肺原发性腺样囊性癌的临床病理特征分析
杨朝晖,王建军,何 燕,余 波,陆珍凤,徐 艳,周晓军,石群立
·临床诊疗提示·
肺原发性腺样囊性癌的临床病理特征分析
杨朝晖,王建军,何 燕,余 波,陆珍凤,徐 艳,周晓军,石群立
目的 分析肺原发性腺样囊性癌的临床病理特征,并探讨其诊断标准及预后。方法 选取2002年1月—2014年1月于南京军区南京总医院行胸部CT或支气管镜检查并诊断为肺原发性腺样囊性癌的患者16例。记录患者的一般资料,包括性别、年龄、临床TNM分期、肿瘤发生部位、临床表现、影像学表现、确诊及治疗方法;病理学检查,包括巨检、镜下特点、免疫表型;检测表皮生长因子受体(EGFR)热点突变基因(19、21外显子)的突变情况。从手术切除及穿刺活检标本确诊后开始电话或门诊随访,半年随访1次,随访至2014-07-31,随访内容为是否放射治疗、是否化学治疗、是否复发转移及死亡日期。结果 肿瘤多发生在气管或主支气管,临床表现为咳嗽及痰中带血,偶有胸痛、胸闷、呼吸困难和发热等症状。影像学表现,10例患者胸部CT检查示:气管或主支气管内、肺门及肺叶结节影或类圆形高密度影,边缘欠清晰;1例支气管镜检查示:左主支气管内新生物,肿瘤表面较光滑。巨检显示气管或主支气管内、肺门和被膜下见肿块,大小1.2 cm×1.2 cm×1.0 cm~6.2 cm×2.5 cm×2.0 cm,切面灰白、实性,质地中等,部分有黏液感,边界不清,其中1例侵及邻近胸膜。镜下特点:癌细胞呈腺管样、筛状、小梁状或巢状排列,腺腔或筛孔中可见均质淡蓝色黏液,癌细胞体积小,大小较一致,细胞质少,细胞卵圆形到多角形,核染色深,核分裂象不常见;肿瘤组织可或不累及气管。癌细胞角蛋白(CK)8/18、CK34βE12、平滑肌肌动蛋白(SMA)、p63、ki-67阳性表达。1例患者行EGFR热点突变基因检测示:19、21外显子未发生突变。生存时间为5~96个月,平均44个月。6例局部复发或伴有肺门、纵隔淋巴结及骨转移。结论 肺原发性腺样囊性癌是一种少见的低度恶性肿瘤,组织形态学及生物学行为与涎腺的腺样囊性癌相似。确诊主要靠组织病理学检查,并辅以免疫组织化学标记。此类型肿瘤生长缓慢,早期发现并采取手术联合放化疗是最好的治疗手段。
肺肿瘤;癌,腺样囊性;病理学,临床;预后
杨朝晖,王建军,何燕,等.肺原发性腺样囊性癌的临床病理特征分析[J].中国全科医学,2015,18(24):2961-2965.[www.chinagp.net]
Yang ZH,Wang JJ,He Y,et al.Clinicopathologic study of pulmonary primary adenoid cystic carcinoma[J].Chinese General Practice,2015,18(24):2961-2965.
肺原发性腺样囊性癌是一类与唾液腺相应名称肿瘤组织学形态一致的恶性上皮肿瘤[1],主要发生在气管及主支气管,伴有上皮样细胞独特的生长方式,筛状、小管和腺样排列,周围有不定的黏液性和丰富的透明变性基底膜样细胞外基质围绕,肿瘤细胞显示衬附导管上皮和肌上皮的分化特征。该病生长缓慢,临床症状出现较晚,且缺乏特异性,较易延误诊断。为此,本研究总结了16例肺原发性腺样囊性癌患者的临床病理特征和预后,以加深对该病的认识。
1 资料与方法
1.1 纳入与排除标准 纳入标准:经手术切除或支气管镜活检标本,由2位及以上病理科医师根据2004年《WHO肺、胸膜、胸腺及心脏肿瘤病理学和遗传学分类》[2]确诊为肺原发性腺样囊性癌。排除标准:涎腺确诊为腺样囊性癌和涎腺有过手术史,但很难确定当时的病变性质。
1.2 临床资料 选取2002年1月—2014年1月于南京军区南京总医院行胸部CT或支气管镜检查并诊断为肺原发性腺样囊性癌的患者16例。
1.3 仪器与试剂 一抗细胞角蛋白(CK)8/18、CK34βE12、平滑肌肌动蛋白(SMA)、p63购自Dako公司,ki-67购自Neomarkers公司。荧光定量聚合酶链式反应(PCR)仪器(型号:ABI7500)购自ABI。
1.4 研究方法
1.4.1 处理方法 所有标本经4%中性甲醛溶液固定,常规脱水、石蜡包埋,4 μm连续切片,分别行HE染色及免疫组织化学染色(采用SP法)。用磷酸盐缓冲液(PBS)代替一抗作为空白对照。
1.4.2 免疫表型 采用二氨基联苯胺(DAB)显色,以细胞内特定部位出现棕色颗粒为阳性信号。观察5个以上高倍镜视野(×400),计数不少于1 000个细胞中的阳性细胞数,阳性细胞数<10%为阴性,≥10%为阳性。其中CK8/18、CK34βE12、SMA阳性表达均定位于细胞质,p63、ki-67阳
本文要点:
(1)肺原发性腺样囊性癌和肺原发性黏液表皮样癌同属于肺原发性涎腺型肿瘤,有很多相似的临床表现和镜下特征,需要鉴别。
(2)肺原发性腺样囊性癌生长缓慢,预后较好,早期发现并采取手术联合放化疗是最好的治疗手段。
性表达定位于细胞核。
1.4.3 表皮生长因子受体(epithelial growth factor receptor,EGFR)热点突变基因检测 采用扩增阻滞突变系统(amplification refractory mutation system,ARMS)法检测EGFR热点突变基因(19、21外显子)的突变情况。
1.5 随访 从手术切除及穿刺活检标本确诊后开始电话随访,半年随访1次,患者失访或死亡即终止随访,随访至2014-07-31,随访内容为是否放射治疗、是否化学治疗,是否复发转移及死亡日期。
1.6 观察指标 记录患者的一般资料,包括性别、年龄、临床TNM分期、肿瘤发生部位、临床表现、影像学表现、确诊及治疗方法;病理学检查,包括巨检、镜下特点、免疫表型;EGFR热点突变基因检测及预后分析。
2 结果
2.1 一般资料 南京军区南京总医院2002年1月—2014年1月共收治原发性肺肿瘤患者4 867例,其中肺原发性腺样囊性癌16例(0.3%)。16例肺原发性腺样囊性癌患者中男6例(37.5%),女10例(62.5%);年龄26~65岁,平均42.6岁;临床TNM分期,Ⅰ~Ⅱ期10例(62.5%),Ⅲ~Ⅳ期6例(37.5%);肿瘤发生在气管或主支气管11例(68.8%),左、右肺叶5例(31.2%,见表1)。临床表现:14例(87.5%)以无明显诱因的咳嗽、咳痰为首发症状,其中6例伴有胸闷、气喘(1例间断胸壁疼痛),2例痰中带血丝,2例伴有陈旧性肺结核,1例腰腿疼痛,1例髋关节疼痛,1例突发呼吸困难,1例治疗过程中继发肺结核;2例(12.5%)无任何临床不适,为常规体检发现。影像学表现:10例患者胸部CT检查示:气管或主支气管内、肺门及肺叶结节影或类圆形高密度影,边缘欠清晰,肿瘤直径1.2~5.5 cm,平均约3.3 cm(见图1);1例支气管镜检查示:左主支气管内新生物,大小约2.0 cm×2.0 cm×1.2 cm,肿瘤表面较光滑(见图2);5例为外院支气管镜检查,资料不详细。确诊及治疗方法:单侧全肺叶切除标本5例(包括2例化学治疗);气管袖状切除标本4例(包括2例局部放射治疗);单纯肺叶切除标本+化学治疗1例;经支气管镜活检证实6例,其中3例行全身化学治疗(包括2例Ⅳ期伴发远处骨转移者),1例伴发活动期结核者行对症治疗,1例行动脉灌注化学治疗+栓塞术,1例因术后未来本院治疗及复查而失访、资料不详。
2.2 病理学检查
2.2.1 巨检 单侧全肺叶切除、气管袖状切除、单纯肺叶切除标本,气管或主支气管内、肺门和被膜下见肿块,大小1.2 cm×1.2 cm×1.0 cm~6.2 cm×2.5 cm×2.0 cm,切面灰白、实性,质地中等,部分有黏液感,边界不清,其中1例侵及邻近胸膜。
2.2.2 镜下特点 癌细胞呈腺管样(见图3)、筛状(见图4)、小梁状或巢状(见图5)排列,腺腔或筛孔中可见均质淡蓝色黏液,癌细胞体积小,大小较一致,细胞质少,细胞卵圆形到多角形,核染色深,核分裂象不常见。肿瘤组织可或不累及气管(见图6)。细胞偶尔形成由2~3个细胞衬附的小管,腔内细胞低立方,周围有肌上皮(层)围绕。
2.2.3 免疫表型 癌细胞CK8/18(见图7)、CK34βE12(见图8)、SMA(见图9)、p63(见图10)、ki-67阳性表达(见图11)。
2.3 EGFR热点突变基因检测 1例患者的19、21外显子未发生突变。
2.4 预后分析 生存时间为5~96个月,平均44个月。6例局部复发或伴有肺门、纵隔淋巴结及骨转移;7例因未来院复查治疗或死亡而失访(见表1)。
3 讨论
3.1 发病情况 肺原发性腺样囊性癌是一类伴有上皮样细胞独特的生长方式、与唾液腺相应名称肿瘤组织学形态一致的恶性上皮肿瘤,其发病率低,为原发性肺肿瘤的0.1%~0.5%[3-5]。本研究结果显示,肺原发性腺样囊性癌16例,占同期原发性肺肿瘤的0.3%。本病男女发病无明显差异,发病年龄在40~50岁左右[6-7]。本研究患者男女比为1∶1.67,年龄26~65岁,平均42.6岁,与文献报道一致[8]。肺原发性腺样囊性癌来源于支气管黏膜的腺管或腺体的黏液分泌细胞,多发生在第6级支气管以上、近中心的、有黏液腺结构的气管、支气管内。有文献报道,气管腺样囊性癌在原发性气管恶性肿瘤中约占33%,为气管肿瘤的第二位[9]。本研究结果显示,肿瘤发生在气管或主支气管11例,占68.8%。

图1 胸部CT检查示:左下肺近肺门处见软组织阴影,大小约6.2 cm×2.5 cm,边缘欠清晰
Figure 1 Chest CT inspected an approximate 6.2 cm×2.5 cm soft tumor shadows near the hilum of left lung,with less clear margin
图2 支气管镜检查示:支气管内见息肉样肿物,大小约2.0 cm×2.0 cm×1.2 cm,肿瘤表面较光滑
Figure 2 Bronchoscopy revealed an approximate 2.0 cm×2.0 cm×1.2 cm smooth-surfaced polypoid tumor inside the bronchus
图3 病理学检查镜下示:癌细胞呈腺管样排列,腺管衬附低立方上皮(HE染色,×200)
Figure 3 Tumor cells were arranged in an adenoid pattern lined by low cuboidal epithelium
图4 病理学检查镜下示:癌细胞呈筛状排列,中央可见黏液,癌细胞形态、大小较一致(HE染色,×200)
Figure 4 Tumor cells were uniform in size,shape and arranged in the cribriform structures,containing mucoid in the central
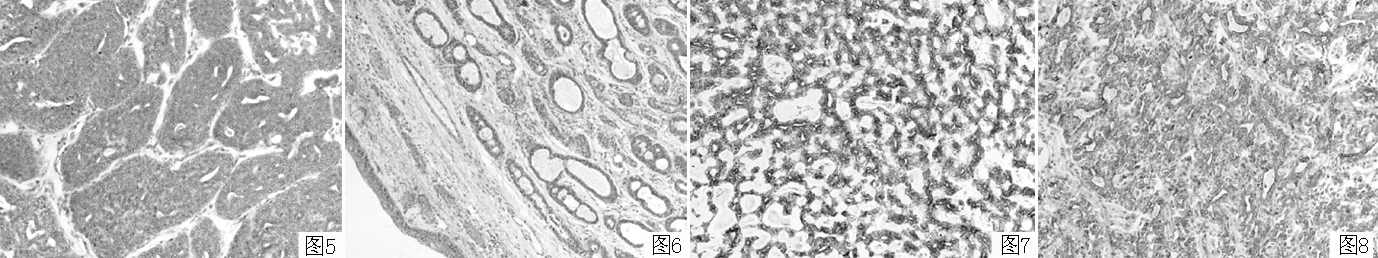
图5 病理学检查镜下示:癌细胞排列成巢状结构,伴部分腺管样结构(HE染色,×200) 图7 CK8/18阳性表达(SP法,×200)
Figure 5 Tumor cells were arranged in the nest-like pattern and partially in adenoid structures Figure 7 Positive expression of CK8/18
图6 病理学检查镜下示:癌组织位于气管黏膜下,未累及气管(HE染色,×200) 图8 CK34βE12阳性表达(SP法,×200)
Figure 6 Tumor cells were close to the tracheal mucosa,without involving the trachea Figure 8 Positive expression of CK34βE12
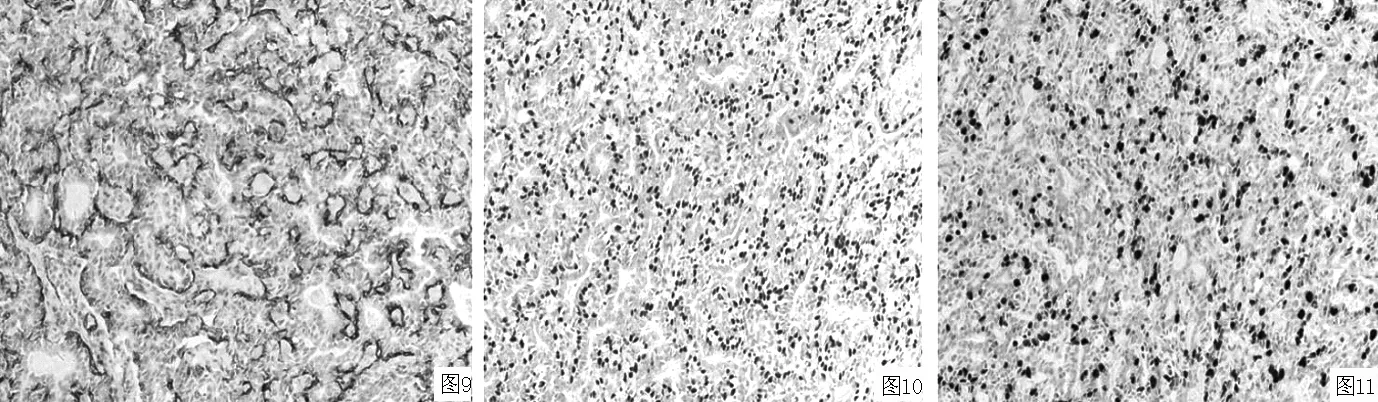
图9 SMA阳性表达(SP法,×200) 图10 p63阳性表达(SP法,×200) 图11 ki-67阳性表达(SP法,×200)
Figure 9 Positive expression of SMA Figure 10 Positive expression of p63 Figure 11 Positive expression of ki-67
表1 16例肺原发性腺样囊性癌患者一般资料
Table 1 General informations of 16 patients of pulmonary primary adenoid cystic carcinoma
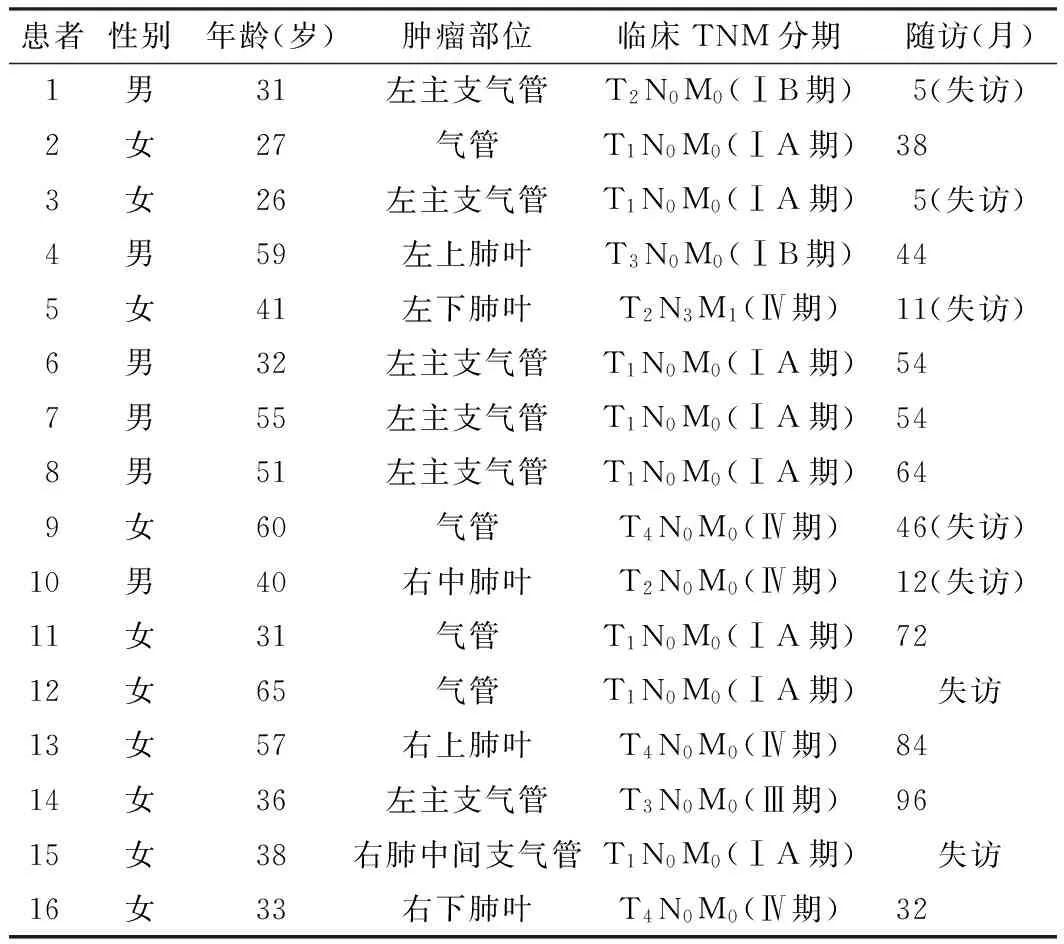
患者性别年龄(岁)肿瘤部位临床TNM分期随访(月)1男31左主支气管T2N0M0(ⅠB期)5(失访)2女27气管T1N0M0(ⅠA期)383女26左主支气管T1N0M0(ⅠA期)5(失访)4男59左上肺叶T3N0M0(ⅠB期)445女41左下肺叶T2N3M1(Ⅳ期)11(失访)6男32左主支气管T1N0M0(ⅠA期)547男55左主支气管T1N0M0(ⅠA期)548男51左主支气管T1N0M0(ⅠA期)649女60气管T4N0M0(Ⅳ期)46(失访)10男40右中肺叶T2N0M0(Ⅳ期)12(失访)11女31气管T1N0M0(ⅠA期)7212女65气管T1N0M0(ⅠA期)失访13女57右上肺叶T4N0M0(Ⅳ期)8414女36左主支气管T3N0M0(Ⅲ期)9615女38右肺中间支气管T1N0M0(ⅠA期)失访16女33右下肺叶T4N0M0(Ⅳ期)32
3.2 临床病理特征 从临床症状看,肺原发性黏液表皮样癌并无特殊性,位于主支气管内中央型者主要表现为呼吸系统症状,可出现呼吸困难、咯嗽、喘息、咳痰、咯血或痰中带血、声音嘶哑、咽部异物感、发热等[10-11]。肺原发性腺样囊性癌生长缓慢,临床症状出现较晚,与病灶大小相关。本研究中,87.5%患者首发症状为气促、呼吸困难、咳嗽、痰中带血等,另外少数(12.5%)无明显临床症状,为体检发现。因此,有长期刺激性咳嗽且阵发性加重、不明原因咯血、顽固性咽部异物感、进食梗阻者,均应提高警惕,必要时行纤维支气管镜检查及胸部CT检查,以除外气管腺样囊性癌的可能[12-13]。本研究中,10例患者胸部CT检查示:气管或主支气管内、肺门及肺叶结节影或类圆形高密度影,边缘欠清晰;1例支气管镜检查示:左主支气管内新生物突向腔内生长,大小约2.0 cm×2.0 cm×1.2 cm,肿瘤表面较光滑。
从病理学特点看,肺原发性腺样囊性癌病理形态特点多在支气管壁上为灰白或褐色息肉状、球状、半球状肿物,也可使支气管壁黏膜增厚,在支气管黏膜下沿长轴和管周形成弥漫性浸润性肿块。本研究中,肿瘤的巨检特征为多数位于气管与主支气管内或周围,部分位于肺门或肺叶,大小1.2 cm×1.2 cm×1.0 cm~6.2 cm×2.5 cm×2.0 cm;肿瘤切面为柔软的、通常带有光泽的黏液息肉样外观,呈粉色或棕色,息肉样肿物表面可有坏死。肺原发性腺样囊性癌可能起源于气管、支气管的原始细胞,该细胞显示导管和肌上皮分化特点。肺原发性腺样囊性癌一般情况下诊断并不困难。镜下肿瘤部分呈实性、管状或筛状结构,腺腔或筛孔中可见均质淡蓝色黏液;肿瘤间质可见黏液变性,有时透明变性显著,则压迫上皮性条索呈窄带状;实性巢外周偶成栅栏状,如基底样构型;肿瘤坏死及核分裂象不常见;侵犯神经是该肿瘤的一个特征;免疫组织化学标记显示肿瘤细胞呈导管和肌上皮表型,癌细胞CK8/18阳性表达,但也阳性表达vimentin、SMA和p63,有助于诊断[14-15];周围基质出现Ⅳ型胶原、laminin和硫酸肝素抗体染色阳性的基底膜样物质[16-17]。本研究中16例肺原发性腺样囊性癌患者具有上述特征。
3.3 治疗及预后 涎腺型肿瘤是一种异质性较高的肿瘤,主要包括黏液表皮样癌和腺样囊性癌,此类肿瘤大部分恶性程度低,预后较好[18]。发生腮腺、颌下腺、舌下腺及口腔小涎腺等部位的腺样囊性癌因部位特殊,常不易切净,术后极易复发。而肺原发性腺样囊性癌手术易于切除肿瘤,预后相对较好[19]。该类型肿瘤病程缓慢,发生远处转移较少且复发时间较晚[20-21]。极少数肺原发性腺样囊性癌具有较强的侵袭性及转移能力,发现时已是临床晚期,无法切除肿瘤,导致预后不良。目前治疗主要是外科手术,手术方式包括肺叶或全肺切除、支气管袖状切除+端端吻合、支气管内肿物局部切除+电灼肿物蒂部等,临床晚期可行全身化学治疗,也可行经动脉灌注化学治疗或局部放射治疗,但至于辅助治疗的作用到底有多大,目前还没有统一的认识[22-24]。本研究中,10例患者采取手术治疗,其中单侧全肺叶切除标本5例(包括2例化学治疗),气管袖状切除标本4例(包括2例局部放射治疗),单纯肺叶切除标本+化学治疗1例(EGFR热点突变基因19、21外显子未发生突变,未行靶向治疗);患者生存时间5~96个月,平均48个月。6例经支气管镜活检证实的肺原发性腺样囊性癌中3例行全身化学治疗(包括2例Ⅳ期伴发远处骨转移者),生存时间均超过1年,生活质量较佳;1例伴发活动期结核者行对症治疗,生存时间为7年;1例双肺多发肿瘤灶,经行动脉灌注化学治疗+栓塞术,患者生命体征平稳,生活质量较佳;1例确诊后失访,资料不详。
本研究尚有一些局限性,首先,收集和随访的资料有限;其次,本研究虽然跨度12年,但是例数较少,缺乏精确的统计学资料,可能会对研究结果带来一定影响。
总之,肺原发性腺样囊性癌是一种易多次复发、常伴有晚期转移的低度恶性肿瘤,生存期需要通过长期观察分析。其好发于气管及支气管,左肺下叶发生率也较高。临床上呈良性经过或低度侵袭性,主要表现为干咳及痰中带血;支气管镜检查并活检通常可获得明确诊断;确诊者首选手术治疗并辅以放化疗,不能手术者在内镜下介入治疗也是一种较好的治疗方法。
[1]Moran CA.Primary salivary gland-type tumors of the lung[J].Semin Diagn Pathol,1995,12(2):106-122.
[2]William DT,Elizabeth B,Konrad MH,et al.Pathology and genetics of tumours of the lung,pleura,thymus and heart[M].France:International Agency for Research on Cancer,2004:65-66.
[3]Gaissert HA,Mark EJ.Tracheobronchial gland tumors[J].Cancer Control,2006,13(4):286-294.
[4]Pellegrino R,Viegi G,Brusasco V,et al.Interpretative strategies for lung function tests[J].Eur Respir J,2005,26(5):948-968.
[5]Kanematsu T,Yohena T,Uehara T,et al.Treatment outcome of resected and nonresected primary adenoid cystic carcinoma of the lung[J].Ann Thorac Cardiovasc Surg,2002,8(2):74-77.
[6]Park CM.Tumors in the tracheobronchial tree:CT and FDG PET features[J].Radiographics,2009,29(1):55-71.
[7]Hua L,Li Q,Bai C,et al.Literatures analysis of tracheal adenoid cystic carcinoma about 5 cases and other domestic 131 cases[J].China Journal of Modern Medicine,2005,15(15):2319-2321,2325.(in Chinese) 华丽,李强,白冲,等.气管腺样囊性癌5例报告及国内131例文献分析[J].中国现代医学杂志,2005,15(15):2319-2321,2325.
[8]Kurul IC,Demiroz SM,Celik A,et al.Primary pulmonary adenoid cystic carcinoma:report of two cases[J].Asian Cardiovasc Thorac Ann,2012,20(5):604-606.
[9]Kwak SH,Lee KS,Chung MJ,et al.Adenoid cystic carcinoma of the airways:helical CT and histopathologiccorrelation[J].AJR Am J Roentgenol,2004,183(2):277-281.
[10]Albers E,Lawrie T,Harrell JH,et al.Tracheobronchial adenoid cystic carcinoma:a clinicopathologic study of 14 cases[J].Chest,2004,125(3):1160-1165.
[11]Yang PY,Liu MS,Chen CH,et al.Adenoid cystic carcinoma of the trachea:a report of seven cases and literature review[J].Chang Gung Med J,2005,28(5):357-363.
[12]Monaco SE,Khalbuss WE,Ustinova E,et al.The cytomorphologic spectrum of salivary gland type tumors in the lung and mediastinum:a report of 16 patients[J].Diagn Cytopathol,2012,40(12):1062-1070.
[13]Wu J,Zhao SH,Guo AT,et al.CT characteristics of primary salivary gland-type lung cancer[J].Chinese Journal of Oncology,2011,33(4):313-315.
[14]Hu MM,Hu Y,He JB,et al.Primary adenoid cystic carcinoma of the lung:Clinicopathological features,treatment and results[J].Oncol Lett,2015,9(3):1475-1481.
[15]Nagao T,Sato E,Inoue R,et al.Immunohistochemical analysis of salivary gland tumors:application for surgical pathology practice[J].Acta Histochem Cytochem,2012,45(5):269-282.
[16]Anupriya S,Mahesh P,Sharada P,et al.Immunohistochemical analysis of laminin expression in adenoid cystic carcinoma[J].J Oral Maxillofac Pathol,2014,18(Suppl 1):S26-31.
[17]Huo Z,Meng Y,Wu H,et al.Adenoid cystic carcinoma of the tracheobronchial tree:clinicopathologic and immunohistochemical studies of 21 cases[J].Int J ClinExp Pathol,2014,7(11):7527-7535.
[18]Lee CL,Wang TY,Tzen CY,et al.Primary high-grade salivary-type duct carcinoma of the lung:a case report[J].Int J Surg Pathol,2014,22(6):536-539.
[19]Kang DY,Yoon YS,Kim HK,et al.Primary salivary gland-type lung cancer:surgical outcomes[J].Lung Cancer,2011,72(2):250-254.
[20]Elnayal A,Moran CA,Fox PS,et al.Primary salivary gland-type lung cancer:imaging and clinical predictors of outcome[J].AJR Am J Roentgenol,2013,201(1):W57-63.
[21]Zhu F,Liu Z,Hou Y,et al.Primary salivary gland-type lung cancer:clinicopathological analysis of 88 cases from China[J].J Thorac Oncol,2013,8(12):1578-1584.
[22]Kitada M,Ozawa K,Sato K,et al.Adenoid cystic carcinoma of the peripheral lung:a case report[J].World J Surg Oncol,2010,8:74.
[23]Shimizu J,Oda M,Matsumoto I,et al.Clinicopathological study of surgically treated cases of tracheobronchial adenoid cystic carcinoma[J].Gen Thorac Cardiovasc Surg,2010,58(2):82-86.
[24]Allen AM,Rabin MS,Reillv JJ,et al.Unresectable adenoid cystic carcinoma of the trachea treated with chemoradiation[J].J Clin Oncol,2007,25(34):5521-5523.
(本文编辑:崔丽红)
Clinicopathologic Study of Pulmonary Primary Adenoid Cystic Carcinoma
YANGZhao-hui,WANGJian-jun,HEYan,etal.
DepartmentofPathology,NanjingGeneralHospitalofNanjingMilitaryCommand,Nanjing210002,China
Objective To investigate the clinical and pathological characteristics of primary pulmonary adenoid cysticcarcinoma and explore its diagnostic criteria and prognosis.Methods 16 patients diagnosed as pulmonary primary adenoid cystic carcinoma by chest CT or bronchoscopy test in the Nanjing General Hospital of Nanjing Military Command were enrolled in the study from January 2002 to January 2014.General information,including gender,age,clinical TNM staging,tumor location,clinical manifestations,imaging manifestations,diagnostic and therapeutic methods,were documented.Pathological features were inspected by gross investigation,microscopy and immunophenotype analysis.The mutations of hot-spot-mutation exons(19th,21th exon) of epidermal growth factor receptor(EGFR) gene were determined.The telephone or outpatient follow-ups were carried out from confirmed surgical diagnosis to 31 July 2014,with a period of half year.Items of follow-up included whether the patients received radiotherapy or chemotherapy,whether tumors metastasized and the date of death.Results Tumors tended to occur in trachea and primary bronchus.Cough and bloody sputum were main symptoms,while chest pain,chest distress,breathing difficulty and fever were occasionally seen.Chest CT scanning demonstrated that 10 of the 16 patients had shadows or quasi-circular high density shadows in rachea,primary bronchus,hiluspulmonis and lung nodules,with fade border.Bronchoscopy showed that a neoplasm sprout out of left primary bronchus in one patient but the surface was smooth.Gross investigation showed there were lumps in rachea,primary bronchus,hiluspulmonis and under envelopes,with the size of 1.2 cm×1.2 cm×1.0 cm-6.2 cm×2.5 cm×2.0 cm.The cross sections of lumps were white,solid,moderate-textured,partly sticky and with blurring borders.In one case,the lumps have infiltrated into adjacent pleura.Microscopy showed that cancer cells distributed as adenoid tubular like,cribrose,trabecular or nest-like arrangement;there was blue mucus in adenoid cavities and sieve pores;the sizes of cancer cells were small and uniform;those cells had little cytoplasm,round or polygonal shapes,deep-stained nucleuses but splintering nucleuses were rare;only a part of the tumors involved the trachea.The expresses of CK8/18,CK34βE12,SMA,p63,ki-67 in cancer cells were positive.The 19th and 21th exons were not mutated in one case.The survival time span of patients was 5-96 months,44 months in average.Six cases occurred local relapse or tumor metastasis in hiluspulmonis,mediastinal lymphnodes and bones.Conclusion Pulmonary primary adenoid cystic carcinoma is an infrequent low-degree-malignant tumor.The morphological features and biological behaviors of this carcinoma are similar to their counterparts in the salivary gland adenoid cystic carcinoma.Definite diagnosis should be mainly based on pathological examination in concert with immunohistochemical analysis.This type of tumor grows slowly,and early detection and surgery combined with radiotherapy and chemotherapy has an excellent prognosis.
Lung neoplasms;Carcinoma,adenoid cystic;Pathology,clinical;Prognosis
国家自然科学基金资助项目(81171391)
210002 江苏省南京市,南京军区南京总医院病理科 南京大学医学院临床学院(杨朝晖,王建军,何燕,余波,陆珍凤,徐艳,周晓军,石群立);浙江省台州市中心医院病理科(杨朝晖)
石群立,210002 江苏省南京市,南京军区南京总医院病理科 南京大学医学院临床学院;
E-mail:shiqunli2005@aliyun.com
R 734.2
B
10.3969/j.issn.1007-9572.2015.24.018
2014-12-26;
2015-02-28)

