小细胞肺癌分子遗传学研究进展
刘笛 樊旼
复旦大学附属肿瘤医院放疗科,复旦大学上海医学院肿瘤学系,上海200032
小细胞肺癌分子遗传学研究进展
刘笛综述 樊旼审校
复旦大学附属肿瘤医院放疗科,复旦大学上海医学院肿瘤学系,上海200032
小细胞肺癌(small cell lung cancer,SCLC)恶性程度高、预后差,现有的靶向药物治疗基本无效,迫切需要深入了解其分子特征从而筛选有效的治疗靶点。二代测序等全基因组研究技术为肿瘤的研究、诊治批量增加遗传标志物,SCLC的遗传位点亦不断被发现和解读。本文对近年SCLC分子遗传特征的研究进展进行综述。
小细胞肺癌;遗传变异;基因表达谱;致癌信号通路
肺癌目前是全世界最常见的恶性实体肿瘤,其中小细胞肺癌(small cell lung cancer,SCLC)约占13%[1],绝大多数罹患者有长期吸烟史。与发病率较高的非小细胞肺癌(non-small cell lung cancer,NSCLC)相比,SCLC侵袭性更强,倍增时间更短,转移更早,预后较差。尽管初治时SCLC对化疗及放疗的敏感性较高,但缓解期通常不持久,极易复发或转移,二线治疗疗效差。
经过40余年多项随机研究的积累,SCLC临床治疗已经形成规范,但近年来缺乏突破性进展,患者生存水平局限期停留在20%,广泛期则停留在2%[1-2],全面、深入了解SCLC的分子遗传特征,为后续研究提供研究基础显得尤为迫切。
基因组研究技术的不断进步使研究者有可能从肿瘤全基因组层面来观察基因改变,从而加速寻找和设计抗肿瘤药物。本文将综述近年全基因组技术在SCLC中的重要研究成果,描述所获基因组结构变异、突变谱、表达谱的特征,并阐述在SCLC中具有重要作用的下游信号转导事件,希望为后续研究提供依据。
1 基因组特征
1.1 基因组结构变异
基因组结构变异(genomic structural variations,SVs)指DNA序列长度>1 kb的差异,包括DNA片段缺失、插入、重复、重排、倒位以及拷贝数变化(copy number variations,CNVs)。表1归纳了SCLC常见的SVs位点及所含基因,其中抑癌基因TP53、RB1、PTEN、FHIT、RASSF1A缺失及MYC基因家族(c-MYC,L-MYC,N-MYC)、bcl-2基因扩增已在早年的研究中被证实。随着全基因组分析技术在SCLC中的广泛应用,更多的功能位点逐渐浮出水面。Dooley等[2]通过基于二代测序的CNV分析方法在小鼠SCLC模型中发现了核因子Ⅰ/B(nuclear factor Ⅰ/B,NFⅠB)基因扩增,并在人类SCLC中得到证实,而之前该基因的扩增只在前列腺癌和乳腺癌中被发现。该基因编码与CATT盒结合的转录因子,文章作者进一步在小鼠SCLC模型中证实了其癌基因功能,但该基因在人类SCLC中的作用还有待深入研究。之后发表的两项SCLC全基因组研究亦发现了NFIB扩增的存在,并提示其可能在SCLC发展的后期发挥作用[3-5]。此外,3项SCLC的全基因组研究均观察到基因SOX2扩增,并完成了初步功能验证[3-5]。性别确定区Y框蛋白2(sex determine region Y-box 2,SOX2)是一种干细胞相关核转录因子,通过与靶基因HMG结构域特异结合而在调控胚胎及组织的发育、维持干细胞多能性、细胞不均等分裂和决定细胞命运方面发挥作用。该基因过表达已在包括肺鳞癌(约20%)在内的多种肿瘤中被检测到,影响这些肿瘤的生物学行为及预后,且其扩增范围在SCLC中更加广泛。有研究报道,SOX2可促使呼吸道前体细胞向基底细胞和神经内分泌细胞转化,其持续高表达可以更好地维持端粒酶活性和稳定性、激活Wnt和Notch等干细胞通路[6]。SOX2的上述作用与SCLC的临床特征显著相关,为治疗这一高度恶性肿瘤提供了新的方向。

表1 SCLC患者常见基因组结构变异Tab. 1 Recurrent genomic structural variations in SCLC patients
1.2 突变谱及单核苷酸多态性
基因突变所致的癌基因活化和抑癌基因失活是肿瘤发生、发展中出现频率较高的分子事件。在SCLC中,除早期发现的TP53、RB1、PIK3CA、CDKN2A、PTEN[1]突变外,其他位点因技术所限鲜有报道。Peifer等[3]通过对27例组织和2株SCLC细胞株进行全外显子组测序发现,SCLC突变率(平均7.4蛋白改变突变/100万个碱基对)明显高于乳腺癌、卵巢癌等8种肿瘤,且大部分突变为C∶G>T∶A颠换,提示吸烟在该肿瘤发生中的作用[5,20]。作者进一步结合CNVs和转录组分析结果,提出基因TP53、RB1、PTEN、CREBBP、EP300、SLIT2、MLL、COBL、EPHA7驱动突变及MYC、MYCL、FGFR1扩增是SCLC重要的分子特征,而其中CREBBP、EP300、SLIT2均与组蛋白修饰过程相关。另一项基于42对SCLC及癌旁组织基因测序的研究[5]找出了22个体细胞热点突变,并对这些突变聚集的基因家族和信号通路进行总结。该研究还发现,与NSCLC相比,几乎所有的SCLC样本中都未检测到K-ras基因突变。
单核苷酸多态性(single nucleotide polymorphisms,SNPs)作为第3代遗传标志,决定基因的功能单位和人群遗传变异的内在特征,能够反映个体表型、疾病易感性和对药物、环境因子反应的差异。近年的研究表明,SCLC的SNPs多发生于DNA修复基因、癌基因和抑癌基因、药物代谢相关基因及凋亡相关基因等。现有的研究显示:碱基切除修复(base excision repair,BER)途径中的8-羟基鸟嘌呤核苷酸酶编码基因Val Met[21]、癌基因MYCL1 rs3134615[22]、抑癌基因TP53 Arg72Pro[23]可以增加罹患SCLC的易感性,X线修复交叉互补基因1(X-ray repair cross complementing group 1,XRCC1)Arg399Gln、8-羟基鸟嘌呤-DNA糖苷酶编码基因Ser326Cys、嘌呤/嘧啶核酸内切酶编码基因Asp148Glu与SCLC的易感性可能无关[24-26]。此外,有研究报道上述基因中与XRCC1表达相关的SNPs[27]及TP53 Arg72Pro[28]高表达的SCLC患者的生存相关;此外,癌基因YAP rs10895256、rs716274[29]、多药耐药基因2[30]、BCL-2-938CC[31]、端粒酶反转录酶基因rs402710[32]也与SCLC患者预后相关。
在一定程度上,SNPs还可用来预测化疗疗效。核苷酸切除修复途径中的切除修复交叉互补基因ERCC6 C6530G等SNPs可能与SCLC患者对含铂方案化疗有效率及预后相关[33-34];多药耐药基因1 2677G>T和3435C>T可作为EP方案化疗疗效预测因子[35];死亡相关蛋白激酶3 rs11169748、甲基转移酶样6 rs2440915可辅助预测铂类化疗疗效[36]。然而,上述SNPs位点的生物学意义还有待更大样本研究的验证。
2 转录组特征
2.1 基因表达谱
表2总结了在SCLC中常见的过表达基因。SCLC往往高表达PCNA、端粒酶基因等高增殖活性相关基因,BCL-2等凋亡抑制基因和SYP(Syn)、CHGA(CgA)、NCAM1(CD56)、ASCL1、IA-1、GRP等神经内分泌基因。近期,Byers等[17]综合应用蛋白组和转录组分析SCLC和NSCLC在分子水平差异时发现,SCLC的一些受体酪氨酸激酶表达水平较低,PI3K和RAS/MAPK/ERK信号通路下调,但包括组蛋白甲基化转移酶(enhancer of zeste homolog 2,EZH2)在内的E2F1调节因子都明显上调,特别是作为DNA修复蛋白及E2F1共激活因子的聚腺苷酸二磷酸核糖转移酶-1[(poly (ADP-ribose) polymerase-1,PARP1],其mRNA和蛋白均过表达,当PARP1和EZH2基因敲除后SCLC细胞生长受到明显抑制。Sato等[18]又进一步证实,EZH2所在的polycomb抑制复合体家族在SCLC中均过表达,且与患者的预后不良相关。已有临床研究显示,SCLC对 PARP1抑制剂有很强的敏感性,可延缓SCLC患者的进展,有良好的临床应用前景。
2.2 融合基因
随着EML4-ALK融合基因在NSCLC中致病机制的阐释,融合基因在肿瘤中的作用日益受到重视,转录组测序技术加速了该方面研究。Rudin等[5]在SCLC中发现了4个高频融合基因并进行了RT-PCR验证。其中RLF-MYCL1是SCLC中发生频率最高(5/55)、报道最多的融合基因[4-5,41],融合形式致使癌基因MYCL1激活并过表达,导致了细胞的恶性转化;通过siRNA抑制融合基因后SCLC细胞增殖明显减慢。有研究提示[4-5,41],融合基因多发生在拷贝数扩增的位点,提示两种事件可能并非独立发生,或许可以用染色体碎裂(chromothripsis)解释。SCLC患者中有少部分是从不吸烟者,融合基因在这些肿瘤中所起作用值得进一步研究。
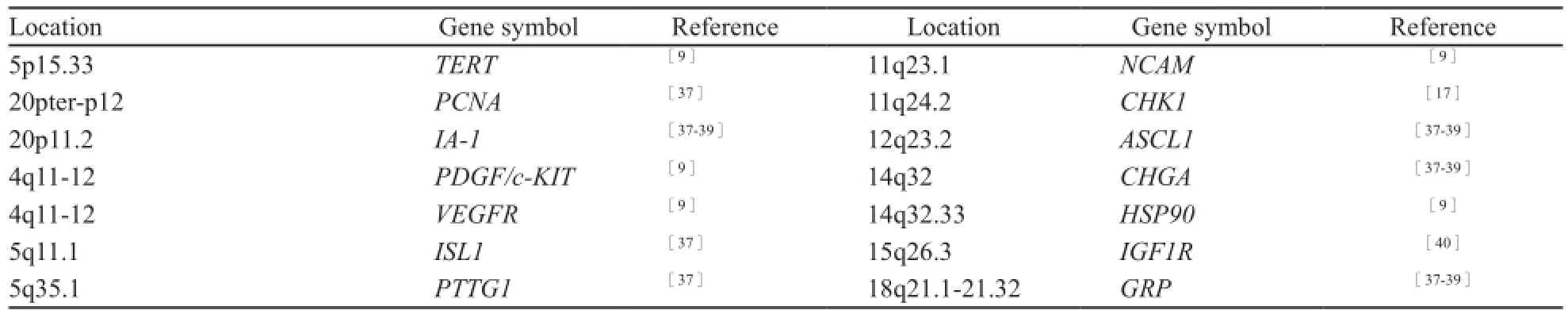
表2 小细胞肺癌常见过表达基因Tab. 2 Genes commonly overexpressed in SCLC
3 信号转导通路
3.1 PI3K/AKT/mTOR信号通路
以往的研究提示,SCLC中存在磷脂酰肌醇3激酶(PI3K)信号通路的组成性活化[42]和PI3K、PTEN的失活突变。此外,多种酪氨酸激酶受体(IGF1R、c-KIT、c-MET等)的过表达、70%患者AKT的磷酸化及细胞株mTOR和4EBP1蛋白表达的增加,似乎提示激活的PI3K/AKT/ mTOR信号通路影响着SCLC的增殖、抗凋亡及迁移。然而,PI3K抑制剂在目前的临床研究中并未展现出预期疗效,结合前述Byers等[17]发表的近乎相反的发现,似乎提示PI3K通路在SCLC中可能并没有像在NSCLC中那样作用显著,但不排除PI3K通路在SCLC某些亚型中的活化,故PI3K在SCLC中的地位还有待证实。
3.2 干细胞信号通路
SCLC是表达神经内分泌标志、临床上最易复发和耐药、分化程度最低的肺部肿瘤,发育及干细胞相关Hedgehog/Notch/Wnt通路为SCLC的研究及治疗提供了新的视角。Notch通路可以调控气管上皮是否向神经内分泌分化;气管上皮前体细胞中Hedgehog通路的激活则可直接加速该细胞向神经内分泌细胞的转化;支气管上皮在烟草的作用下Wnt通路将激活,导致细胞增殖和肿瘤形成[43]。在SCLC中,Hedgehog通路呈现配体依赖的活化,其阻滞剂可在体内及体外明显抑制SCLC细胞的增殖[44],现已有多种Hedgehog抑制剂进入了临床研究阶段,结果令人期待。
4 结语
SCLC的发生、发展与多种分子遗传变异相关,全基因组技术的进步使得大量新位点甚至低频、稀有变异的发现成为可能,这种以数据为导向的研究模式极大地提高了疾病研究的效率,为疾病的诊治提供了更为有效的手段。但另一方面,人类基因组仍有很多未知,肿瘤基因组的复杂性对全基因组研究技术和生物信息学分析手段提出了更高要求,而高通量筛选结果仍需经过传统实验的功能和机制验证。新技术有望不断推动SCLC的研究,从而为进一步理解、治疗该疾病带来希望。
[1] VAN MEERBEECK J P, FENNELL D A, De RUYSSCHER D K. Small-cell lung cancer [J]. Lancet, 2011, 378(9804): 1741-1755.
[2] DOOLEY A L, WINSLOW M M, CHIANG D Y, et al. Nuclear factor Ⅰ/B is an oncogene in small cell lung cancer [J]. Genes Dev, 2011, 25(14): 1470-1475.
[3] PEIFER M, FERNANDEZ-CUESTA L, SOS M L, et al. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer [J]. Nat Genet, 2012, 44(10): 1104-1110.
[4] IWAKAWA R, TAKENAKA M, KOHNO T, et al. Genomewide identification of genes with amplification and/or fusion in small cell lung cancer [J]. Genes Chromosomes Cancer, 2013.
[5] RUDIN C M, DURINCK S, STAWISKI E W, et al. Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer [J]. Nat Genet, 2012, 44(10): 1111-1116.
[6] WANG S, CHANDLER-MILITELLO D, LU G, et al. Prospective identification, isolation, and profiling of a telomerase-expressing subpopulation of human neural stem cells, using sox2 enhancer-directed fluorescence-activated cell sorting [J]. J Neurosci, 2010, 30(44): 14635-14648.
[7] SOZZI G, VERONESE M L, NEGRINI M, et al. The FHIT gene 3p14.2 is abnormal in lung cancer [J]. Cell, 1996, 85(1): 17-26.
[8] DAMMANN R, LI C, YOON J H, et al. Epigenetic inactivation of a RAS association domain family protein from the lung tumour suppressor locus 3p21.3 [J]. Nat Genet, 2000, 25(3): 315-319.
[9] D’ANGELO S P, PIETANZA M C. The molecularpathogenesis of small cell lung cancer [J]. Cancer Biol Ther, 2010, 10(1): 1-10.
[10] TESTA J R, LIU Z, FEDER M, et al. Advances in the analysis of chromosome alterations in human lung carcinomas [J]. Cancer Genet Cytogenet, 1997, 95(1): 20-32.
[11] LEVIN N A, BRZOSKA P M, WARNOCK M L, et al. Identification of novel regions of altered DNA copy number in small cell lung tumors [J]. Genes Chromosomes Cancer, 1995, 13(3): 175-185.
[12] RIED T, PETERSEN I, HOLTGREVE-GREZ H, et al. Mapping of multiple DNA gains and losses in primary small cell lung carcinomas by comparative genomic hybridization[J]. Cancer Res, 1994, 54(7): 1801-1806.
[13] MIURA I, GRAZIANO S L, CHENG J Q, et al. Chromosome alterations in human small cell lung cancer: frequent involvement of 5q [J]. Cancer Res, 1992, 52(5): 1322-1328.
[14] KIM Y H, GIRARD L, GIACOMINI C P, et al. Combined microarray analysis of small cell lung cancer reveals altered apoptotic balance and distinct expression signatures of MYC family gene amplification [J]. Oncogene, 2006, 25(1): 130-138.
[15] SCHAFFER B E, PARK K S, YIU G, et al. Loss of p130 accelerates tumor development in a mouse model for human small-cell lung carcinoma [J]. Cancer Res, 2010, 70(10): 3877-3883.
[16] LEVIN N A, BRZOSKA P, GUPTA N, et al. Identification of frequent novel genetic alterations in small cell lung carcinoma[J]. Cancer Res, 1994, 54(19): 5086-5091.
[17] BYERS L A, WANG J, NILSSON M B, et al. Proteomic profiling identifies dysregulated pathways in small cell lung cancer and novel therapeutic targets including PARP1 [J]. Cancer Discov, 2012, 2(9): 798-811.
[18] SATO T, KANEDA A, TSUJI S, et al. PRC2 overexpression and PRC2-target gene repression relating to poorer prognosis in small cell lung cancer [J]. Sci Rep, 2013, 3: 1911.
[19] ZHAO X, WEIR B A, LAFRAMBOISE T, et al. Homozygous deletions and chromosome amplifications in human lung carcinomas revealed by single nucleotide polymorphism array analysis [J]. Cancer Res, 2005, 65(13): 5561-5570.
[20] PLEASANCE E D, STEPHENS P J, O’MEARA S, et al. A small-cell lung cancer genome with complex signatures of tobacco exposure [J]. Nature, 2010, 463(7278): 184-190.
[21] KOHNO T, SAKIYAMA T, KUNITOH H, et al. Association of polymorphisms in the MTH1 gene with small cell lung carcinoma risk [J]. Carcinogenesis, 2006, 27(12): 2448-2454.
[22] XIONG F, WU C, CHANG J, et al. Genetic variation in an miRNA-1827 binding site in MYCL1 alters susceptibility to small-cell lung cancer [J]. Cancer Res, 2011, 71(15): 5175-5181.
[23] FERNANDEZ-RUBIO A, LOPEZ-CIMA M F, GONZALEZARRIAGA P, et al. The TP53 Arg72Pro polymorphism and lung cancer risk in a population of Northern Spain [J]. Lung Cancer, 2008, 61(3): 309-316.
[24] DAI L, DUAN F, WANG P, et al. XRCC1 gene polymorphisms and lung cancer susceptibility: a meta-analysis of 44 casecontrol studies [J]. Mol Biol Rep, 2012, 39(10): 9535-9547.
[25] LI Z, GUAN W, LI M X, et al. Genetic polymorphism of DNA base-excision repair genes (APE1, OGG1 and XRCC1) and their correlation with risk of lung cancer in a Chinese population [J]. Arch Med Res, 2011, 42(3): 226-234.
[26] DUAN W X, HUA R X, YI W, et al. The association between OGG1 Ser326Cys polymorphism and lung cancer susceptibility: a meta-analysis of 27 studies [J]. PLoS One, 2012, 7(4): e35970.
[27] SUN Z, CHEN J, AAKRE J, et al. Genetic variation in glutathione metabolism and DNA repair genes predicts survival of small-cell lung cancer patients [J]. Ann Oncol, 2010, 21(10): 2011-2016.
[28] SREEJA L, SYAMALA V, RAVEENDRAN P B, et al. p53 Arg72Pro polymorphism predicts survival outcome in lung cancer patients in Indian population [J]. Cancer Invest, 2008, 26(1): 41-46.
[29] WU C, XU B, YUAN P, et al. Genome-wide interrogation identifies YAP1 variants associated with survival of small-cell lung cancer patients [J]. Cancer Res, 2010, 70(23): 9721-9729.
[30] MULLER P J, DALLY H, KLAPPENECKER C N, et al. Polymorphisms in ABCG2, ABCC3 and CNT1 genes and their possible impact on chemotherapy outcome of lung cancer patients [J]. Int J Cancer, 2009, 124(7): 1669-1674.
[31] KNOEFEL L F, WERLE-SCHNEIDER G, DALLY H, et al. Polymorphisms in the apoptotic pathway gene BCL-2 and survival in small cell lung cancer [J]. J Thorac Oncol, 2011, 6(1): 183-189.
[32] XUN W W, BRENNAN P, TJONNELAND A, et al. Singlenucleotide polymorphisms (5p15.33, 15q25.1, 6p22.1, 6q27 and 7p15.3) and lung cancer survival in the European Prospective Investigation into Cancer and Nutrition (EPIC)[J]. Mutagenesis, 2011, 26(5): 657-666.
[33] YU D, ZHANG X, LIU J, et al. Characterization of functional excision repair cross-complementation group 1 variants and their association with lung cancer risk and prognosis [J]. Clin Cancer Res, 2008, 14(9): 2878-2886.
[34] 刘炬, 张雪梅, 张湘茹, 等. ERCC6 C6530G单核苷酸多态与局限期小细胞肺癌患者生存相关 [J]. 中国癌症杂志, 2006(11): 943-947.
[35] SOHN J W, LEE S Y, LEE S J, et al. MDR1 polymorphisms predict the response to etoposide-cisplatin combination chemotherapy in small cell lung cancer [J]. Jpn J Clin Oncol, 2006, 36(3): 137-141.
[36] TAN X L, MOYER A M, FRIDLEY B L, et al. Geneticvariation predicting cisplatin cytotoxicity associated with overall survival in lung cancer patients receiving platinumbased chemotherapy [J]. Clin Cancer Res, 2011, 17(17): 5801-5811.
[37] PEDERSEN N, MORTENSEN S, SORENSEN S B, et al. Transcriptional gene expression profiling of small cell lung cancer cells [J]. Cancer Res, 2003, 63(8): 1943-1953.
[38] SUGITA M, GERACI M, GAO B, et al. Combined use of oligonucleotide and tissue microarrays identifies cancer/testis antigens as biomarkers in lung carcinoma [J]. Cancer Res, 2002, 62(14): 3971-3979.
[39] BHATTACHARJEE A, RICHARDS W G, STAUNTON J, et al. Classification of human lung carcinomas by mRNA expression profiling reveals distinct adenocarcinoma subclasses [J]. Proc Natl Acad Sci U S A, 2001, 98(24): 13790-13795.
[40] BADZIO A, WYNES M W, DZIADZIUSZKO R, et al. Increased insulin-like growth factor 1 receptor protein expression and gene copy number in small cell lung cancer[J]. J Thorac Oncol, 2010, 5(12): 1905-1911.
[41] MAKELA T P, SAKSELA K, EVAN G, et al. A fusion protein formed by L-myc and a novel gene in SCLC [J]. EMBO J, 1991, 10(6): 1331-1335.
[42] MOORE S M, RINTOUL R C, WALKER T R, et al. The presence of a constitutively active phosphoinositide 3-kinase in small cell lung cancer cells mediates anchorageindependent proliferation via a protein kinase B and p70s6kdependent pathway [J]. Cancer Res, 1998, 58(22): 5239-5247.
[43] LEMJABBAR-ALAOUI H, DASARI V, SIDHU S S, et al. Wnt and Hedgehog are critical mediators of cigarette smokeinduced lung cancer [J]. PLoS One, 2006, 1: e93.
[44] GARCIA C M, ALONSO C G, APARICIO G G, et al. Stem cell and lung cancer development: blaming the Wnt, Hh and Notch signalling pathway [J]. Clin Transl Oncol, 2011, 13(2): 77-83.
Recent advances of molecular genetic characteristics of small cell lung cancer
LIU Di, FAN Min
(Department of Radiation Oncology, Fudan University Shanghai Cancer Center, Department of Oncology, Shanghai Medical College, Fudan University, Shanghai 200032, China)
FAN Min E-mail: fanming@fudan.edu.cn
Small cell lung cancer (SCLC) is an aggressive malignancy with fairly poor prognosis. Innovative treatment based on improved understanding of the genetic alterations of SCLC is awaited. Recently, a number of potential targets or important oncogenic pathways have been identified by the next generation sequencing or other systematic genomic analysis in SCLC. In this review, we summarised the new findings of genetic characteristics in SCLC.
Small cell lung cancer; Genomic variation; Gene expression pro filing; Oncogenic pathway
10.3969/j.issn.1007-3969.2014.08.013
R734.2
A
1007-3639(2014)08-0636-06
2013-09-31
2014-01-09)
卫生部临床学科重点项目(No:卫规财函[2010]439号)。
樊旼 E-mail:fanming@fudan.edu.cn

