基于甲烷化反应的催化剂颗粒设计与过程强化
李军,朱庆山,李洪钟
基于甲烷化反应的催化剂颗粒设计与过程强化
李军,朱庆山,李洪钟
(中国科学院过程工程研究所多相复杂系统国家重点实验室,北京100190)
甲烷化反应过程的主要问题是“烧结”和“积炭”。基于甲烷化反应的强放热、减分子特性和对反应机理的认识,从催化剂与反应器的匹配性角度,论述了当前的主要甲烷化工艺、甲烷化催化剂、甲烷化反应及过程强化方法。流化床技术可有效防止催化剂的积炭和烧结,从与流化床反应器匹配的催化剂结构设计源头出发,制备具有耐磨损、易流化、低密度的高活性甲烷化催化剂,是流化床甲烷化发展的一个重要途径。
甲烷化;流化床反应器;强放热;减分子;镍催化剂;积炭
引 言
我国“富煤、贫油、少气”的能源结构特点决定了煤炭在能源利用中占主导地位,并且在今后相当长的时期内不会改变。然而,持续增加的天然气需求及日益严格的环保要求促使人们寻求新的煤炭利用途径和天然气来源。这使得煤制天然气技术的迅速发展成为煤炭洁净利用的选择之一。
煤制天然气是煤经过煤气化、合成气变换、净化、甲烷化等化学反应最终获得清洁燃料甲烷的过程[1]。其中,煤气化和合成气甲烷化是煤制天然气技术体系的核心。到目前为止,作为煤炭三大利用途径之一的煤气化技术已经成熟。然而,甲烷化技术仅有国外少数几个公司掌握,尚未实现国产化。甲烷化技术的核心是甲烷化催化剂和甲烷化反应器的研制和开发,其关键在于如何有效控制催化剂床层温度,避免因反应的强放热导致床层局部飞温现象[2-3]。
本文系统地论述了当前主要的甲烷化工艺、甲烷化催化剂和甲烷化反应机理的主要进展,提出了基于甲烷化反应机理的流化床反应器和催化剂颗粒设计是未来流化床甲烷化工艺的发展方向,以期为甲烷化工艺的进一步应用开发提供指导。
1 甲烷化工艺研究现状
甲烷化反应的一个重要工业应用是合成氨、燃料电池等富氢气体中痕量CO的脱除[4-9],但更加引人关注的应用是煤/生物质气化甲烷化制天然气工艺[10-17]。煤制天然气工艺大致包括煤气化、合成气变换、净化和甲烷化等,如图1所示。首先煤气化使煤颗粒与水蒸气和氧气在高温下反应得到粗合成气,主要成分包括H2、CO、CO2、H2O、CH4和少量碳氢化合物,含S、Cl杂质,其组分含量与气化工艺条件、反应器类型、气化剂等密切相关;由于粗合成气中焦油、含S/Cl等微量杂质气体对后续的反应器甲烷化催化剂有损害,需要经过气体净化装置处理;净化处理后的气体经水煤气变换反应调整H2和CO比例为3左右;进入甲烷化反应装置和提纯装置得到甲烷(>95%)。
表1概括了甲烷化过程主要反应,反应(1)为甲烷化反应的主反应,但实际反应过程中,存在CO歧化(2)、甲烷分解(3)等副反应,使催化剂表面积炭,降低催化剂的活性和使用寿命。同时甲烷也可能通过CO2甲烷化反应(4)和逆CO2-CH4重整反应(5)获得。其中反应(5)可以认为是甲烷化反应和水蒸气变换反应(6)的叠加。从热力学上分析,甲烷化反应为减分子的强放热反应,增加压力和低温对甲烷化反应有利。但在动力学上高温有利于提高甲烷化反应速率,同时从能量利用角度考虑,高温甲烷化有利于提高能量利用率,近年来受到研究者们的高度关注[18-19]。然而,在高温条件下催化剂积炭和活性金属烧结等问题是严重影响高温甲烷化的不利因素。因此,甲烷化工艺的难点在于如何有效控制反应区域的温度,防止催化剂积炭、烧结失活。其关键是甲烷化反应器和甲烷化催化剂的开发。
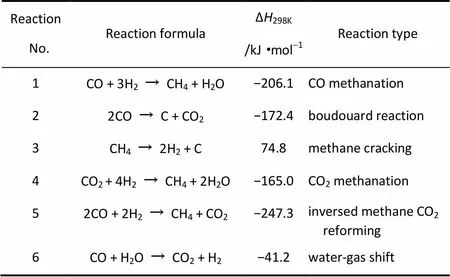
表1 甲烷化工艺过程主要反应和副反应[20-21]
1.1 固定床甲烷化工艺
甲烷化反应器与甲烷化催化剂并列为甲烷化技术的两大核心[22]。自20世纪50年代,研究者就开始致力于甲烷化反应器的开发,如固定床、流化床和浆态床甲烷化反应器[23-25]。由于固定床具有反应速率高、催化剂用量少、催化剂不易磨损等优点,已经成熟的工业化甲烷化技术普遍采用绝热多段固定床甲烷化反应器,包括丹麦托普索公司(Topsoe)的TREMPTM技术[26]、英国戴维公司(Davy)的CRG技术[27]和德国鲁奇公司(Lurgi)的甲烷化技术[28],均采用了固定床甲烷化反应器。但由于固定床反应器传热性能差,如何移出甲烷化反应大量放热是固定床甲烷化技术的关键。通常采用多段绝热式固定床反应器的串联方式,通过控制各段反应器的转化率、部分产品气体循环和内置或外置预热器等方法实现反应过程的温度控制。根据催化剂的耐受温度范围不同,其甲烷化工艺的操作温度和回收热量的方式有所不同。比如,丹麦的TREMPTM技术的特点是采用MCR-2X催化剂具有宽的温度窗口(250~700℃),在较高温度下(600℃)运行,可减少气体循环量和回收高压蒸汽热量,能量利用率高[29]。Davy的CRG甲烷化技术的特点是采用CRG催化剂具有变换功能,不需要调节合成气的H/C比,并且在250~700℃具有较高活性[30]。鲁奇甲烷化工艺的特点是甲烷化反应温度较低(450℃),采用气体循环限制原料气的进口温度(<300℃),防止催化剂积炭[31]。最近报道显示,鲁奇甲烷化工艺为了提高竞争力,开发了高温甲烷化催化剂,提高了甲烷化反应温度[32]。3种甲烷化技术各有特色,鲁奇和戴维的甲烷化技术得到了美国大平原项目的长期验证[33-34],引进托普索甲烷化技术的新疆庆华年产55亿立方米煤制天然气项目一期已于2013年8月竣工投产,产出的煤制天然气已送入西气东输管线[35]。而采用戴维甲烷化工艺的大唐内蒙古克什克腾旗年产40亿立方米煤制天然气一期示范项目已于2013 年12月投运,正式向北京供气[36]。
甲烷化反应器的设计通常与甲烷化工艺和催化剂配套,其反应器结构中很多经验取值与其配套工艺和催化剂密切相关,是甲烷化技术的关键技术之一。由于甲烷化反应具有反应迅速、放热量大、易积炭等特点,在反应器设计中,除了防止催化剂床层飞温、积炭失活问题外,还需要考虑诸如床层热点穿出、水浸入催化剂结构性破坏和反应器冷热位移等问题[37]。由于甲烷化工艺与催化剂高度匹配,目前只有丹麦托普索公司、英国戴维公司和德国鲁奇公司等少数公司掌握固定床甲烷化技术,国内鲜有关于固定床甲烷化反应器结构的文献报道。
多级串联的固定床反应器结构使得整体设备和流程相对复杂,工艺参数控制相对较难,同时需要返回大量的产品气稀释原料气,限制了生产能力,并且增加了动力消耗,因而操作成本较高,影响了工艺的整体经济性。为克服工业固定床工艺中的缺点,许多研究机构对甲烷化工艺及其设备进行改进,开发了流化床工艺和浆态床工艺。
1.2 流化床甲烷化工艺
与固定床反应器比较,流化床反应器具有相间接触良好、床层温度均匀的特点,易于规模化连续化操作的优势,特别适合于应用于强放热的甲烷化反应。1950~1980年间,先后有多个国家参与开发流化床甲烷化工艺,主要有美国矿务局(Bureau of Mines)建立的多段流化床甲烷化工艺[38]、美国Bituminous Coal Research Inc. (BCR, United States)公司的Bi-Gas流化床甲烷化工艺[39]和德国卡尔斯鲁厄大学(University of Karlsruhe)与Thyssenga公司合作开发的Comflux甲烷化工艺[40]。其工艺参数和运行状况列于表2中[38-42]。
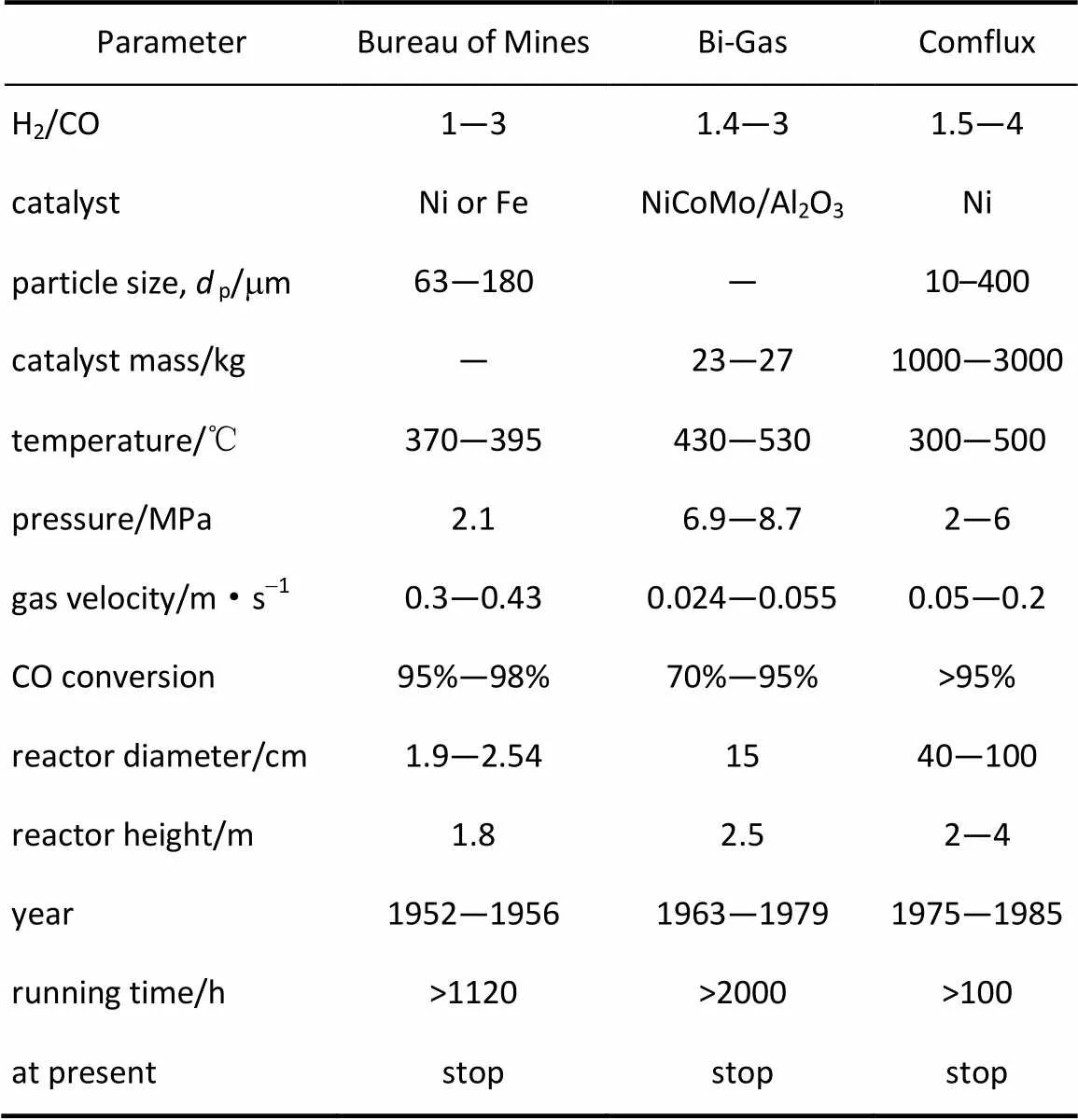
表2 典型的流化床甲烷化工艺参数
从表2中看出,美国矿务局(Bureau of Mines)建立的煤气化甲烷化制天然气流化床工艺的规模较小,其流化床直径仅为1.9~2.54 cm。催化剂采用Fe基或Ni基催化剂(p=63~180mm),运行结果显示镍基催化剂优于Fe基催化剂,床层温度控制较好,CO和H2转化率95%~98%,但该工艺自1956年后未见有报道[38]。
Bi-Gas工艺流化床反应器直径15 cm,反应区高度2.5 m。催化剂采用NiCoMo/Al2O3催化剂,具有水煤气变换和甲烷化功能[41]。但运行结果显示,在催化剂量23~27 kg,H2/CO比1.4~3,表观气速2.4~5.5 cm·s-1(8~18倍mf)条件下,CO和H2转化率70%~95%,还需要进一步提高转化率。但在运行过程中发现,在甲烷化反应初期催化剂颗粒的磨损较为严重。自1979年Cobb等[39]利用其运行数据计算了CO反应动力学和建立了两相流数学模型之后,未见与Bi-Gas甲烷化工艺及流化床反应器的文献报道。
Comflux工艺的突出特点是水煤气变换反应和甲烷化反应集中在一个流化床中进行。与前两个流化床甲烷化工艺比较,Comflux工艺规模显著提高,流化床反应器直径为40~100 cm,能容纳1000~3000 kg催化剂(p=10~400mm),SNG 生产规模达到2000 m3·h-1。考虑到省去了变换单元和产品循环气压缩机,该工艺降低了投资运行的成本,比固定床工艺减少了将近10%的成本。该工艺通过了中试和半商业运营,尚无商业化规模的运营。受石油价格影响,该装置自20世纪80年代中期终止运行。
除了以上3种煤基甲烷化流化床工艺外,自20世纪90年代,瑞士PSI (Paul-Scherrer Institut, Switzerland)公司开始致力于生物质转化制SNG技术开发,称为PSI流化床甲烷化工艺[43-46]。其核心技术源于Comflux流化床甲烷化技术,催化剂同时具有水煤气变换反应和甲烷化反应的功能。该工艺于2007年在10 kW SNG中试规模的装置上运行了1000 h,结果显示产品气含有高达40%的CH4和极少量的CO,并于2009年在1 MW SNG PDU规模装置上完成验证。2010年初,PSI工艺与奥地利的快速内循环流化床气化工艺(FICFB gasifier)嫁接形成具有竞争力的甲烷化技术,预计2016年建成100 MW SNG的甲烷化工艺。
1.3 浆态床甲烷化工艺
浆态床反应器以液态惰性烃为反应介质,涉及气、液、固三相反应器,由于其反应系统的热稳定性高,系统温度可以达到瞬间平衡的特点,非常适用于甲烷化反应。其基本工艺原理是反应器下部通入原料气和流化用液体,与流化床中悬浮的Ni 催化剂作用进行甲烷化反应。反应热被液体吸收。由于液体热容量大,反应基本是在等温条件下进行。气化的流化液体与产品气体在反应器外部用热交换器进行冷却分离,液体进行循环再利用。美国化学系统研究所(Chem. System)开发了LPM(liquid phase methanation)工艺,该工艺在bench-scale unit (BSU)、process development unit(PDU)、pilot plant(PP)3种规模的实验装置进行了验证,其工艺条件和装置规模见表3[46-48]。在PP装置上运行300 h结果显示,该工艺存在甲烷合成效率较低、催化剂损失严重的问题,于1981年被终止。
国内太原理工大学、中国矿业大学(北京)等科研机构也对浆态床甲烷化进行了研究[49-51]。太原理工大学的研究表明,浆态床CO甲烷化在280℃的反应温度下,CO的转化率保持在96%以上,取得了很好的反应结果[49-50]。目前,该工艺还在研究开发阶段,未见到工业化项目相关报道。

表3 浆态床甲烷化工艺参数
1.4 甲烷化反应器性能对比分析
从以上甲烷化工艺及甲烷化反应器的研究结果可以看出,甲烷化反应器的设计是整个甲烷化工艺的关键技术。一个理想的甲烷化反应器应具有高效的传热性能、防止催化剂积炭失活和减少催化剂损失等优点。
表4列出了固定床、流化床和浆态床甲烷化工艺的性能,可以看出,浆态床反应器的CO转化率低、催化剂磨损严重,仍然处于实验室研究阶段,距离工业化较远。固定床具有CO转化率高、催化剂用量少、催化剂无磨损等优点。但固定床工艺流程和结构复杂,运行成本高。流化床甲烷化工艺CO转化率高,具有流程结构简单、生产能力大等优势,操作成本较固定床低,但存在催化剂磨损严重问题,制约了其工业化进展。因此,未来流化床甲烷化工艺的研发重点在于如何防止催化剂磨损和研制抗磨损的甲烷化催化剂。
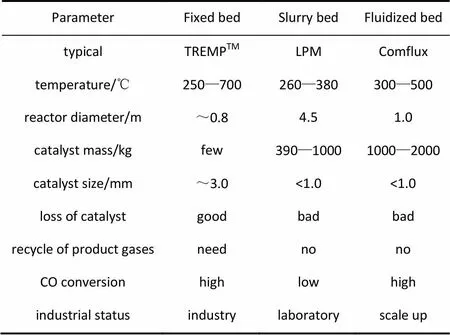
表4 固定床、流化床和浆态床甲烷化工艺对比[35,46]
2 甲烷化催化剂研究现状
自1902年Sabatier等[52]发现在Ni及其他金属(Ru、Rh、Pt、Fe、Co)催化剂能催化CO甲烷化反应以来,甲烷化催化剂的研究一直是该技术关注的焦点,涉及了甲烷化反应热力学、反应动力学、催化反应机理、失活机理等多个方面[53-60]。大量的研究表明第Ⅷ族金属及Ag和Mo均有甲烷化活性,其单位金属表面的甲烷化催化活性顺序依次为Ru>Ir>Rh>Ni>Co>Os>Pt>Fe>Mo>Pd> Ag[20]。在众多的金属催化剂中,具有高甲烷化催化活性的有贵金属Ru、Rh及过渡金属元素Ni、Co、Fe、Mo等[61-66]。Fe、Co作为甲烷化催化剂的选择性较差,且易积炭失活[67-68]。具有工业化应用前景的催化剂主要是Ru基和Ni基催化剂,Ru基催化剂比Ni基催化剂的催化活性高,为最理想的甲烷化催化剂,但因其价格昂贵,限制了它的工业使用[69-71]。镍基催化剂由于其较好的甲烷化催化活性、选择性高且价格相对低廉,是工业化甲烷化催化剂的主要选择[72-76]。从目前已经工业化的甲烷化催化剂看,如托普索公司的MCR-2X[19,77]、戴维的CRG[78-79]以及鲁奇公司的Cl-85[46,80],均是镍基催化剂,其典型的催化剂特点见表5。可以看出,高温甲烷化技术和高温甲烷化催化剂是未来甲烷化工艺的重点发展方向。
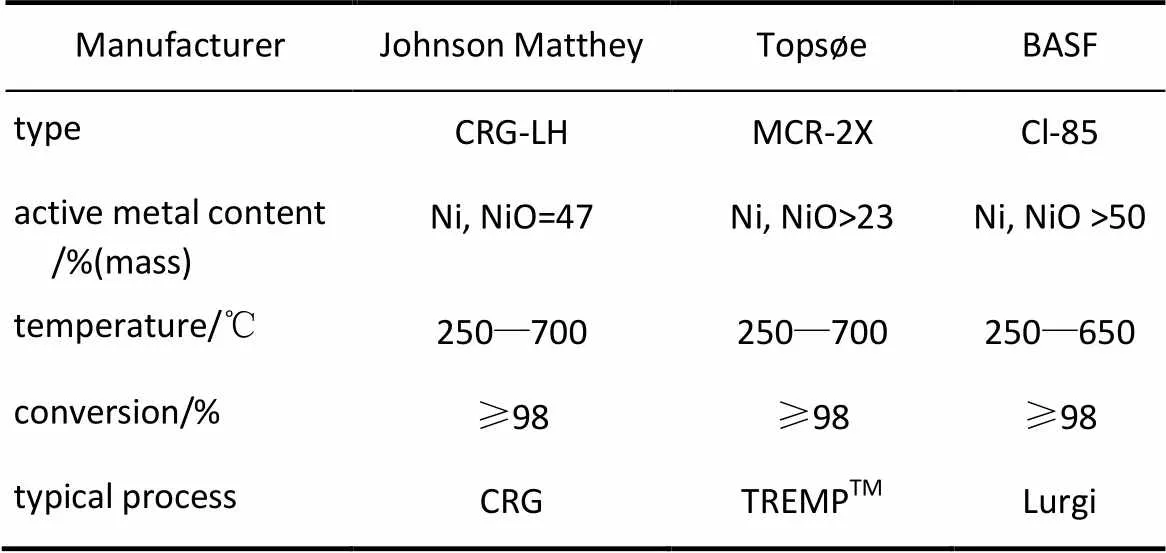
表5 商业化甲烷化催化剂的特点
到目前为止,国内研究机构开发的甲烷化催化剂主要是应用于中低温微量CO脱除方面(部分甲烷化),而针对完全甲烷化的高温甲烷化催化剂,由于没有配套的甲烷化工艺的支撑,其甲烷化催化剂正处于研发阶段,主要以实验室研究为主,缺乏中试示范装置和工业装置验证。国内中国科学技术大学研制的KD-306催化剂的CO转化率仅40%~60%,甲烷选择性>70%,上海煤气公司研发的SG-100镍基催化剂的CO转化率为60%~74%,甲烷选择性50%~63%[81],远低于工业化的甲烷化催化剂[82-83]。近年来,中科院过程所在催化剂载体、镍粒子调控及甲烷化催化剂抗积炭方面进行详细的研究,开发出多种活性高的Ni基催化剂[25,61,84-86]。北京低碳清洁能源研究所在耐硫型催化剂和宽温型(250~700℃)Ni基甲烷化催化剂开发方面取得了进展,通过添加助剂MgO提高了NiO热稳定性和甲烷选择性[87-91]。但缺乏长时间的稳定性实验验证该催化剂的高温稳定性。中科院大连化物所开发了高温和低温甲烷化催化剂,完成的5000 m3·d-1煤制天然气甲烷化工业中试装置已连续稳定运行超过1000 h[24],为中国煤制天然气技术的产业化发展向前迈出了关键一步。
在高温甲烷化反应过程中,原料气与甲烷化催化剂(镍、助剂和载体组成)颗粒表面的Ni原子接触并反应,其甲烷化催化剂活性决定于CO解离能和主要中间体在金属催化剂表面的稳定性[58-60],理想的催化剂是在两个因素之间取得平衡。要求甲烷化催化剂具有高比表面积、高镍分散性及与载体的强相互作用[92-94]。大量研究表明,导致甲烷化催化剂失活的原因有:(1)积炭[95-97];(2)烧结[19,56,98-100];(3)镍流失[67,101];(4)硫中毒[44,102-103]。针对硫中毒和镍的流失,工业上一般采用对原料气体进行深度预脱硫,高于生成Ni(CO)4温度操作,使硫中毒和镍流失问题得以解决。然而,高温下积炭和镍的烧结仍然是镍基催化剂甲烷化工艺面临的两个技术难题。工业上,通常以牺牲生产能力和耗费能量来减少催化剂的积炭和烧结,如鲁奇甲烷化工艺采用产品气循环以稀释原料气控制反应器温升,托普索公司就是采用从第二反应器出来的部分气体循环到第一反应器入口来控制反应器温度[31,104]。
催化剂积炭主要来源于CO的歧化反应和甲烷分解反应,积炭通常发生在催化剂床层上部和固定床反应器入口处[105]。生成的碳晶须或聚合炭会沉积在催化剂表面而覆盖其金属活性位,阻塞催化剂载体的孔道,使活性组分与载体分离,不仅造成催化剂的失活,缩短催化剂寿命[106-107],还会增加催化床层阻力。Czekaj等[43]给出了积炭机理,该机理认为催化剂表面上的NiO 和Ni(OH)2不具催化活性,只有被H2还原后的金属态Ni才具有甲烷化催化活性。催化剂活性降低的原因是金属态Ni 晶格和-Al2O3晶格不匹配而形成了由Ni和NiC或Ni3C组成的一个薄层界面,造成活性组分镍与载体间作用力弱,从而导致具有活性的Ni粒子从载体上脱落。另一方面,积炭会形成惰性炭层或低反应活性的碳化物覆盖在催化剂表面,阻止反应进行。因此,选择与镍兼容性好的催化剂载体材料,以增强活性镍与载体之间的作用力,是防止积炭的有效方法。
高温或低温高CO浓度甲烷化过程均会导致镍催化剂烧结失活,镍基催化剂的烧结失活存在两种烧结机理[108]:一种是粒子迁移机理,即金属晶粒在催化剂表面上迁移、碰撞、聚并长大;另一种是原子迁移机理,认为金属原子从金属晶粒上脱离开,在催化剂表面迁移,并被另一个晶粒捕获。无论是哪一种烧结机理,都与催化剂载体结构、活性金属含量、镍与载体的相互作用密切相关[99-100]。由于甲烷化反应的强放热特性,高温下引起床层局部过热是导致催化剂烧结失活的另一个主要原因。
由此可见,催化剂积炭和活性金属烧结是甲烷化催化剂失活的两个主要原因,并且受催化剂结构和操作条件影响较大。无论是积炭,还是镍粒子烧结都与甲烷化的强放热特性引起的床层过热有关。一方面,从甲烷化催化剂本身出发,研制具有高热稳定性的新型抗积炭抗烧结催化剂;另一方面从甲烷化工艺入手,比如产品气循环以稀释原料气、通入水蒸气以调节CO分压等手段稳定床层温度。另外,通过流化床反应器强化传热是甲烷化一个重要途径。
3 甲烷化反应过程强化
关于CO甲烷化的动力学和反应机理的研究很多。早期的研究认为,氧中间体(CHO)是甲烷化反应的中间体[20]。但在甲烷化过程的红外研究中没有发现CHO物种的存在。Wise[109-111]提出了表面碳中间体机理并给出了甲烷化反应路径,即CO 在催化剂表面解离得到表面碳原子(Cs),部分加氢(CH)后通过CO 的不断插入和部分氢解作用使链增长,最终加氢使链终止。表面碳机理得到了大多数实验的证实,但对氢气在甲烷化中的作用和控速步骤目前尚未达成共识,最大分歧在于是CO直接解离还是氢助解离[112-114],以及速控步骤是CO解离还是表面碳加氢[115-117]。甲烷化反应机理的争论也反映了甲烷化反应的复杂性,但无论是何种加氢机理,CO在催化剂表面吸附都是关键。
大量研究表明,CO在催化剂表面解离生成的吸附碳是甲烷化反应的前驱体。催化剂表面的碳可以分为Cα、Cβ、石墨碳以及碳须[118]。其中,Cα是原子状态的碳,低温下易于加氢,Cβ在较高温度下才能加氢,活性约为Cα的1/100[119];温度在600 K以上,Cα可以缓慢转变为Cβ,Cβ在高温下转变为石墨碳。碳须由吸附碳原子在金属表面进行扩散,并在金属和载体界面处成核并生长而成[120]。碳须不占据金属表面,对催化活性没有太大影响。但是,由于具有很高机械强度,大量碳须生成会使催化剂强度严重下降,甚至粉化。因此,从催化剂结构和反应器出发,降低催化剂积炭和烧结仍然是甲烷化反应研究的前沿领域。
基于以上认识,从甲烷化反应过程强化扩散、传热角度认识甲烷化反应和降低催化剂积炭和烧结速率引起研究者的关注[121-122]。流化床反应器具有高效的气固传质传热效率,反应床层内温度和催化剂颗粒均匀分布等优点,有利于实现对甲烷化反应温度的控制,抑制床层温度过热和防止烧结。研究表明,流化床甲烷化反应可以分为富CO区和贫CO区,高流化气速有利于强化传热,避免出现局部热点,但高流化气速也会带来气泡快速的穿越催化剂床层,降低反应转化率[123-124]。同时,催化剂颗粒在流化床中循环流动使得流化床成为积炭的缓冲器,能够有效防止催化剂积炭,使得流化床甲烷化重新引起重视[45,105,121]。许光文课题组[125-128]研究表明,流化床具有比固定床高的CO转化率和CH4选择性,并进一步证实了流化床的强化传热效果。
早期的流化床甲烷化工艺研究中发现催化剂颗粒的磨损严重[39],但对流化床甲烷化催化剂的流化行为及催化剂颗粒的流化质量对甲烷化反应的影响很少有文献报道。近年来,Kai等[129-131]研究表明,甲烷化减分子反应造成催化剂流化质量降低,甚至失流。在其他反应中也发现了减分子反应对流化床反应和反应器放大的不利影响[132-133]。而且,催化剂的磨损使得催化剂颗粒变细,也会导致催化剂的黏结失流,造成CO转化率和甲烷选择性降低。Li等[134]也通过添加B类颗粒,改善了催化剂的流化质量,提高了CO转化率和甲烷选择性。纳米催化剂颗粒具有比普通负载催化剂更高的甲烷化活性,但由于其强的黏结性,极易团聚而导致失流[135-136],通过外加磁场[137]和预烧结造粒[138],显著提高了纳米催化剂的流化质量,抑制催化剂烧结和积炭。宗保宁等[139-143]利用磁场强化受传热和传质限制的反应过程,如己内酰胺加氢精制,显示出良好的工业应用前景。
以往的研究中,忽略了催化剂与反应器的匹配性及甲烷化反应的强放热和减分子反应特性,是导致流化床甲烷化失败的一个重要因素。通过颗粒及反应器结构设计,强化传递和反应的研究成为新的研究方向[144-156]。微反应器(微通道)具有狭窄的通道、大的比表面积和体积比,大大强化了传热和传质速率,明显优于传统的反应器,特别是在甲烷化反应中起到了独特的作用[144-148]。程易等[121]考虑到选择适于流化床的甲烷化催化剂颗粒,以易于流化的耐磨损的-Al2O3为载体,通过浸渍法制备了镍基催化剂。从与流化床反应器匹配的催化剂结构设计源头出发,采用非常规制备技术手段(如超重力)[153-154]制备具有耐磨损、易流化、低密度的高活性甲烷化催化剂,可能是流化床甲烷化发展的一个重要途径。
4 结论和展望
甲烷化反应过程的主要问题是“烧结”和“积炭”。现有固定床甲烷化工艺以牺牲生产能力和耗费能量来减少催化剂的积炭和烧结。这为流化床甲烷化反应器及配套催化剂设计带来了机遇和挑战。
基于甲烷化反应特点和对甲烷化反应机理的认识,从与流化床反应器-催化剂结构的匹配角度,制备具有耐磨损、易流化、低密度的高活性甲烷化催化剂,可能是流化床甲烷化发展方向。
[1] Ding Y J, Han W J, Chai Q H, Yang S H, Shen W. Coal-based synthetic natural gas (SNG): a solution to China’s energy security and CO2reduction? [J]., 2013, 55: 445-453.
[2] Gao J J, Wang Y L, Ping Y, Hu D C, Xu G W, Gu F N, Su F B. A thermodynamic analysis of methanation reactions of carbon oxides for the production of synthetic natural gas [J]., 2012, 2: 2358-2368.
[3] Liu Z H, Chu B Z, Zhai X L, Jin Y, Cheng Y. Total methanation of syngas to synthetic natural gas over Ni catalyst in a micro-channel reactor [J]., 2012, 95: 599-605.
[4] Zhao Lijun(赵利军), Lin Hualin(蔺华林). Methanation history and catalytic mechanisms [J].(神华科技), 2010, (5): 80-84.
[5] Liu B, Ji S F. Comparative study of fluidized-bed and fixed-bed reactor for syngas methanation over Ni-W/TiO2-SiO2catalyst [J]., 2013, 22: 740-746.
[6] Kustov A L, Frey A M, Larsena K E, Johannessen T, Nørskov J K, Christensen C H. CO methanation over supported bimetallic Ni-Fe catalysts: from computational studies towards catalyst optimization [J].:, 2007, 320: 98-104.
[7] Liu Qihai(刘其海), Liao Liewen(廖列文), Liu Zili(刘自力), Dong Xinfa(董新法). Effect of ZrO2crystalline phase on the performance of Ni-B/ZrO2catalyst for the CO selective methanation [J].(中国化学工程学报), 2011, 19: 434-438.
[8] Zhang Cheng(张成). Research progress of methanation of carbon monoxide and carbon dioxide [J].(化工进展), 2007, 26: 1269-1273.
[9] Zhang Y F, Zhang G J, Wang L P, Xu Y, Sun Y L. Selective methanation of carbon monoxide over Ru-based catalysts in H2-rich gases [J]., 2012, 18: 1590-1597.
[10] Bai X B, Wang S, Sun T J, Wang S D. Influence of operating conditions on carbon deposition over a Ni catalyst for the production of synthetic natural gas (SNG) from coal [J]., 2014, 144: 2157-2166.
[11] Lebarbier V M, Dagle R A, Kovarik L, Albrecht K O, Li X H, Li L Y, Taylor C E, Bao X H, Wang Y. Sorption-enhanced synthetic natural gas (SNG) production from syngas: a novel process combining CO methanation, water-gas shift, and CO2capture [J].:, 2014, 144: 223-232.
[12] Rezvani S, McIlveen-Wright D, Huang Y, Dave A, Mondol J D, Hewitt N. Comparative analysis of energy storage options in connection with coal fired integrated gasification combined cycles for an optimised part load operation [J]., 2012, 101: 154-160
[13] Karellas S, Panopoulos K D, Panousis G, Rigas A, Karl J, Kakaras E. An evaluation of substitute natural gas production from different coal gasification processes based on modeling [J]., 2012, 45: 183-194.
[14] Lou Ren(楼韧), Ren Xiaoxian(任筱娴), Zhong Yongfang(钟永芳). A discuss on application of isothermal methanation technology in coal to gas [J].(天然气化工), 2013, 38: 42-45.
[15] Jin Yong(金涌), Zhou Yucheng(周禹成), Hu Shanying(胡山鹰). Discussion on development of coal chemical industry using low-carbon concept [J].(化工学报), 2012, 63: 3-8.
[16] Liu Huazhang(刘化章). Catalysis function in energy source conversion [J].(工业催化), 2011, 19: 1-12.
[17] van Heek K H. Progress of coal science in the 20thcentury [J]., 2000, 79: 1-26.
[18] Lin Hualin(蔺华林), Li Kejian(李克健), Zhao Lijun(赵利军). Research progress of coal-based high temperature methanation catalyst for synthetic natural gas [J].(化工进展), 2011, 30: 1739-1743.
[19] Rostrup-Nielsen J R, Pedersen K, Sehested J. High temperature methanation sintering and structure sensitivity [J].:, 2007, 330: 134-138.
[20] Mills G A, Steffgen F W. Catalytic methanation [J]., 1973, 8: 159-210.
[21] Nahar G A, Madhani S S. Thermodynamics of hydrogen production by the steam reforming of butanol: analysis of inorganic gases and light hydrocarbons [J]., 2010, 35: 98-109.
[22] Lom W L, Williams A F. Substitute natural gas [M]. NewYork: Wiley, 1976: 167-184.
[23] Penniine H W, Schehl R R, Haynes W P. Operation of a tube wall methanation reactor //2nd Joint Conference CIC/ACS[C], Montreal, Canada, 1977.
[24] Seglin L, Geosits R, Franko B R, Gruber G. Survey of methanation chemistry and processes [J]., 1975, 146: 1-30.
[25] Hu D C, Gao J J, Ping Y, Jia L H, Gunawan P, Zhong Z Y, Xu G W, Gu F N, Su F B. Enhanced investigation of CO methanation over Ni/Al2O3catalysts for synthetic natural gas production [J]., 2012, 51: 4875-4886.
[26] Harms H, Höhlein B, Skov A. Methanisierung kohlenmonoxidreicher gase beim energie-transport [J]., 1980, 52: 504-515.
[27] Zhu Ruichun(朱瑞春), Gong Weiheng(公维恒), Fan Shaofeng(范少锋). Research on technology of synthetic natural gas from coal [J].(洁净煤技术), 2011, 17: 81-85.
[28] Eisenlohr K H, Moeller F W, Dry M. Effect of certain reacation parameters on methanation of coal gas to SNG [J]., 1975, 146: 113-122.
[29] Hohlein B, Menzer R, Range J. High temperature methanation in the long-distance nuclear energy transport system [J]., 1981, 1: 125-139.
[30] Qian Wei(钱卫), Huang Yuyi(黄于益), Zhang Qingwei(张庆伟), Du Minghua(杜铭华), Xie Qiang(解强). Development of synthetic technique of substitute natural gas (SNG) from coal [J].(洁净煤技术), 2011, 17: 27-32.
[31] Moeller F W, Ros H, Britz B. Methanation of coal gas for SNG [J]., 1974, 53: 69-74
[32] Lurgi/BASF. Advanced coal based SNG technology// 3rdCoal to SNG conference[C]. Beijing, 2012: 09-10.
[33] Zhao Zhenben(赵振本). Great plains coal gasification plant [J].(煤炭加工与综合利用), 1986, (2): 51-55.
[34] Li Yao(李瑶), Zheng Hua’an(郑化安), Zhang Shengjun(张生军), Fu Gang(付刚), Zhang Hexiang(赵鹤翔), Li Xueqiang(李学强), Liu Shuangtai(刘双泰). Status and development of synthetic natural gas (SNG) from coal [J].(洁净煤技术), 2013, (6): 62-67.
[35] Zhu Yanyan(朱艳艳), Yuan Hui(袁慧), Guo Lei(郭雷), Hou Jianguo(侯建国), Gao Zhen(高振). Research progress in methanation technology at home and abroad [J].(天然气化工), 2014, 39: 77-82.
[36] Li Anxue(李安学), Li Chunqi(李春启), Zuo Yubang(左玉帮), Liu Yongjian(刘永健). Analysis on the present situation and prospect of China’s synthetic natural gas [J].(煤炭加工与综合利用), 2014, (10): 1-10.
[37] Song Pengfei(宋鹏飞), Hou Jianguo(侯建国), Wang Xiulin(王秀林), Gao Zhen(高振), Zhang Yu(张瑜), Yao huichao(姚辉超), Mu Xiangyu(穆祥宇). Several design problems of multi-stage fixed bed adiabatic methanation reactor [J].(现代化工), 2014, 34(10): 143-147.
[38] Schlesinger M D, Demeter J J, Greyson M. Catalyst for producing methane from hydrogen and carbon monoxide [J]., 1956, 48: 68-70.
[39] Cobb Jr T J, Streeter R C. Evaluation of fluidized-bed methanation catalysts and reactor modeling [J]., 1979, 18: 672-679.
[40] Cheng Y-H. Untersuchungen zur Gleichzeitigen methanisierung und konvertierung CO-reicher synthesegase in Gegenwart von Schwefelwasserstoff [D]. Germany: Universität Karlsruhe, 1983.
[41] Alcorn W R, Cullo L A. Nickel-copper-molybdenum methanation catalyst [P]: US, 3962140. 1976-06-08.
[42] Graboski M S, Donath E E. Combined shift and methanation reaction process for the gasification of carbonaceous materials [P]: US, 3904386. 1975-09-09.
[43] Czekaj I, Loviat F, Raimondi F, Wambach J, Biollaz S, Wokaun A. Characterization of surface processes at the Ni-based catalyst during the methanation of biomass-derived synthesis gas: X-ray photoelectron spectroscopy (XPS) [J].:, 2007, 329: 68-78.
[44] Struis R P W J, Schildhauer T J, Czekaj I, Janousch M, Ludwig C, Biollaz S M A. Sulphur poisoning of Ni catalysts in the SNG production from biomass: a TPO/XPS/XAS study [J].:, 2009, 362: 121-128.
[45] Seemann M C, Schildhauer T J, Biollaz S M A, Stucki S, Wokaun A. The regenerative effect of catalyst fluidization under methanation conditions [J].:, 2006, 313: 14-21.
[46] Kopyscinski J, Schildhauer T J, Biollaz S M A. Production of synthetic natural gas (SNG) from coal and dry biomass—a technology review from 1950 to 2009 [J]., 2010, 89: 1763-1783.
[47] Frank M E, Sherwin M B, Blum D B, Mednick R L. Liquid phase methanation—shift PDU results and pilot plant status// Proceeding of eighth synthetic pipeline gas symposium [C]. Chicago: American Gas Association, 1976: 159-79.
[48] Frank M E, Mednick R L. Liquid phase methanation pilot plant results//Proceeding of ninth synthetic pipeline gas symposium [C]. Chicago: American Gas Association; 1977: 185-191.
[49] Meng Fanhui(孟凡会), Chang Huirong(常慧蓉), Li Zhong(李忠). Catalytic performance of Ni-Mn/Al2O3catalyst for CO methanation in slurry-bed reactor [J].(化工学报), 2014, 65: 2997-3003.
[50] Ji Keming(吉可明), Meng Fanhui(孟凡会), Gao Yuan(高源), Li Zhong(李忠). Solution combustion prepared Ni-based catalysts and their catalytic performance for slurry methanation [J].(无机化学学报), 2015, 31: 267-274.
[51] He Long(贺龙), Wang Yonggang(王永刚), Gong Weibo(公维博), Yang Fangfang(杨芳芳), Xu Deping(许德平), Zhang Haiyong(张海永). Research of the catalysts for methanation in slurry bed reactor [J].(化工进展), 2012, 31: 311-314.
[52] Sabatier P, Senderens J B. New methane synthesis [J]., 1902, 134: 514-516.
[53] Vannice M A. The catalytic synthesis of hydrocarbons from carbon monoxide and hydrogen [J].:, 1976, 14: 153-191.
[54] Ponec V. Some aspects of the mechanism of methanation and Fischer-Tropsch synthesis [J].:, 1978, 18: 151-171.
[55] Sehested J, Dahl S, Jacobsen J, Rostrup-Nielsen J R. Methanation of CO over nickel: mechanism and kinetics at high H2/CO ratios [J]., 2005, 109: 2432-2438.
[56] Agnelli M, Kolb M, Mirodatos C. CO hydrogenation on a nickel catalyst (Ⅰ): Kinetics and modeling of a low-temperature sintering process [J]., 1994, 148: 9-21.
[57] Keyser M J, Everson R C, Espinoza R L. Fischer-Tropsch kinetic studies with cobalt-manganese oxide catalysts [J]., 2000, 39: 48-54.
[58] Bligaard T, Norskov J K, Dahl S, Matthiesenb J, Christensena C H, Sehested J. The Brønsted-Evans-Polanyi relation and the volcano curve in heterogeneous catalysis [J]., 2004, 224: 206-217.
[59] Andersson M P, Bligaard T, Kustov A,. Larsen K E, Greeley J, Johannessen T, Christensen C H, Nørskov J K. Toward computational screening in heterogeneous catalysis: pareto-optimal methanation catalysts [J]., 2006, 239: 501-506.
[60] Norskov J K, Bligaard T, Logadottir A, Bahn S, Hansen L B, Bollinger M, Bengaard H, Hammer B, Sljivancanin Z, Mavrikakis M, Xu Y, Dahl S, Jacobsen C J H. Universality in heterogeneous catalysis [J]., 2002, 209: 275-278.
[61] Liu J, Shen W L, Cui D M, Yu J, Su F B, Xu G W. Syngas methanation for substitute natural gas over Ni-Mg/Al2O3catalyst in fixed and fluidized bed reactors [J]., 2013, 38: 35-39.
[62] Masini F, Strebel C E, McCarthy D N, Nierhoff A U F, Kehres J, Fiordaliso E M, Nielsena J H, Chorkendorff I. Methanation on mass-selected Ru nanoparticles on a planar SiO2model support: the importance of under-coordinated sites [J]., 2013, 308: 282-290.
[63] Szailer T, Novák É, Oszkó A, Erdőhelyi A. Effect of H2S on the hydrogenation of carbon dioxide over supported Rh catalysts [J]., 2007, 46: 79-86.
[64] Liu J X, Su H Y, Li W X. Structure sensitivity of CO methanation on Co (0001), (10-12) and (11-20) surfaces: density functional theory calculations [J]., 2013, 215; 36-42.
[65] Govender A, Ferre D C, Niemantsverdriet J W. A density functional theory study on the effect of zero-point energy corrections on the methanation profile on Fe(100) [J]., 2012, 13: 1591-1596.
[66] Chen J, Li S L, Xu Q, Tanaka K. Synthesis of open-ended MoS2nanotubes and the application as the catalyst of methanation [J]., 2002, 16: 1722-1723.
[67] Enger B C, Holmen A. Nickel and Fischer-Tropsch synthesis [J].:, 2012, 54: 437-488.
[68] Rojanapipatkul S, Jongsomjit B. Synthesis of cobalt on cobalt-aluminatesolvothermal method and its catalytic properties for carbon monoxide hydrogenation [J]., 2008, 10: 232-236.
[69] Tada S, Kikuchi R, Urasaki K, Satokawa S. Effect of reduction pretreatment and support materials on selective CO methanation over supported Ru catalysts [J].:, 2011, 404: 149-154.
[70] Eckle S, Anfang H G, Behm R J. What drives the selectivity for CO methanation in the methanation of CO2-rich reformate gases on supported Ru catalysts? [J].:, 2011, 391: 325-333.
[71] Kamble V S, Londhe V P, Gupta N M, Thampi K R, Grätzel M. Studies on the sulfur poisoning of Ru-RuO/TiO2catalyst for the adsorption and methanation of carbon monoxide [J]., 1996, 158:427-438.
[72] Barrientos J, Lualdi M, Boutonnet M, Järås S. Deactivation of supported nickel catalysts during CO methanation [J].:, 2014, 486: 143-149.
[73] Zhang Jiaying(张加赢), Xin Zhong(辛忠), Meng Xin(孟鑫), Tao Miao(陶淼). Activity and stability of nickel based MCM-41 methanation catalysts for production of synthetic natural gas [J].(化工学报), 2014, 65: 160-168.
[74] Tian D Y, Liu Z H, Li D D, Shi H L, Pan W X, Cheng Y. Bimetallic Ni-Fe total-methanation catalyst for the production of substitute natural gas under high pressure [J]., 2013, 104: 224-229.
[75] Zeng Y, Ma H F, Zhang H T, Ying W Y, Fang D Y. Highly efficient NiAl2O4-free Ni/-Al2O3catalysts prepared by solution combustion method for CO methanation [J]., 2014, 137: 155-163.
[76] Munnik P, Velthoen M E Z, deJongh P E, deJong K P, Gommes C J. Nanoparticle growth in supported nickel catalysts during methanation reaction—larger is better [J]., 2014, 53(36): 9493-9497.
[77] Harms H, Hohlein B, Jorn E. High-temp methanation tests run [J]., 1980, 14: 120-135.
[78] Liu Zhiguang(刘志光), Gong Huajun(龚华俊), Yu Liming(余黎明). SNG development in China [J].(煤化工), 2009, (2): 1-5.
[79] Zhao Liang(赵亮), Chen Yunjie(陈允捷). Development phenomina of foreign methanation progress [J].(化工进展), 2012, 31: 176-178.
[80] Yang Bolun(杨伯伦), Li Xingxing(李星星), Yi Chunhai(伊春海), Jiang Xuedong(蒋雪冬), Zhang Yong(张勇), Zhou Xiaoqi(周晓奇). Technological progress of synthetic natural gas [J].(化工进展), 2011, 30: 110-116.
[81] Dai Chen(代陈), Liu Zhiming(刘志铭), Xie Jianrong(谢建榕), Lin Guodong(林国栋), Zhang Hongbin(张鸿斌). A highly efficient Sc2O3-promoted Ni-ZrO2catalyst for methanation of coal-based syngas to produce synthesis natural gas [J].(厦门大学学报), 2013, 52: 650-654.
[82] Zhao Gangwei(赵钢炜), Xiao Yunhan(肖云汉), Wang Yu(王钰). Analysis and discussion of process coal based synthetic natural gas and the factors effecting the catalysts [J].(陶瓷), 2009, (11): 21-26.
[83] Zhao Lijun(赵利军), Lin Hualin(蔺华林). Methanation catalyst design by virtue of catalytic echanisms and new fields in methanation studies [J].(神华科技), 2011, (9): 87-91.
[84] Liu Y J, Gao J J, Liu Q, Gu F N, Lu X P, Jia L H, Xu G W, Zhong Z Y, Su F B. Preparation of high-surface-area Ni/-Al2O3catalysts for improved CO methanation [J]., 2015, 5: 7539-7546.
[85] Liu Q, Gu F N, Lu X P, Liu Y J, Li H F, Zhong Z Y, Xu G W, Su F B. Enhanced catalytic performances of Ni/Al2O3catalystaddition of V2O3for CO methanation [J].:, 2014, 488: 37-47.
[86] Gao J J, Jia C M, Li J, Gu F N, Xu G W, Zhong Z Y, Su F B. Nickel catalysts supported on barium hexaaluminate for enhanced CO methanation [J]., 2012, 51: 10345-10353.
[87] Tian Dayong(田大勇), Yang Xia(杨霞), Qin Shaodong(秦绍东), Sun Shouli(孙守理), Sun Qi(孙琦). Preparation of Ni-based catalyst with wide working temperature and its performance for methanation reaction [J].(工业催化), 2013, 21: 26-29.
[88] Jiang M H, Wang B W, Yao Y Q, Wang H Y, Li Z H, Ma X B, Qin S D, Sun Q. Effect of stepwise sulfidation on a MoO3/CeO2-Al2O3catalyst for sulfur-resistant methanation [J].:, 2014, 469: 89-97.
[89] Jiang M H, Wang B W, Yao Y Q, Li Z H, Ma X B, Qin S D, Sun Q. Effect of sulfidation temperature on CoO-MoO3/-Al2O3catalyst for sulfur-resistant methanation [J]., 2013, 3: 2793-2800.
[90] Jiang M H, Wang B W, Yao Y Q, Li Z H, Ma X B, Qin S D, Sun Q. A comparative study of CeO2-Al2O3support prepared with different methods and its application on MoO3/CeO2-Al2O3catalyst for sulfur-resistant methanation [J].,2013, 285: 267-277.
[91] Li Z H, Liu J, Wang H Y, Wang E D, Wang B W, Ma X B, Qin S D, Sun Q. Effect of sulfidation temperature on the catalytic behavior of unsupported MoS2catalysts for synthetic natural gas production from syngas [J].:, 2013, 378: 99-108.
[92] Araki M, Ponec V. Methanation of carbon monoxide on nickel and nickel-copper alloys [J]., 1976, 44: 439-488
[93] Schoubye P. Methanation of CO on some Ni catalysts [J]., 1969, 14: 238-246.
[94] Vannice M A, Garten R L. Metal-support effects on the activity and selectivity of Ni catalysts in CO/H2synthesis reactions [J]., 1979, 56: 236-248.
[95] Erekson E J, Sughrue E L, Bartholomew C H. Catalyst degradation in high temperature methanation [J]., 1981, 5: 91-101.
[96] Gierlich H H, Fremery M, Skov A, Rostrup-Nielsen J R. Deactivation phenomena of a Ni-based catalyst for high temperature methanation [J]., 1980, 6: 459-469.
[97] Zhang J, Fatah N, Capela S, Kara Y, Guerrini O, Khodakov A Y. Kinetic investigation of carbon monoxide hydrogenation under realistic conditions of methanation of biomass derived syngas [J]., 2013, 111: 845-854.
[98] Hwang S, Lee J, Hong U G, Seo J G, Jung J C, Koh D J, Lim H, Byun C, Song I K. Methane production from carbon monoxide and hydrogen over nickel-alumina xerogel catalyst: effect of nickel content [J]., 2011, 17: 154-157.
[99] Bai X B, Wang S, Sun T J, Wang S D. The sintering of Ni/Al2O3methanation catalyst for substitute natural gas production [J]., 2014, 112: 437-451.
[100] Zhang J Y, Xin Z, Meng X, Tao M. Synthesis, characterization and properties of anti-sintering nickel incorporated MCM-41 methanation catalysts [J]., 2013, 109: 693-701.
[101] Hu Xianhui(胡贤辉), Wang Xingjun(王兴军), Xu Chao(徐超), Yu Guangsuo(于广锁), Wang Fuchen(王辅臣). Influence of precipitant on the performance of nickel catalyst for methanation [J].(燃料化学学报), 2012, 40: 430-435.
[102] Legras B, Ordomsky V V, Dujardin C, Virginie M, Khodakov A Y. Impact and detailed action of sulfur in syngas on methane synthesis on Ni/-Al2O3catalyst [J]., 2014, 4: 2785-2791.
[103] Czekaj I, Struis R, Wambach J, Biollaz S. Sulphur poisoning of Ni catalysts used in the SNG production from biomass: computational studies [J]., 2011, 176: 429-432.
[104] Topsoe. Coal to gas high temperature methanation process// The 16thnational fertilizer and methanol technical annual meeting [C]. Nanjing, China, 2007: 470-472.
[105] Schildhauer T J, Kopyscinski J, Biollaz S M A. Fluidized-bed methanation: interaction between kinetics and mass transfer [J]., 2011, 50: 2781-2790
[106] Bartholomew C H. Mechanisms of catalyst deactivation [J].:, 2001, 212: 17-60.
[107] Bartholomew C H. Carbon deposition in steam reforming and methanation [J].:, 1982, 24(1): 67-112.
[108] Sehested J. Sintering of nickel steam-reforming catalysts [J]., 2003, 217:417-426.
[109] Wentrcek P R, Wood B J, Wise H. Role of surface carbon in catalytic methanation [J]., 1976, 43: 363-366.
[110] Goodman D W, Kelley R D, Madey T E, White J M. Measurement of carbide buildup and removal kinetics on Ni(100) [J]., 1980, 64: 479-481.
[111] Stone F S. Research perspectives during 40 years of the Journal of Catalysis [J]., 2003, 216: 2-11.
[112] Wang S, Moon S, Vannice M A. The effect of SMSI (strong metalsupport interaction) behavior on CO adsorption and hydrogenation on Pd catalysts (Ⅱ): Kinetic behavior in the methanation reaction [J]., 1981, 71:167-174.
[113] Ojeda M, Nabar R, Nilekar A U, Ishikawa A, Mavrikakis M, Iglesia E. CO activation pathways and the mechanism of Fischer-Tropsch synthesis [J]., 2010, 272: 287-297.
[114] Cant N W, Bell A T. Studies of carbon monoxide hydrogenation over ruthenium using transient response techniques [J]., 1982, 73: 257-271.
[115] Engbaek J, Lytken O, Nielsen J H, Chorkendorff I. CO dissociation on Ni: the effect of steps and of nickel carbonyl [J]., 2008, 602: 733-743.
[116] Polizzotti R S, Schwarz J A. Hydrogenation of CO to methane: kinetic studies on polycrystalline nickel foils [J]., 1982, 77(1): 1-15.
[117] Goodman D W, Kelley R D, Madey T E. Kinetics of the hydrogenation of CO over a single crystal nickel catalyst [J]., 1980, 63(1): 226-234.
[118] Moeller A D, Bartholomew C H. Deactivation by carbon of nickel, nickel-ruthenium, and nickel-molybdenum methanation catalysts [J]., 1982, 21: 390-397.
[119] Arkatova L A. The deposition of coke during carbon dioxide reforming of methane over intermetallides [J]., 2010, 157: 170-176.
[120] Chen Y G, Tomishige K, Fujimoto K. Formation and characteristic properties of carbonaceous species on nickel-magnesia solid solution catalysts during CH4-CO2reforming reaction [J].:, 1997, 161: L11-L17.
[121] Li Dandan(李丹丹), Liu Zhihong(刘志红), Guo Fen(郭奋), Cheng Yi(程易). Preparation and performance of the nickel-based catalyst for the methanation [J].(化工进展), 2011, 30: 139-142.
[122] Li Yakun(李亚坤), Zhang Qiaofei(张巧飞), Chai Ruijuan(柴瑞娟), Lu Yong(路勇). Structured metal carrier catalyst// Performance, heat transfer enhancement and CFD simulation of CO methanation catalyst [C]. Beijing: 2014.
[123] Kopyscinski J, Schildhauer T J, Biollaz S M A. Methanation in a fluidized bed reactor with high initial CO partial pressure (Ⅱ): Modeling and sensitivity study [J]., 2011, 66: 1612-1621.
[124] Kopyscinski J, Schildhauer T J, Biollaz S M A. Methanation in a fluidized bed reactor with high initial CO partial pressure (Ⅰ): Experimental investigation of hydrodynamics, mass transfer effects, and carbon deposition [J]., 2011, 66: 924-934.
[125] Liu J, Cui D M, Yu J, Su F B, Xu G W. Performance characteristics of fluidized bed syngas methanation over Ni-Mg/Al2O3catalyst [J]., 2015, 23: 86-92.
[126] Liu J, Yu J, Su F B, Xu G W. Intercorrelation of structure and performance of Ni-Mg/Al2O3catalysts prepared with different methods for syngas methanation [J]., 2014, 4: 472-481.
[127] Liu J, Shen W L, Cui D M, Yu J, Su F B, Xu G W. Syngas methanation for substitute natural gas over Ni-Mg/Al2O3catalyst in fixed and fluidized bed reactors [J]., 2013, 38: 35-39.
[128] Cui D M, Liu J, Yu J, Yue J R, Su F B, Xu G W. Necessity of moderate metal-support interaction in Ni/Al2O3for syngas methanation at high temperatures [J]., 2015, 5: 10187-10196.
[129] Kai T, Furukawa M, Nakazato T, Tsutsui T, Mizuta K. Analysis of fluidization quality of a fluidized bed with staged gas feed for reactions involving gas-volume reduction [J]., 2010, 56: 2297-2303.
[130] Kai T, Furukawa M, Nakazato T, Nakajima M. Prevention of defluidization by gas dilution for reactions involving gas-volume reduction [J]., 2011, 166: 1126-1131.
[131] Kai T, Toriyama K, Nishie K, Takahashi T, Nakajima M. Effect of volume decrease on fluidization quality of fluidized catalyst beds [J]., 2006, 52: 3210-3215.
[132] Chu Y, Chu B Z, Wei X B, Zhang Q, Wei F. An emulsion phase condensation model to describe the defluidization behavior for reactions involving gas volume reduction [J]., 2012, 198/199: 364-370.
[133] Knowlton T, Karri S, Issangya A. Scale-up of fluidized-bed hydrodynamics [J]., 2005, 150: 72-77.
[134] Li J, Zhou L, Li P C, Zhu Q S, Gao J J, Gu F N, Su F B. Enhanced fluidized bed methanation over a Ni/Al2O3catalyst for production of synthetic natural gas [J]., 2013, 219: 183-189.
[135] Geldart D. Types of gas fluidization [J]., 1973, 7: 285-292.
[136] Jin Yong(金涌),. Fluidization Engineering Principles(流态化工程原理) [M]. Beijing: Tsinghua University Press, 2001.
[137] Li J, Zhou L, Zhu Q S, Li H Z. Enhanced methanation over aerogel NiCo/Al2O3catalyst in a magnetic fluidized bed [J]., 2013, 52: 6647-6654.
[138] Li P C, Li J, Zhu Q S, Cui L J, Li H Z. Effect of granulation on the activity and stability of a Co-Al2O3aerogel catalyst in a fluidized-bed reactor for CH4-CO2reforming [J]., 2013, 3: 8939–8946.
[139] Zong B N, Zhang X X, Qiao M H. Integration of methanation into the hydrogenation process of benzoic acid [J]., 2009, 55: 192-197.
[140] Pan Z Y, Dong M H, Meng X K, Zhang X X, Mu X H, Zong B N. Integration of magnetically stabilized bed and amorphous nickel alloy catalyst for CO methanation [J]., 2007, 62: 2712-2717.
[141] Wu Hao(吴浩), Pan Zhiyong(潘智勇), Zong Baoning(宗保宁), Shen Shikong(沈师孔). Study of low temperature methanation on nickel based amorphous alloy catalyst [J].(化工进展), 2005, 24: 299-302.
[142] Zong B N, Meng X K, Mu X H, Zhang X X. Magnetically stabilized bed reactors [J]., 2013, 34: 61-68.
[143] Zong B N. Applications of the amorphous alloy catalyst and magnetically stabilized bed technology in petrochemical processes [J].(催化学报), 2008, 29: 873-877.
[144] Lee C B, Cho S H, Lee D W, Hwang K R, Park J S, Kim S H. Combination of preferential CO oxidation and methanation in hybrid MCR (micro-channel reactor) for CO clean-up [J].2014, 78: 421-425.
[145] Men Y, Kolb G, Zapf R, Hessel V, Löwe H. Selective methanation of carbon oxides in a microchannel reactor—primary screening and impact of gas additives [J]., 2007, 125: 81-87.
[146] Gao Jun(高军), Dong Xinfa(董新法), Lin Weiming(林维明). Selective catalytic methanation of CO in hydrogen-rich gas with a metal foam micro reactor [J].(燃料化学学报), 2010, 38: 337-342.
[147] Dong Xinfa(董新法), Liu Wenyue(刘文跃), Gao Jun(高军), Lin Weiming(林维明). Purification of COselective methanation using microchannel reactor [J].(华南理工大学学报), 2009, 37: 44-49.
[148] Liu Wenyue(刘文跃), Dong Xinfa(董新法), Lin Weiming(林维明). Selective methanation of CO over Ni-Ru/ZrO2catalyst in micro channel reactor [J].(石油化工), 2009, 38: 711-715.
[149] Ryi S K, Lee S W, Hwang K R, Park Jo S. Production of synthetic natural gas by means of a catalytic nickel membrane [J]., 2012, 94: 64-69.
[150] Li C M, Zhang S T, Zhang B S, Su D S, He S, Zhao Y F, Liu J, Wang F, Wei M, Evans D G, Duan X. Photohole-oxidation-assisted anchoring of ultra-small Ru clusters onto TiO2with excellent catalytic activity and stability [J]., 2013, 1: 2461-2467.
[151] He S, Li C M, Chen H, Su D S, Zhang B S, Cao X Z, Wang B Y, Wei M, Evans D G, Duan X. A surface defect-promoted Ni nanocatalyst with simultaneously enhanced activity and stability [J].,2013, 25: 1040-1046.
[152] Cheng M Y, Pan C J, Hwang B J. Highly-dispersed and thermally-stable NiO nanoparticles exclusively confined in SBA-15: blockage-free nanochannels [J]., 2009, 19: 5193-5200.
[153] Zou Haikui(邹海魁), Chu Guangwen(初广文), Zhao Hong(赵宏), Xiang Yang(向阳), Chen Jianfeng(陈建峰). Process intensification of high-gravity reactor for enviromental engineering: from fundamental to industrialization [J].(中国科学:化学), 2014, 44: 1413-1422.
[154] Sang Le(桑乐), Luo Yong(罗勇), Chu Guangwen(初广文), Zou Haikui(邹海魁), Xiang Yang(向阳), Chen Jianfeng(陈建峰). Research progress of gas-liquid mass transfer enhancement in high gravity field [J].(化工学报), 2015, 66(1):14-31.
[155] Zhang Jianwen(张建文), Gao Dongxia(高冬霞), Li Yachao(李亚超), Chen Jianfeng(陈建峰). Research progress of multiphase transport in high gravity environment in rotating packed bed [J].(化工学报), 2013, 64:243-251.
[156] Sun Hongwei(孙宏伟), Chen Jianfeng(陈建峰). Advances in fundamental study and application of chemical process intensification technology in China [J].(化工进展), 2011, 30: 1-15.
Process intensification and catalysts particle design for CO methanation
LI Jun,ZHU Qingshan,LI Hongzhong
State Key Laboratory of Multiphase Complex SystemsInstitute of Process EngineeringChinese Academic SciencesBeijingChina
Carbon deposition and sintering of metal particles are the two dominating reasons for deactivation of the methanation catalyst. Based on the strong exothermic reaction accompanied by a large decrease in mole number and methanation mechanism,from the perspective of the matching of catalyst and reactor, this paper summarizes the development of main CO methanation techniques, CO methanation catalysts, reaction mechanism of CO methanation and its process intensifications. Fluidized bed reactors have the advantages in preventing the carbon deposition and sintering of Ni catalysts. Thus, the design of wear-resistant, easy fluidized and low density catalyst structure particles that applicable to fluidized bed reactors should be a feasible way and the new direction for the development of methanation techniquesfluidized bed reactors.
methanation; fluidized bed reactor; strong exothermic; molecular reduction; Ni catalyst; carbon deposition
2015-05-29.
Prof. ZHU Qingshan, qszhu@ipe.ac.cn
10.11949/j.issn.0438-1157.20150748
TQ 032.4
A
0438—1157(2015)08—2773—11
朱庆山。
李军(1979—),男,博士,副研究员。
国家自然科学基金项目(91334108);国家重大科学仪器设备开发专项项目(2011YQ12003908)。
2015-05-29收到初稿,2015-06-10收到修改稿。
supported by the National Natural Science Foundation of China (91334108) and the National Special Project for Development of Major Scientific Equipment (2011YQ12003908).

