PC组无显著性差异。
与PC组相比,F-AIP引起的胰管扩张往往相对温和[2],其病理原因可能是炎症浸润造成的不规则狭窄引起的扩张程度往往较肿块压迫造成的完全阻塞引起的扩张程度较轻所致。本研究的F-AIP组胰管扩张阳性率为20%,虽然低于PC组的40%,但差异无统计学意义,考虑与本研究样本量较小、未对胰管扩张程度进一步分级有关。延迟扫描对F-AIP与PC无鉴别诊断价值可能因非感染性炎症以及肿瘤异质性是削弱双时相研究诊断功能的因素之一[16]。
既往研究表明,92.2%的AIP患者表现为胰腺外病灶侵犯,包括唾液腺(47.5%)、肺门淋巴结(78.3%)、胆管壁增厚(77.8%)、后腹膜纤维化(19.8%)等[17]。当AIP侵犯肝内胆管及胰周、纵膈淋巴结时,往往表现为FDG代谢增高[18]。Ozaki等[14]的结果显示AIP组的肺门淋巴结显示率显著高于PC组。本研究的F-AIP组肺门淋巴结显示率高于PC组,与Ozaki等的结果一致,其原因可能为亚洲人群好发I型AIP,为IgG4系统性疾病之一,而IgG4疾病易发生肺门淋巴结侵犯。AIP和PC均可侵犯腹腔淋巴结,但PC的淋巴结转移率达37.2%[19]。本研究的PC患者腹腔淋巴结侵犯率也显著高于F-AIP患者。
综上所述,18F-FDG PET/CT的代谢参数SUVmax、SUVmean、MTV等可能为F-AIP与PC的鉴别提供有价值的信息。
参考 文 献
[1]Matsubayashi H, Kakushima N, Takizawa K, et al. Diagnosis of autoimmune pancreatitis[J]. World J Gastroenterol, 2014, 20(44): 16559-16569.DOI:10.3748/wjg.v20.i44.16559.
[2]Sahani DV, Kalva SP, Farrell J, et al. Autoimmune pancreatitis: imaging features[J]. Radiology, 2004, 233(2): 345-352.DOI:10.1148/radiol.2332031436.
[3]Park DH, Kim MH, Chari ST. Recent advances in autoimmune pancreatitis[J]. Gut, 2009, 58(12): 1680-1689.DOI:10.1136/GUT.2008.155853.
[4]Zhang L, Chari S, Smyrk TC, et al. Autoimmune pancreatitis (AIP) type 1 and type 2: an international consensus study on histopathologic diagnostic criteria[J]. Pancreas, 2011, 40(8): 1172-1179.DOI:10.1097/MPA.0b013e318233bec5.
[5]Yang DH, Kim KW, Kim TK, et al. Autoimmune pancreatitis: radiologic findings in 20 patients[J]. Abdom Imaging, 2006, 31(1): 94-102.DOI:10.1007/S00261-005-0047-8.
[6]Rosenbaum SJ, Lind T, Antoch G, et al. False-positive FDG PET uptake-the role of PET/CT[J]. Eur Radiol, 2006, 16(5): 1054-1065.DOI:10.1007/S0030-005-0088-Y.
[7]Crosara S, D′onofrio M, De Robertis R, et al. Autoimmune pancreatitis: Multimodality non-invasive imaging diagnosis[J]. World J Gastroenterol, 2014, 20(45): 16881-16890.DOI:10.3748/wjg.v20.i45.16881.
[8]Sun GF, Zuo CJ, Shao CW, et al. Focal autoimmune pancreatitis: radiological characteristics help to distinguish from pancreatic cancer[J]. World J Gastroenterol, 2013, 19(23): 3634-3641.DOI:10.3748/wjg.v19.i23.3634.
[9]Shimosegawa T, Chari ST, Frulloni L, et al. International consensus diagnostic criteria for autoimmune pancreatitis guidelines of the international association of pancreatology[J]. Pancreas, 2011, 40(3): 352-358.DOI:10.1097/MPA.0b013e31821429012.
[10]Kozoriz MG, Chandler TM, Patel R, et al. Pancreatic and extrapancreatic features in autoimmune pancreatitis[J]. Can Assoc Radiol J, 2015, 66(3): 252-258.DOI:10.1016/j.carj.2014.10.001.
[11]Lee TY, Kim MH, Park Do H, et al. Utility of 18F-FDG PET/CT for differentiation of autoimmune pancreatitis with atypical pancreatic imaging findings from pancreatic cancer[J]. Am J Roentgenol, 2009, 193(2): 343-348.DOI:10.2214/AJR.08.2297.
[12]Finkelberg DL, Sahani D, Deshpande V, et al. Autoimmune pancreatitis[J]. N Engl J Med, 2006, 355(25): 2670-2676.
[13]Metser U, Miller E, Lerman H, et al. Benign nonphysiologic lesions with increased 18F-FDG uptake on PET/CT: characterization and incidence[J]. Am J Roentgenol, 2007, 189(5): 1203-1210.DOI:10.2214/AJR.07.2083.
[14]Ozaki Y, Oguchi K, Hamano H, et al. Differentiation of autoimmune pancreatitis from suspected pancreatic cancer by fluorine-18 fluorodeoxyglucose positron emission tomography[J]. J Gastroenterol, 2008, 43(2): 144-151.DOI:10.1007/S00535-007-2132-Y.
[15]Son SH, Lee SW, Jeong SY, et al. Whole-body metabolic tumor volume, as determined by (18)F-FDG PET/CT, as a prognostic factor of outcome for patients with breast cancer who have distant metastasis[J]. Am J Roentgenol, 2015, 205(4): 878-885.DOI:10.2214/AJR.14.13906.
[16]Cheng G, Torigian DA, Zhuang H, et al. When should we recommend use of dual time-point and delayed time-point imaging techniques in FDG PET[J]? Eur J Nucl Med Mol Imaging, 2013, 40(5): 779-787.DOI:10.1007/S00259-013-2343-9.
[17]Fujinaga Y, Kadoya M, Kawa S, et al. Characteristic findings in images of extra-pancreatic lesions associated with autoimmune pancreatitis[J]. Eur J Radiol, 2010, 76(2): 228-238.DOI:10.1016/j.ejrad.2009.06.010.
[18]Nishino T, Oyama H, Hashimoto E, et al. Clinicopathological differentiation between sclerosing cholangitis with autoimmune pancreatitis and primary sclerosing cholangitis[J]. J Gastro-enterol, 2007, 42(7): 550-559.
[19]Kamisawa T, Nakajima H, Egawa N, et al. IgG4-related sclerosing disease incorporating sclerosing pancreatitis, cholangitis, sialadenitis and retroperitoneal fibrosis with lymphadenopathy[J]. Pancreatology, 2006, 6(1-2): 132-137.DOI:10.1159/000090033.
(本文编辑:吕芳萍)
Application of18F-FDG PET/CT metabolic parameters in differentiating focal autoimmune pancreatitis from pancreatic cancer
JiaGuorong,ZhangJian,ChengChao,LiCuicui,FengFei,QiuShuang,ZuoChangjing.DepartmentofNuclearMedicine,ChanghaiHospital,SecondMilitaryMedicalUniversity,Shanghai200433,China
【Abstract】ObjectiveTo evaluate the diagnostic value of the metabolic parameters for differentiating focal autoimmune pancreatitis (F-AIP) and pancreatic cancer (PC) by dual time (18)F-FDG PET/CT scan. MethodsTen F-AIP patients and 20 PC patients in Changhai Hospital from May 2011 to November 2014 were enrolled in this study. All the AIP patients were histological confirmed or diagnosed by clinical follow up. The PC patients were histological confirmed and gender- and age-matched with F-AIP patients. 50% SUV(max) was set as the threshold to fine-tune the boundary of interest. The extracted parameters included SUV(max), SUV(mean), metabolic tumor volume (MTV), total lesion glycolysis(TLG), target-to-background ratio (TBR) and the retention indexes(RI) of all the parameters above. The PET/CT imaging features were also observed. Results The high metabolic lesions were observed in both F-AIP patients and PC patients. There were 6 F-AIP patients whose lesion was located in pancreas head, 4 F-AIP patients whose lesion was located in pancreas body and tail. There were 12 PC patients whose lesion was located in pancreas head, 8 PC patients whose lesion was located in pancreas body and tail. In F-AIP patients, 2 cases had dilated pancreatic duct, 6 had dilated biliary duct, 8 had increased metabolism in mediastinal lymph node and 2 had abdominal lymphadenopathy, which were 8, 5, 5 and 14 cases in PC patients. The positive rate of mdeiastinal lymphadenopathy in F-AIP patients was statistically higher than that in PC patients, while the positivity rate of abdominal lymphadenopathy in AIP patients was lower than that in PC patients. The difference was statistically significant (both P<0.05).There were no statistical differences on the positivity rate of the dilated pancreatic duct, intra-and extra-hepatic bile duct between two groups. SUV(max), SUV(mean) and MTV in F-AIP were 5.37±0.88, 3.48±0.66, 21.79±15.60 in early stage and 6.45±1.51, 4.23±1.10, 19.36±14.63 in delayed stage, and those in PC were 8.31±3.08, 5.41±1.95, 9.26±8.35 in early stage, and 9.75±3.86, 6.36±2.56, 9.09±10.71 in delayed stage. SUV(max) and SUV(mean) in F-AIP were lower than those in PC, whereas MTV were larger in F-AIP than that in PC. ROC curves for SUV(max), SUV(mean) and MTV were made. The AUC of SUV(mean) was the highest at 0.85, the cut-off value was 4.45, the corresponding sensitivity was 65% and the specificity was 90%. TLG, TBR and RI of all the parameters were not statistically different in F-AIP and PC. ConclusionsThe (18)F-FDG PET/CT metabolic parameters, such as SUV(max), SUV(mean), MTV, could be of special diagnostic significance in discriminating F-AIP from PC.
【Key words】Autoimmune disease;Pancreatitis;Pancreatic neoplasms;Tomography, emission-computed;Tomography, X-ray computed;Deoxyglucose;Diagnosis, differential
(收稿日期:2015-12-25)
Corresponding author:Zuo Changjing, Email:changjing.zuo@qq.com
通信作者:左长京,Email:changjing.zuo@qq.com
DOI:10.3760/cma.j.issn.1674-1935.2016.02.005

