精子功能相关的蛋白质调控受精过程的研究进展
陈志林,冯美莹,陈预明,卫恒习,李莉,吴同山,张守全
1. 华南农业大学动物科学学院,国家生猪种业工程技术研究中心,广州 510642;
2. 东莞市畜牧科学研究所,东莞 523086
哺乳类动物受精是一个精细的程序化复杂过程。受精过程中任何的偏差往往会导致动物的低繁殖力或不育[1]。具有良好受精能力的精子是受精成功的先决条件。精子在睾丸曲精细管中不断生成,随后离开睾丸进入到附睾中进行成熟。在这一过程中,精子发生了若干的生理变化,其主要表现为附睾及副性腺分泌的蛋白因子在精子头部的组装和附着,精子质膜表面蛋白构象的改变,向前运动能力的获得等[2]。精子在雌性生殖道里需要依次经历超活化和获能反应,以获得与卵子受精的能力。获能的精子需要依次通过子宫与输卵管接合部、输卵管壶-峡连接部等屏障才可以与卵子相遇。只有运动活力极强的精子才能够通过雌性生殖道中的这一系列屏障。在精卵相遇后,精子仍需要经历复杂的过程才能完成受精过程。其中,精卵开始接触的过程中,精子受卵丘细胞和透明带的作用,逐步诱发了顶体反应;反应过程释放多种顶体内的水解酶以促进精子溶解透明带;同时,顶体膜与精子质膜的融合暴露出精子头部的卵识别和结合位点[3],为随后的精卵结合做好准备;精子溶解透明带后进入到卵周间隙,精子头部的识别和结合位点在若干蛋白因子的协助下,识别和结合卵膜受体蛋白[4],从而导致精卵的膜融合(图1),并最终形成完整的受精卵。
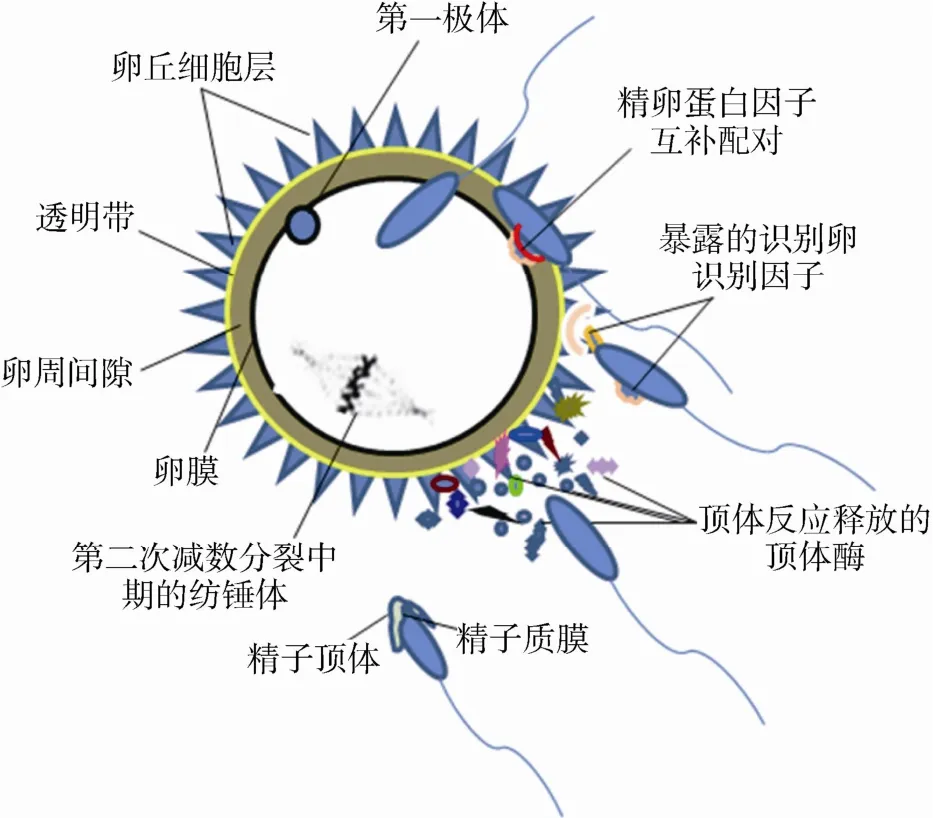
图1 受精过程
在整个受精过程中,精子功能相关的蛋白分子参与其中的信号通路调控以及一系列的生理生化反应。随着蛋白组学的发展和基因敲除技术的应用,哺乳动物精子功能相关的蛋白质不断涌现。本文主要对精子相关的信号通路及其他受精过程相关的精子功能蛋白质进行总结,阐述这些蛋白质分子与精子运动活力、精子获能、顶体反应、透明带穿入以及精卵融合方面的关系,并对其在改善哺乳动物繁殖力上的应用前景提出展望。
1 精子运动活力及获能反应的信号通路
在受精过程中,精子不仅需要在雌性生殖道中游动到与卵子结合的位点,而且还要获得与卵子受精的能力,故精子运动活力及获能反应是影响受精的两个关键因素。精子运动活力主要由精子尾部的长鞭毛摆动而形成[5],除了受外界环境影响外,也受到内部若干种信号通路的调节。精子获能是精子获得与卵子结合的能力的生理过程,是精子在受精前必须经历的一个重要反应。环腺苷酸(Cyclic adenodine monophosphate, cAMP)-环腺苷酸依赖性蛋白激酶A(CAMP-dependent protein kinase, cAMPPKA)信号通路和钙离子信号通路是调控哺乳动物精子运动活力和获能反应的两个最重要的信号通路,其信号通路调控模式相对比较清晰[6](图2)。这两种信号通路的活化过程分别是由精子细胞内的 HCO3-和Ca2+水平来调节[7]。自精子从雄性生殖道射出后,精清富含的HCO3-可以通过Na+/ HCO3-协同转运蛋白(Cotransporter, nbc)进入精子细胞内,引起胞内的pH改变[8]。与此同时,由精子膜产生的胆固醇外流作用促使膜通透性的增强,HCO3-也因此可以快速进入精子细胞内[9],引起细胞内 HCO3-浓度的升高,导致精子胞质的碱性化。胞质碱性化在刺激精子加速新陈代谢的同时激活了胞内的可溶性腺苷酸环化酶(Soluble adenyl cyclase, sAC)[10]。sAC的活化过程是增强精子运动活力和诱发精子获能的必要过程。sAC的主要作用是引起细胞内cAMP水平的提高,并致使 PKA的激活[6]。活化的 PKA可以使精子细胞内的蛋白磷酸酶(Protein phosphatases,PPs)活性受到抑制,同时也不断地激活蛋白酪氨酸激酶(Protein tyrosine kinases, PTK),使胞内蛋白酪氨酸磷酸化作用得到增强。另外,精子内PKA的活化还有利于钙离子通道蛋白(CatSper)的激活,从而达到诱发精清中的Ca2+内流的效果[11~13]。胞内Ca2+浓度的升高不仅可以引起精子细胞膜的超极化[14],增强精子鞭毛的运动活力[15,16],也能够维持sAC的活化程度[6],从而持续激活 PTK,增强胞内蛋白酪氨酸磷酸化的水平。事实上,PTK的活化能够促使凝溶胶蛋白(Gelsolin)发生磷酸化[17],并使其处于抑制状态,进而引起肌动蛋白的聚合作用,最后引发精子发生超活化反应[18,19]。另外,由于PPs去磷酸化活性被抑制以及PTK磷酸化作用的持续,细胞内的蛋白酪氨酸磷酸化水平不断提高,从而诱发精子超活化运动和获能反应[20,21]。精子的超活化和获能不仅直接活化了精子鞭毛轴丝[16],增强鞭毛运动性和尾部侧摆幅度[15,22],还解除了对精子顶体反应的抑制,有利于精卵的结合。研究表明,胞内 HCO3-和 Ca2+高浓度水平的状态不仅是维持精子超活化运动和获能反应的关键[11,23],也是精子发生顶体反应的重要因素[6]。
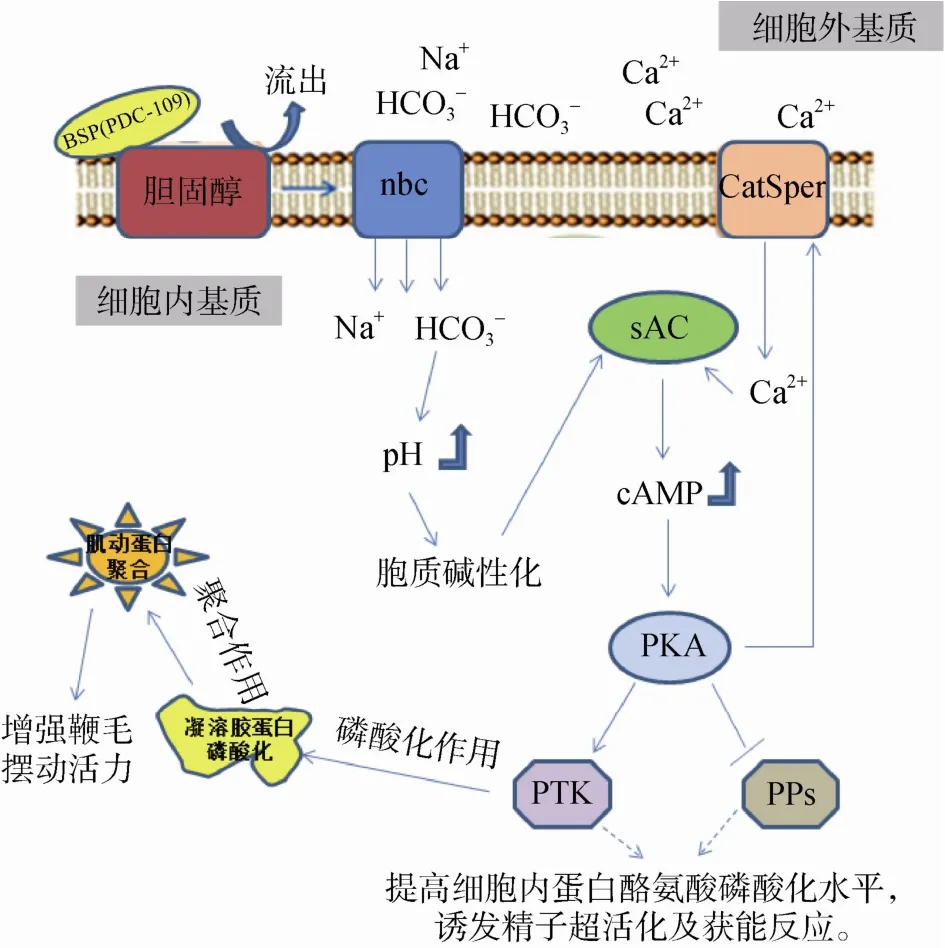
图2 cAMP/PKA信号通路和钙离子信号通路
2 参与精子运动活力调节及获能反应的精子功能蛋白
虽然cAMP/PKA信号通路和钙离子信号通路的具体分子调控机理仍不明确,但近年来的研究结果表明,多种精子和精清蛋白质参与这些过程(表 1)。其中锌 a2糖蛋白(Zn-a2-glycoprotein, ZAG)是调节cAMP/PKA通路中的关键因子cAMP的蛋白分子。ZAG抗体能够显著地降低精子细胞内的cAMP水平,致使PKA蛋白激酶不能维持活化状态,从而抑制精子的超活化,影响精子的运动活力[24]。附睾活力抑制因子II (Motility inhibiting factor, MIF-II)[25]和精浆活力抑制因子(Seminal plasma motility inhibitor,SPMI)[26]与 ZAG抗体作用相似,同样具有降低精子细胞内cAMP浓度的功能,但其具体作用靶点仍不清楚。此外,CatSper是钙离子信号通路的关键蛋白。活化的 CatSper能够引起 Ca2+内流,诱发精子发生超活化反应。研究表明,缺失catsper基因的小鼠精子失去超活化的能力以及表现出极弱的运动力[27]。另外,小鼠附睾分泌的钙离子-腺苷三磷酸膜蛋白(Plasma membrane Ca2+-ATPase 4a, PMCA4a)能够作为一种 Ca2+流泵,影响钙离子信号通路的激活并维持精子内Ca2+的稳态。缺失pmca4基因的精子不能够发生超活化和获能反应[28],因此PMCA4a对精子运动活力和受精具有极其重要的作用。
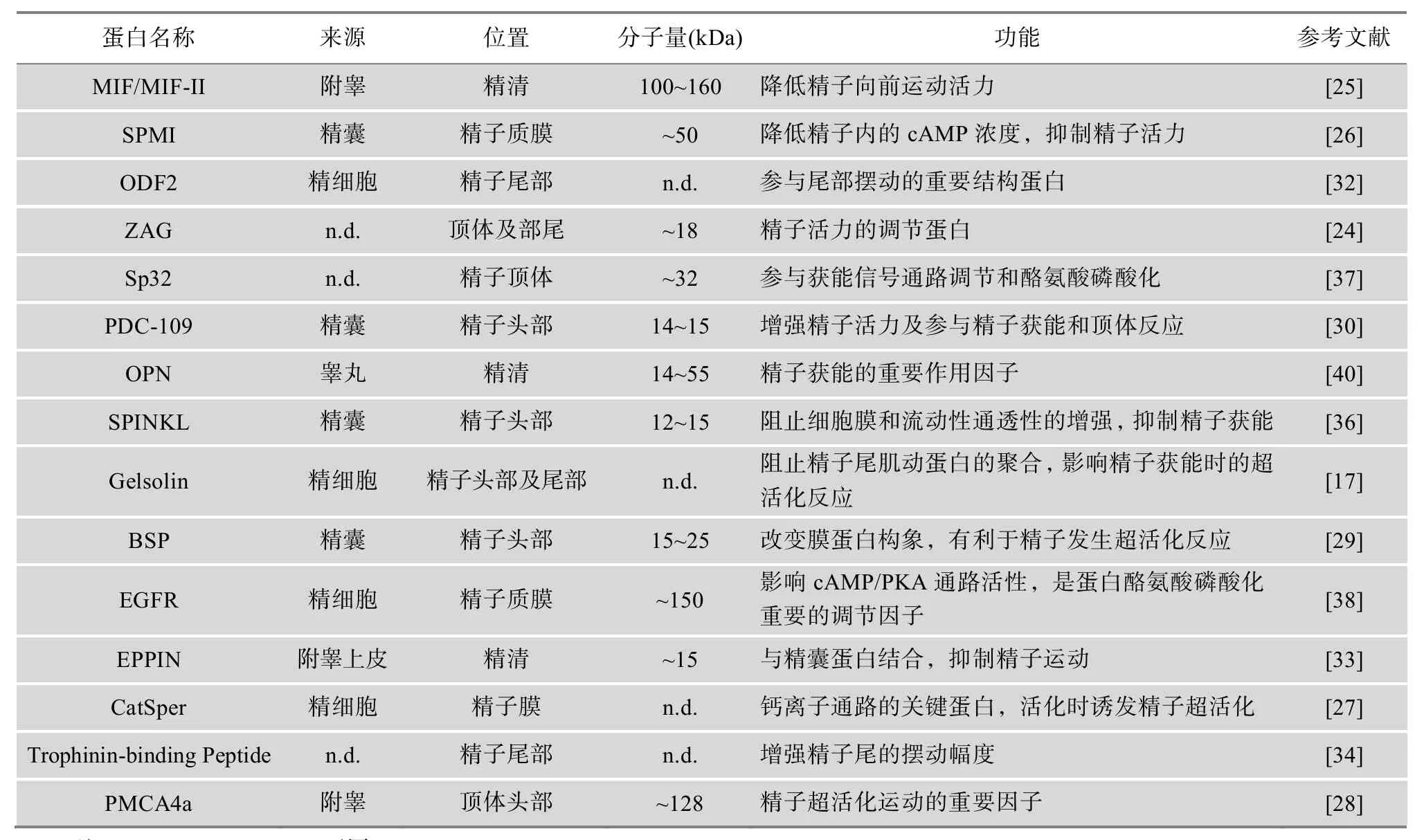
表1 精子运动活力及获能相关的蛋白质
公牛精清蛋白(Bovine seminal plasma, BSP)是与公牛繁殖力相关的蛋白质。BSP可以改变精子膜表面的蛋白构象,增强精子膜的流动性,调节膜表面的胆固醇外流,从而参与精子获能的调节[29]。研究表明,BSP的主要成分PDC-109是引起膜胆固醇流的主要作用蛋白[30]。PDC-109能够改变膜的通透性,提高胞内的HCO3-和Ca2+-ATPase的浓度,影响精子的运动活力以及获能进程[31]。
随着对受精生物标记的深入研究,多种精子活力及获能相关的蛋白质也随之被发现。致密纤维蛋白(Outer dense fiber protein, ODF2)是精子尾部的组成蛋白,对精子尾部的摆动作用十分重要。研究显示,odf2+/-嵌合体小鼠由于产生运动活力极低的精子而重度不育[32]。附睾蛋白酶抑制蛋白(Epididymal protease inhibitor, EPPIN)是一种由睾丸支持细胞分泌的蛋白,能够与精囊蛋白相互结合,抑制精子的活力[33]。Trophinin-binding Peptide是发现于精子尾部的一种内源性蛋白质,能够促进精子尾部的摆动,增强精子的直线游动能力[34]。丝氨酸蛋白酶抑制因子(Serine protease inhibitor kazal-type-like SPINKL)是一种由精囊分泌的蛋白因子[35],能够抑制精子膜的胆固醇外流,阻碍膜通透性的增强和胞质的碱性化,从而抑制精子发生获能反应[36]。Sp32作为前顶体素的结合蛋白,发生磷酸化作用主要是与精子获能相关[37]。表皮生长因子受体(Epidermal growth factor receptor, EGFR)获能时的磷酸化则可能与获能信号通路相关,因为EGFR与表皮生长因子(Epidermal growth factor, EGF)结合后,不仅能够明显地降低精子蛋白质酪氨酸的磷酸化作用[38],且抑制了PKA的活化状态[9]。此外,酶柠檬酸蛋白酶、延胡索酸水化酶、苹果酸酶和脱氢酶在公马精液中存在,其含量与精子运动活力和个体的受精能力呈正相关[39];公牛精液的骨桥蛋白(Osteopontin, OPN)对于维持精子在母性生殖道中的活力及获能状态具有重要作用,其含量与雄性个体繁殖力呈正相关[40]。
上述蛋白对精子运动活力或获能都具有重要的作用,但其作用机理还有待考证。在今后的研究中,通过这些蛋白筛选高繁殖力的个体,检测这些分子标记以判断是否留种,从而指导畜牧生产育种工作,提高选育工作效率。
3 精子功能蛋白质与顶体反应的关系
获能后的精子在输卵管中与卵子相遇后,接触并溶解卵丘细胞层。在该过程中,精子运动活力和透明质酸酶的作用十分关键。在自然受精时精子穿越卵丘层的分子机理至今仍不清楚,一般认为是精子顶体在穿透卵丘细胞时能够诱发顶体膜的融合、顶体内各种酶的释放以及卵透明带被水解等顶体反应现象(图3)。然而有研究显示,在精子成功穿过卵丘细胞层后,顶体的配体与透明带表面的受体相互识别,膜表面的半乳糖基转移酶(GalT)等蛋白分子随后与透明带蛋白(Zona pellucida protein, ZP3)结合,引发顶体反应[41]。尽管顶体反应的发生仍存在争议,但反应形成的入卵通道以及过程中暴露出的卵膜识别位点对精卵融合过程必不可少。
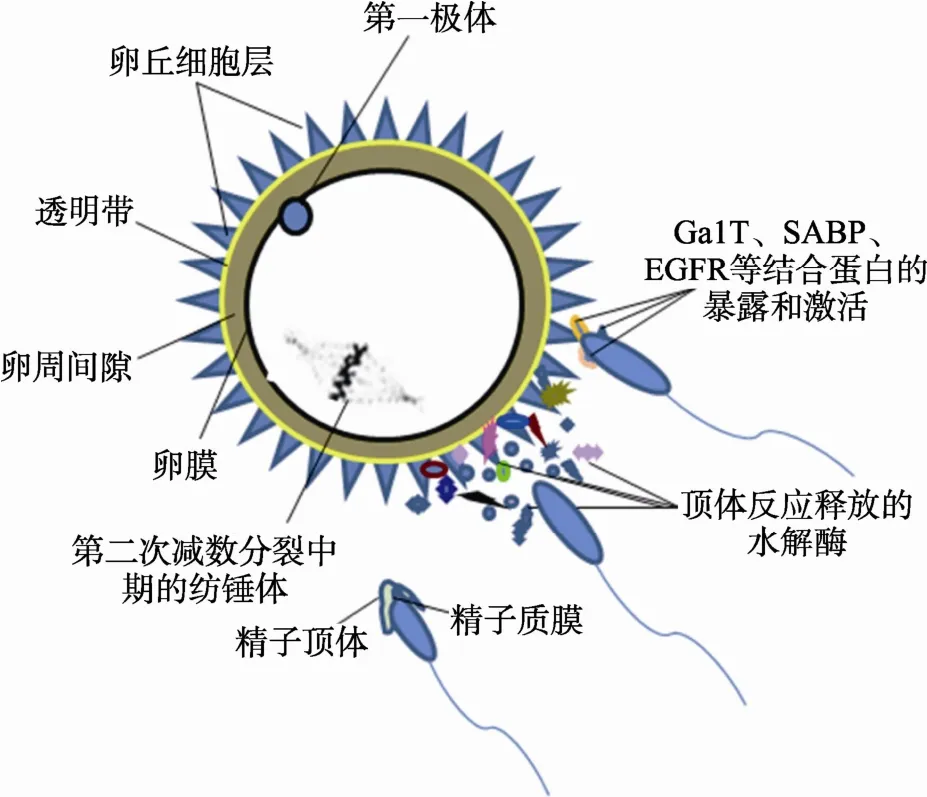
图3 顶体反应的过程
顶体反应是受精的先决条件,只有经过获能和完成顶体反应的精子才能够进入卵周间隙,完成精卵膜融合过程。该反应时间进程长,多种蛋白分子参与其中(表2)。除了GalT能够与ZP3结合外,乙酰胆碱受体(Acetylcholine receptor, α7nAChR)能够致使 EGFR分子活化,介导EGFR和 ZP3结合[42]。EGFR和ZP3的结合有可能是刺激了钙离子通路,影响 Ca2+调节的顶体反应。另外,肌动蛋白结合蛋白(Secretory actin-binding protein, SABP)是一种位于精子尾部的蛋白分子,在顶体反应时与肌动蛋白结合,从而阻断肌动蛋白的聚合[43]。由于精子获能后Gelsolin发生的去磷酸化能使肌动蛋白解聚,并引发顶体反应,因此 SABP很可能是一种诱发顶体反应的重要作用因子。此外,精子头部还存在多种与顶体反应相关但作用位点未知的蛋白。P14是一种附睾来源的顶体蛋白质,在顶体膜与精子质膜发生融合时发挥重要的作用,其抗体能够显著降低顶体反应的发生率[44]。与P14作用相似的还有动力蛋白(Dynamin),Dynamin能够启动顶体内容物胞外分泌,调控顶体膜融合的发生[45]。此外,精子头部的顶体相关蛋白分子(Sperm acrosome associated 7, SPACA7)的释放也是诱发顶体反应的关键因素[46,47]。
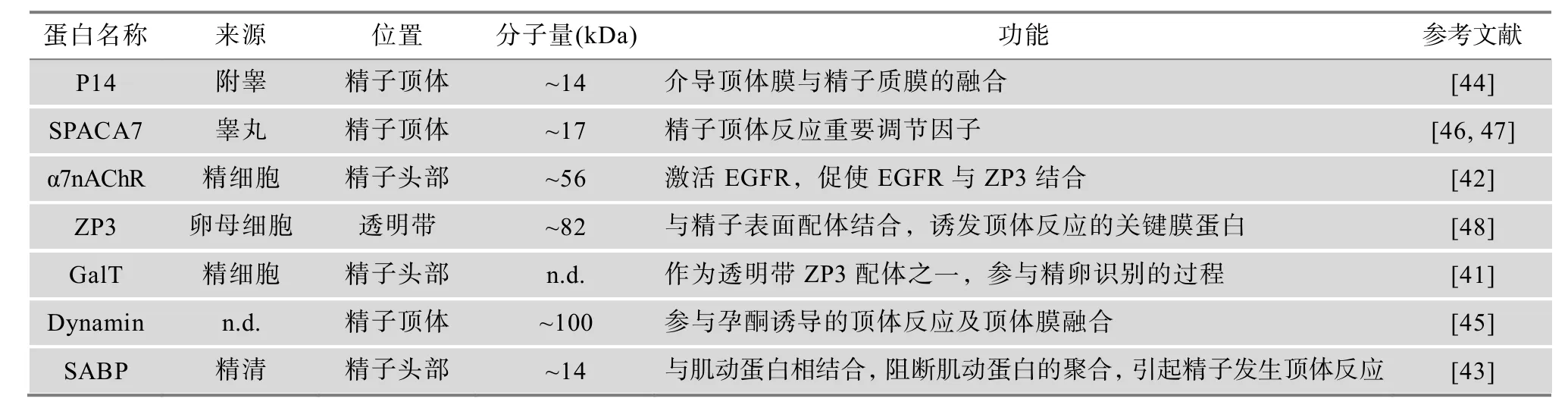
表2 精子顶体反应相关的蛋白质
4 精子功能蛋白质与入卵通道的形成
哺乳动物卵透明带一般由 3~4种糖蛋白(ZP1、ZP2和ZP3等)组成[49,50],构成了精卵受精过程的屏障。自发生顶体反应后,顶体内释放的复合酶(顶体素(Acrosin)、卵结合蛋白、脂酶和唾液酸苷酶等)能溶解卵透明带,协助精子突破此屏障,并形成一条入卵通道。事实上,在形成入卵通道之前,精子头部需要结合在透明带表面以形成一种牢固的状态(图4)。这种状态对于精子随后溶解坚硬的透明带是十分必要的。精子头部内源性或附着的蛋白则是形成该状态的关键分子(表3)。
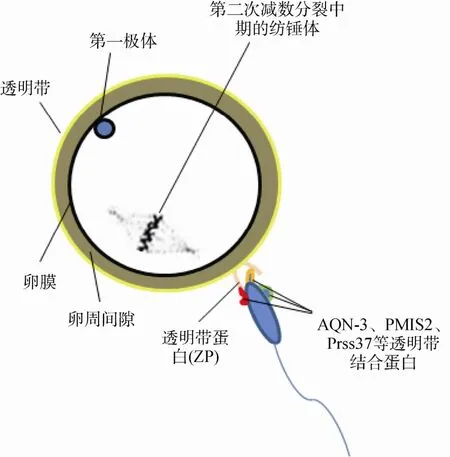
图4 精子穿入透明带的过程
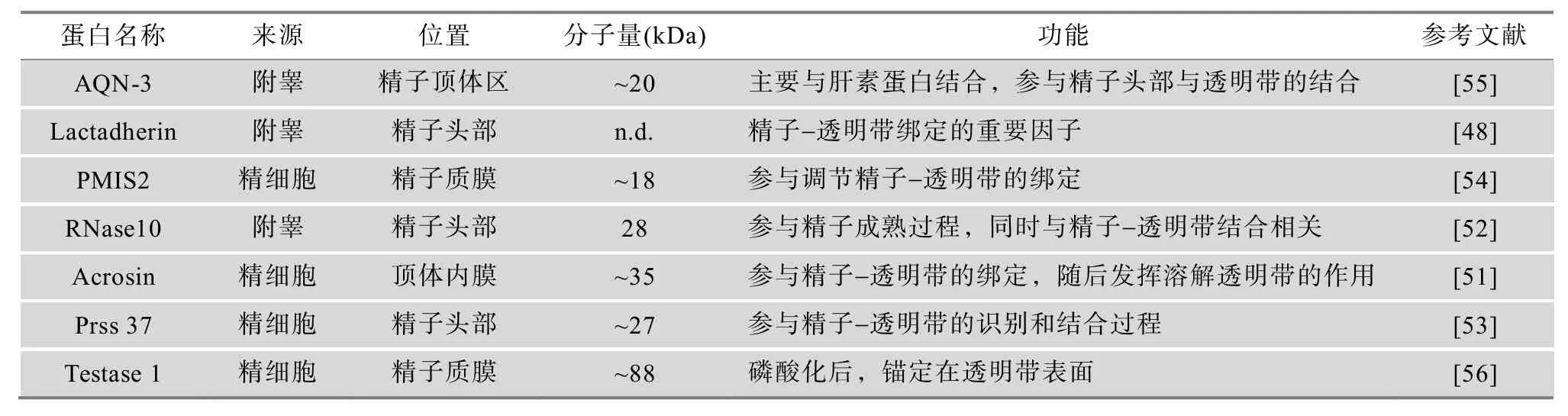
表3 精子穿入透明带相关的蛋白质
近年来相关的研究结果还发现了与穿入透明带相关的 Acrosin[51]、RNase10[52]、Prss37[53]和 PMIS2[54]4种蛋白质。这 4种蛋白质在精子与透明带形成稳定的绑定或结合状态时发挥着必不可少的作用。敲除编码这 4种蛋白的任一基因后,小鼠精子均不能与卵子的透明带产生相互作用,也无法完成两者间的绑定与结合,从而导致受精失败。哺乳动物附睾合成的精子粘附素(Heparin-binding spermadhesins,AQN-3)[55]和 Lactadherin[48]同样能够协助精子与透明带发生结合,使精子可以牢固地绑定在卵子表面。精细胞内源的Testase1蛋白因子则能够发生磷酸化,并作为一种锚定蛋白来引导顶体复合酶溶解透明带,实现入卵通道的形成[56]。虽然这些功能蛋白质的具体作用方式不明确,但目前多数推断是与透明带表面受体相互结合,保证精子稳定地结合在卵子上,为溶解透明带提供一种稳态,也为精子顺利穿入卵子做好铺垫。
5 精子功能蛋白质与精卵融合过程的关系
精子穿过透明带后进入到卵周间隙。此时,精卵膜融合的成功是精子头部内的遗传物质输送入卵细胞内的保证,也是受精过程最重要的一步。虽然精卵融合的具体过程和分子机制目前尚不十分清楚,但是精子蛋白质在这个过程中发挥着不可替代的作用(表4)。其中热休克蛋白(Heat shock 70 kDa protein 2, HSPA2)与精子头部粘附因子(Sperm adhesion molecule 1, SPAM1)形成识别复合物,介导精卵间的识别[57];随后 Phospholipase C-zeta[58]和 Prosaposin[59]能够激活卵子,刺激精卵发生膜融合(图5),实现合子的形成。精卵膜融合的实质是精子膜和卵膜表面互补配对的特异性蛋白分子介导的过程,如精子膜表面的受精素家族(ADAMs)和卵膜上的整合素(Integrin)配对[60,61],精子头部赤道区的IZUMO1蛋白和卵膜上JUNO分子的结合[62~64],精子顶体后膜的 CRISP家族和卵膜上微丝的相互作用[65,66],IAM38[67]和Zonadhedin[48]分别与不同卵膜受体蛋白相互结合等。另外,卵膜上CD9分子、微丝的长度及密度对精卵膜识别和融合至关重要[68]。当CD9与特异性糖蛋白(Pregnancy-specific glycoprotein 17,PSG17)结合后,PSG17占据了配体融合结合位点,因而导致精卵不能发生融合[69]。
总之,只有精卵发生膜融合后,精子才得以穿入卵内并逐渐形成雄原核,此时卵子受到激活并完成第二次减数分裂。随着第二极体的排出,雌原核形成以及随后两性原核发生结合并形成受精卵。随后受精卵不断发育和成长,受精卵开始第一次卵裂并完成精卵受精过程。受精卵在此后也依次经历4-细胞期、8-细胞期、16-细胞期、桑葚胚期、囊胚期等过程,最终形成胚胎乃至动物个体。
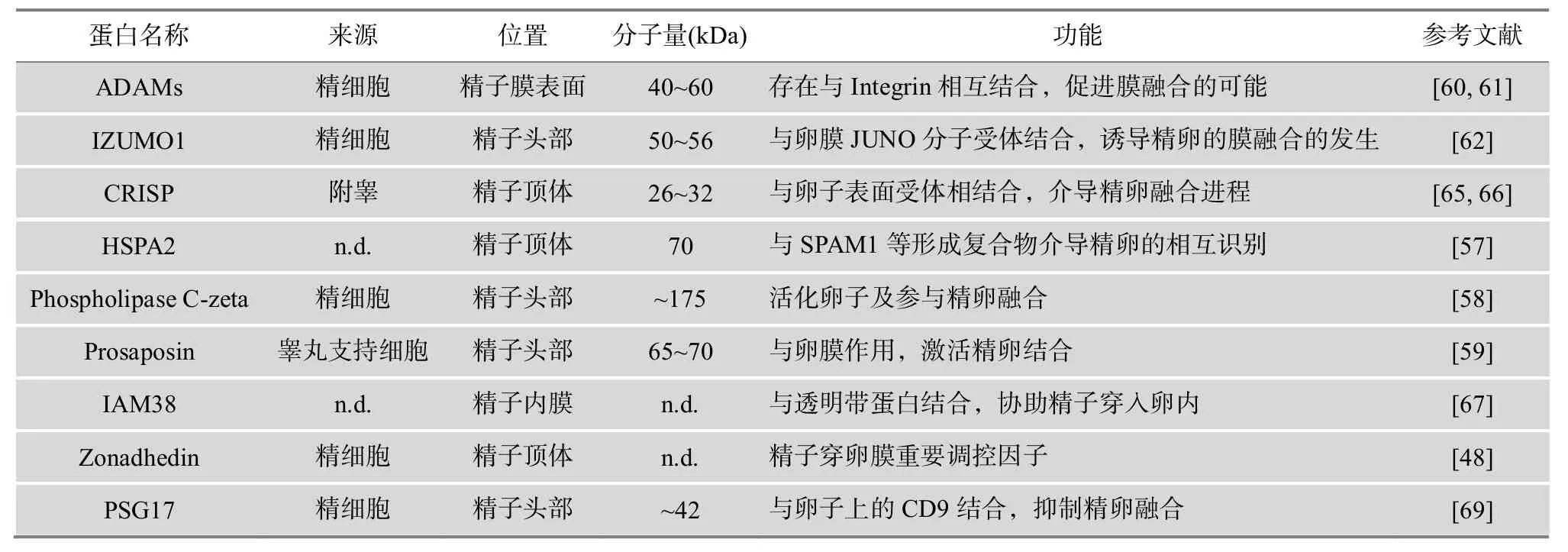
表4 精子识别并融合卵膜的相关蛋白质
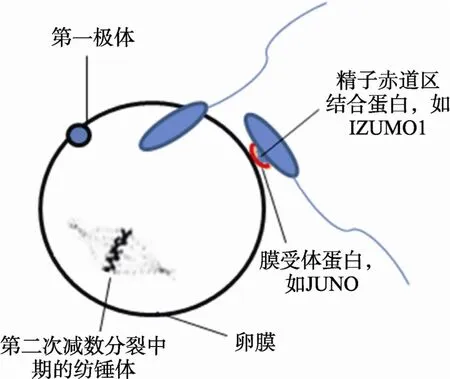
图5 精卵膜融合的过程
6 结 语
哺乳动物受精作用受到多种蛋白分子调控,目前大部分蛋白分子在精子运动活力、精子获能、顶体反应以及精卵融合等方面的详细作用机理仍不清楚。近年来,相关研究显示,精子功能相关的蛋白质能够作为受精能力的标记,评估或改善家畜的繁殖力。人工授精是应用在畜牧业生产上最成功和广泛的一项遗传育种技术,但是如何客观地评估和判断精子受精能力是人工授精技术的关键之一。目前,畜牧生产上常用的评估方法是检测精子的活力、密度以及形态等,然而这些传统的评估方法并不能准确判断出公畜个体间受精能力的差异[70,71]。个体间受精能力的差异是由于精液中受精相关的蛋白分子组成不同所导致[72],检测出这些与受精能力相关的分子标记或许是最为行之有效的评估方法。建立生物标记检测方法,人们可以有效地预测和评定公畜的繁殖力和受精性能,客观地区分高低繁殖力的个体以获得更好的生产效率。
虽然已证实一些蛋白分子(如 BSP、OPN、Acrosin、ADAMs和 CRISP1等)与精子功能以及公畜受精能力相关,但限于其实验的重复性和实际生产的应用性,这些受精生物标记还不足以客观地应用于评估个体的繁殖力。对精子发生、精子成熟、精子获能以及顶体反应等一系列受精过程需要进行更深入地研究和探讨,以寻找出合适于畜牧生产的生物标记分子,进而建立起更加简便、客观和高效的受精能力评定方法。
[1]Ashrafzadeh A, Karsani SA, Nathan S. Mammalian sperm fertility related proteins. Int J Med Sci, 2013, 10(12):1649–1657.
[2]Escoffier J, Jemel I, Tanemoto A, Taketomi Y, Payre C, Coatrieux C, Sato H, Yamamoto K, Masuda S, Pernet- Gallay K,Pierre V, Hara S, Murakami M, De Waard M, Lambeau G,Arnoult C. Group X phospholipase A2 is released during sperm acrosome reaction and controls fertility outcome in mice. J Clin Invest, 2010, 120(5): 1415–1428.
[3]Gupta SK, Bhandari B. Acrosome reaction: relevance of zona pellucida glycoproteins. Asian J Androl, 2011, 13(1): 97–105.
[4]Primakoff P, Myles DG. Cell-cell membrane fusion during mammalian fertilization. FEBS Lett, 2007, 581(11): 2174–2180.
[5]Vernon GG, Woolley DM. Basal sliding and the mechanics of oscillation in a mammalian sperm flagellum. Biophys J,2004, 87(6): 3934–3944.
[6]Signorelli J, Diaz ES, Morales P. Kinases, phosphatases and proteases during sperm capacitation. Cell Tissue Res, 2012,349(3): 765–782.
[7]Visconti PE. Understanding the molecular basis of sperm capacitation through kinase design. Proc Natl Acad Sci USA,2009, 106(3): 667–668.
[8]Demarco IA, Espinosa F, Edwards J, Sosnik J, De La Vega-Beltrán JL, Hockensmith JW, Kopf GS, Darszon A, Visconti PE. Involvement of a Na+/HCO-3cotransporter in mouse sperm capacitation. J Biol Chem, 2003, 278(9): 7001–7009.
[9]Ickowicz D, Finkelstein M, Breitbart H. Mechanism of sperm capacitation and the acrosome reaction: role of protein kinases. Asian J Androl, 2012, 14(6): 816–821.
[10]Lishko PV, Botchkina IL, Fedorenko A, Kirichok Y. Acid extrusion from human spermatozoa is mediated by flagellar voltage-gated proton channel. Cell, 2010, 140(3): 327–337.
[11]Marquez B, Suarez SS. Bovine sperm hyperactivation is promoted by alkaline-stimulated Ca2+influx. Biol Reprod,2007, 76(4): 660–665.
[12]Santi CM, Santos T, Hernández-Cruz A, Darszon A. Properties of a novel pH-dependent Ca2+permeation pathway present in male germ cells with possible roles in spermatogenesis and mature sperm function. J Gen Physiol, 1998,112(1): 33–53.
[13]Kirichok Y, Navarro B, Clapham DE. Whole-cell patchclamp measurements of spermatozoa reveal an alkaline- activated Ca2+channel. Nature, 2006, 439(7077): 737–740.
[14]Navarro B, Kirichok Y, Clapham DE. KSper, a pH-sensitive K+ current that controls sperm membrane potential. Proc Natl Acad Sci USA, 2007, 104(18): 7688–7692.
[15]Giroux-Widemann V, Jouannet P, Pignot-Paintrand I, Feneux D. Effects of pH on the reactivation of human spermatozoa demembranated with Triton X-100. Mol Reprod Dev, 1991,29(2): 157–162.
[16]Ho HC, Granish KA, Suarez SS. Hyperactivated motility of bull sperm is triggered at the axoneme by Ca2+and not cAMP.Dev Biol, 2002, 250(1): 208–217.
[17]Finkelstein M, Megnagi B, Ickowicz D, Breitbart H. Regulation of sperm motility by PIP2(4, 5) and actin polymerization.Dev Biol, 2013, 381(1): 62–72.
[18]Gremm D, Wegner A. Gelsolin as a calcium-regulated actin filament-capping protein. Eur J Biochem, 2000, 267(14):4339–4345.
[19]Janmey PA, Iida K, Yin HL, Stossel TP. Polyphosphoinositide micelles and polyphosphoinositide-containing vesicles dissociate endogenous gelsolin-actin complexes and promote actin assembly from the fast-growing end of actin filaments blocked by gelsolin. J Biol Chem, 1987, 262(25):12228–12236.
[20]Flesch FM, Wijnand E, van de Lest CHA, Colenbrander B,van Golde LMG, Gadella BM. Capacitation dependent activation of tyrosine phosphorylation generates two sperm head plasma membrane proteins with high primary binding affinity for the zona pellucida. Mol Reprod Dev, 2001, 60(1): 107–115.
[21]Visconti PE, Moore GD, Bailey JL, Leclerc P, Connors SA,Pan D, Olds-Clarke P, Kopf GS. Capacitation of mouse spermatozoa. II. Protein tyrosine phosphorylation and capacitation are regulated by a cAMP-dependent pathway. Development, 1995, 121(4): 1139–1150.
[22]Goltz JS, Gardner TK, Kanous KS, Lindemann CB. The interaction of pH and cyclic adenosine 3', 5'-monophosphate on activation of motility in Triton X-100 extracted bull sperm.Biol Reprod, 1988, 39(5): 1129–1136.
[23]Suarez SS. Control of hyperactivation in sperm. Hum Reprod Update, 2008, 14(6): 647–657.
[24]Qu F, Ying X, Guo W, Guo Q, Chen G, Liu Y, Ding Z. The role of Zn-α2 glycoprotein in sperm motility is mediated by changes in cyclic AMP. Reproduction, 2007, 134(4):569–576.
[25]Das S, Saha S, Majumder GC, Dungdung SR. Purification and characterization of a sperm motility inhibiting factor from caprine epididymal plasma. PLoS ONE, 2010, 5(8):e12039.
[26]Iwamoto T, Hiroaki H, Furuichi Y, Wada K, Satoh M, Satoh M, Osada T, Gagnon C. Cloning of boar SPMI gene which is expressed specifically in seminal vesicle and codes for a sperm motility inhibitor protein. FEBS Lett, 1995, 368(3):420–424.
[27]Jin J, Jin N, Zheng H, Ro S, Tafolla D, Sanders KM, Yan W.Catsper3 and Catsper4 are essential for sperm hyperactivated motility and male fertility in the mouse. Biol Reprod, 2007,77(1): 37–44.
[28]Al-Dossary AA, Strehler EE, Martin-Deleon PA. Expression and secretion of plasma membrane Ca2+-ATPase 4a(PMCA4a) during murine estrus: association with oviductal exosomes and uptake in sperm. PLoS ONE, 2013, 8(11):e80181.
[29]Hung PH, Suarez SS. Alterations to the bull sperm surface proteins that bind sperm to oviductal epithelium. Biol Reprod,2012, 87(4): 88.
[30]Srivastava N, Jerome A, Srivastava SK, Ghosh SK, Kumar A.Bovine seminal PDC-109 protein: An overview of biochemical and functional properties. Anim Reprod Sci, 2013,138(1-2): 1–13.
[31]Gwathmey TM, Ignotz GG, Suarez SS. PDC-109 (BSPA1/A2) promotes bull sperm binding to oviductal epithelium in vitro and may be involved in forming the oviductal sperm reservoir. Biol Reprod, 2003, 69(3): 809–815.
[32]Tarnasky H, Cheng M, Ou Y, Thundathil JC, Oko R, van der Hoorn FA. Gene trap mutation of murine outer dense fiber protein-2 gene can result in sperm tail abnormalities in mice with high percentage chimaerism. BMC Dev Biol, 2010, 10(1):67.
[33]O'Rand MG, Widgren EE, Hamil KG, Silva EJ, Richardson RT. Functional studies of eppin. Biochem Soc Trans, 2011,39(5): 1447–1449.
[34]Park SK, Yoon J, Wang L, Shibata TK, Motamedchaboki K,Shim KJ, Chang MS, Lee SH, Tamura N, Hatakeyama S,Nadano D, Sugihara K, Fukuda MN. Enhancement of mouse sperm motility by trophinin-binding peptide. Reprod Biol Endocrinol, 2012, 10(1): 101.
[35]Wang C, Wang L, Su B, Lu N, Song JJ, Yang XQ, Fu WW,Tan WW, Han B. Serine protease inhibitor Kazal type 1 promotes epithelial-mesenchymal transition through EGFR signaling pathway in prostate cancer. Prostate, 2014, 74(7):689–701.
[36]Lin MH, Lee RK, Hwu YM, Lu CH, Chu SL, Chen YJ,Chang WC, Li SH. SPINKL, a Kazal-type serine protease inhibitor-like protein purified from mouse seminal vesicle fluid,is able to inhibit sperm capacitation. Reproduction, 2008,136(5): 559–571.
[37]Dubé C, Leclerc P, Baba T, Reyes-Moreno C, Bailey JL. The proacrosin binding protein, sp32, is tyrosine phosphorylated during capacitation of pig sperm. J Androl, 2005, 26(4):519–528.
[38]Luna C, Colas C, Perez-Pe R, Cebrian-Perez JA, Muino-Blanco T. A novel epidermal growth factor-dependent extracellular signal-regulated MAP kinase cascade involved in sperm functionality in sheep. Biol Reprod, 2012, 87(4): 93.
[39]Novak S, Smith TA, Paradis F, Burwash L, Dyck MK, Foxcroft GR, Dixon WT. Biomarkers of in vivo fertility in sperm and seminal plasma of fertile stallions. Theriogenology, 2010,74(6): 956–967.
[40]Boccia L, Di Francesco S, Neglia G, De Blasi M, Longobardi V, Campanile G, Gasparrini B. Osteopontin improves sperm capacitation and in vitro fertilization efficiency in buffalo(Bubalus bubalis). Theriogenology, 2013, 80(3): 212–217.
[41]Rodeheffer C, Shur BD. Sperm from β1, 4-galactosyltransferase I-null mice exhibit precocious capacitation. Development, 2004, 131(3): 491–501.
[42]Jaldety Y, Glick Y, Orr-Urtreger A, Ickowicz D, Gerber D,Breitbart H. Sperm epidermal growth factor receptor (EGFR)mediates α7 acetylcholine receptor (AChR) activation to pro-mote fertilization. J Biol Chem, 2012, 287(26): 22328– 22340.
[43]Capkova J, Elzeinova F, Novak P. Increased expression of secretory actin-binding protein on human spermatozoa is associated with poor semen quality. Hum Reprod, 2007, 22(5):1396–1404.
[44]Nandi P, Ghosh S, Jana K, Sen PC. Elucidation of the involvement of p14, a sperm protein during maturation, capacitation and acrosome reaction of caprine spermatozoa. PLoS ONE, 2012, 7(1): e30552.
[45]Reid AT, Lord T, Stanger SJ, Roman SD, Mccluskey A, Robinson PJ, Aitken RJ, Nixon B. Dynamin regulates specific membrane fusion events necessary for acrosomal exocytosis in mouse spermatozoa. J Biol Chem, 2012, 287(45):37659–37672.
[46]Korfanty J, Toma A, Wojtas A, Rusin A, Vydra N, Widlak W.Identification of a new mouse sperm acrosome-associated protein. Reproduction, 2012, 143(6): 749–757.
[47]Nguyen EB, Westmuckett AD, Moore KL. SPACA7 is a novel male germ cell-specific protein localized to the sperm acrosome that is involved in fertilization in mice. Biol Reprod,2014, 90(1): 16.
[48]Shur BD. Reassessing the role of protein-carbohydrate complementarity during sperm-egg interactions in the mouse. Int J Dev Biol, 2008, 52(5/6): 703–715.
[49]Conner SJ, Lefièvre L, Hughes DC, Barratt CLR. Cracking the egg: increased complexity in the zona pellucida. Hum Reprod, 2005, 20(5): 1148–1152.
[50]Wassarman P M. Zona pellucida glycoproteins. Annu Rev Biochem, 2008, 283: 24285–24289.
[51]Ferrer M, Rodriguez H, Zara L, Yu Y, Xu W, Oko R. MMP2 and acrosin are major proteinases associated with the inner acrosomal membrane and may cooperate in sperm penetration of the zona pellucida during fertilization. Cell Tissue Res,2012, 349(3): 881–895.
[52]Krutskikh A, Poliandri A, Cabrera-Sharp V, Dacheux JL,Poutanen M, Huhtaniemi I. Epididymal protein Rnase10 is required for post-testicular sperm maturation and male ferti lity. FASEB J, 2012, 26(10): 4198–4209.
[53]Shen C, Kuang Y, Liu J, Feng J, Chen X, Wu W, Chi J, Tang L, Wang Y, Fei J, Wang Z. Prss37 is required for male ferti lity in the mouse. Biol Reprod, 2013, 88(5): 123.
[54]Yamaguchi R, Fujihara Y, Ikawa M, Okabe M. Mice expressing aberrant sperm-specific protein PMIS2 produce normal-looking but fertilization-incompetent spermatozoa.Mol Biol Cell, 2012, 23(14): 2671–2679.
[55]van Gestel RA, Brewis IA, Ashton PR, Brouwers JF, Gadella BM. Multiple proteins present in purified porcine sperm apical plasma membranes interact with the zona pellucida of the oocyte. Mol Hum Reprod, 2007, 13(7): 445–454.
[56]Zhu GZ, Myles DG, Primakoff P. Testase 1 (ADAM 24) a plasma membrane-anchored sperm protease implicated in sperm function during epididymal maturation or fertilization.J Cell Sci, 2001, 114(Pt 9): 1787–1794.
[57]Redgrove KA, Nixon B, Baker MA, Hetherington L, Baker G,Liu DY, Aitken RJ. The molecular chaperone HSPA2 plays a key role in regulating the expression of sperm surface receptors that mediate sperm-egg recognition. PLoS ONE, 2012,7(11): e50851.
[58]Theodoridou M, Nomikos M, Parthimos D, Gonzalez-Garcia JR, Elgmati K, Calver BL, Sideratou Z, Nounesis G, Swann K, Lai FA. Chimeras of sperm PLCζ reveal disparate protein domain functions in the generation of intracellular Ca2+oscillations in mammalian eggs at fertilization. Mol Hum Reprod,2013, 19(12): 852–864.
[59]Morales CR, Hay N, El-Alfy M, Zhao Q. Distribution of mouse sulfated glycoprotein-1 (prosaposin) in the testis and other tissues. J Androl, 1998, 19(2): 156–164.
[60]Kim T, Oh J, Woo JM, Choi E, Im SH, Yoo YJ, Kim DH,Nishimura H, Cho C. Expression and relationship of male reproductive ADAMs in mouse. Biol Reprod, 2006, 74(4):744–750.
[61]Nishimura H, Kim E, Nakanishi T, Baba T. Possible function of the ADAM1a/ADAM2 Fertilin complex in the appearance of ADAM3 on the sperm surface. J Biol Chem, 2004, 279(33):34957–34962.
[62]Bianchi E, Doe B, Goulding D, Wright GJ. Juno is the egg Izumo receptor and is essential for mammalian fertilization.Nature, 2014, 508(7497): 483–487.
[63]Inoue N, Ikawa M, Okabe M. The mechanism of sperm- egg interaction and the involvement of IZUMO1 in fusion. Asian J Androl, 2011, 13(1): 81–87.
[64]Satouh Y, Inoue N, Ikawa M, Okabe M. Visualization of the moment of mouse sperm-egg fusion and dynamic localization of IZUMO1. J Cell Sci, 2012, 125(Pt 21): 4985–4990.
[65]Cohen DJ, Maldera JA, Weigel MM, Ernesto JI, Vasen G,Cuasnicu PS. Cysteine-rich secretory proteins (CRISP) and their role in mammalian fertilization. Biol Res, 2011, 44(2):135–138.
[66]Cohen DJ, Maldera JA, Vasen G, Ernesto JI, Munoz MW,Battistone MA, Cuasnicu PS. Epididymal protein CRISP1 plays different roles during the fertilization process. J Androl,2011, 32(6): 672–678.
[67]Sutovsky P. Sperm-egg adhesion and fusion in mammals.Expert Rev Mol Med, 2009, 11: e11.
[68]Kaji K, Kudo A. The mechanism of sperm-oocyte fusion in mammals. Reproduction, 2004, 127(4): 423–429.
[69]Ellerman DA, Ha C, Primakoff P, Myles DG, Dveksler GS.Direct binding of the ligand PSG17 to CD9 requires a CD9 site essential for sperm-egg fusion. Mol Biol Cell, 2003,14(12): 5098–5103.
[70]Januskauska A, Johannisson A, Rodriguez-Martinez H. Assessment of sperm quality through fluorometry and sperm chromatin structure assay in relation to field fertility of frozen-thawed semen from Swedish AI bulls. Theriogenology,2001, 55(4): 947–961.
[71]Graham JK, Mocé E. Fertility evaluation of frozen/thawed semen. Theriogenology, 2005, 64(3): 492–504.
[72]Govindaraju A, Dogan S, Rodriguez-Osorio N, Grant K,Kaya A, Memili E. Delivering value from sperm proteomics for fertility. Cell Tissue Res, 2012, 349(3): 783–793.

