氦液滴中羰基硫分子的光解动力学
张翠梅 张志国 黄存顺 张 群 陈 旸
(1中国科学技术大学合肥微尺度物质科学国家实验室,化学物理系,合肥230026;2中国科学院大连化学物理研究所,分子反应动力学国家重点实验室,辽宁大连116023)
1 Introduction
Helium droplets have been recognized as ideal cryogenic matrix isolation due to their ultralow temperature and superfluidity.1One of the most remarkable properties is that the rotational temperature of molecules doped into droplets could be quickly and efficiently cooled to as low as 0.37 K.2,3Hence,the spectra,structures,and dynamics of various isolated systems(such as atoms,molecular radicals,and ions)in helium droplets have been extensively studied over decades.4,5
The photodissociation dynamics of doped helium droplets drew attention of people recently.Braun and Drabbels6−8studied the photodissociation of alkyl iodide molecules doped helium droplets with the ion imaging technique.Comparing with the gaseous phase results,the kinetic energy releases from the photodissociation of the doped helium droplets are much lower.A nonthermal“direct”mechanism that the photofragments push away surrounding helium atoms until they reach the droplets surface followed by escaping into the gaseous phase has been proposed.The classical Monte Carlo calculations based on a binary hard-sphere scattering model reproduced the experimental results very well.Besides the translational energy distribution,they observed some special phenomena such as fragment salvation dynamics and recombination process.These observations demonstrate that the finite-sized superfluid helium droplet system exhibits odd characteristic which affects the chemical reactions inside the droplets.Later,using the femtosecond pump-probe technique,Stapelfeldt et al.9studied the alignment effect of the methyl iodide doped helium droplets system.The results show that the rotational dynamics of methyl iodide dissolved inside the helium droplets is much slower than that of the isolated gaseoupus phase value.And the sharp transient alignment recurrence characteristic of gaseous phase molecules are absence in the doped droplets.All previous photochemistry studies on the doped helium droplets presage opportunities to explore both the chemical reaction dynamics inside the helium droplets and the properties of the finite-sized superfluid system.
As a benchmark system,carbonyl sulfide(OCS)has been vastly studied in the gas phase.10−18Although there have been a number of reports on OCS molecules in helium droplets,they mainly concentrated on the spectroscopic aspects.2,19−21For instance,the high resolution double resonance infrared-microwave19and the rotational spectra2of a single OCS molecule in helium droplets have been obtained.To date,the photodissociation dynamics of OCS doped helium droplets are still obscure.As a complementary to the gaseous phase photodissociation dynamics,here we report on the photodissociation dynamics of OCS in helium nanodroplets by means of velocity map ion imaging(VMI)in combination with(2+1)resonance enhanced multiphoton ionization(REMPI)detection.Firstly we report the CO REMPI from the OCS photodissociation.Then we obtain ion images of CO(v=0)and CO(v=1).The photodissociation dynamics of the OCS doped helim droplets have been discussed in light of the previous related works.
2 Experimental
The experiments were performed on a new constructed pulsed helium droplets-VMI apparatus.22Briefly,the apparatus consists of three differentially pumped vacuum chambers named source,doping,and detection chambers.The source chamber is equipped with a large turbo molecular pump with the pumping speed of 2200 L·s−1(STP-A2203C,Edwards,USA)to keep the pressure at 1×10−6Pa without the droplets beam and~2×10−4Pa with the beam.The doping chamber and detection chamber are equipped with nominal helium pumping speeds of 260 L·s−1(TC600,Pfeiffer vacuum,USA)and 1000 l·s−1(STP-1003C,Edwards,USA),respectively.The vacuums of doping and detection chambers are 2.2×10−6and 1×10−6Pa without the beam,respectively.The droplets source consists of an assembly holding a cryogenic Even-Lavie valve(EL-C-C-2009,Israel),which is thermally connected to the cold head of a closed-cycle refrigerator system(RDK-415E,Sumitomo Heavy Industries,Japan).The temperature of the nozzle can be adjusted with an accuracy of 0.5 K by heating the assembly by a 25 W cartridge heater which is powered by a temperature controller(TC202,East Changing,China).The droplets beam is formed by expanding high purity helium gas(4He,99.9999%)with a stagnation pressure of 3.0×106Pa from the pulsed valve whose temperatures were kept in the range of 16−24 K.The expanding gas accelerates and cools adiabatically.Then the helium clusters beam is formed and collimated by a conical skimmer(Beam Dynamics)with a diameter of 2 mm which is placed at a distance of 15 cm from the source.In such conditions,the mean droplet radius ranges from 1.7 nm(consisting of 500 helium atoms on average)to 5.1 nm(consisting of 12740 helium atoms on average).Passing through the skimmer,the droplets beam enters the doping chamber and picks up impurities via collision.The doping sample is introduced into the chamber through a leak valve(LVM940,VG Scienta,Sweden)which is continuously controllable between 10−1and 10−9Pa·s−1.The length of the doping chamber is 8 cm.By adjusting the leak rate the constant scattering gas pressure is obtained.The doping chamber pressure is optimized at 4.6×10−3Pa to maximize the pickup of a single OCS molecule.The doped droplets enter the detection chamber by passing through the other 2 mm skimmer(Beam Dynamics,USA).The photodissociation and ionization laser interact with the doped droplets beam perpendicularly at the center of the VMI spectrometer.The doped beam,laser,and VMI spectrometer are orthogonal in the detection chamber.
In terms of the photodissociation of OCS inside helium droplets,the excitation wavelengths(230.4−229.8 nm)were provided by a Nd:YAG(Continuum PL DLS 8000)pump tunable dye laser(Sirah CBST-G-18)with Coumarin 460 dye.The frequency-doubled laser beam(~230 nm,typical energy ~0.5−1.5 mJ·pulse−1)was focused with a len(f=250 mm)onto the helium droplet beam.The CO+fragment ions formed in the subsequent ionization were accelerated by a set of well adjusted ion optics assembly and detected by a Chevron-type dual MCP's coupled to a P-47 phosphor screen(Burle Electro-Optics).A negative high voltage pulse generator(PVM-4210,DEI;typical duration~200 ns)was used for mass selection.The VMI images from the phosphor screen were recorded by a digital camera(scA780-54gm)and further transferred to a computer.The total fluorescence from the phosphor screen was monitored by a photomultiplier tube(PMT).The timing of the pulse valve,the laser,and the gate pulse applied on the MCP's was controlled by a multichannel digital delay pulse generator(BNC Model 555).
3 Results and discussion
The(2+1)REMPI spectra(229.8−230.3 nm;Q branch of the B1Σ+←←X1Σ+transition17)of CO fragments dissociated from OCS molecules are shown in Fig.1.The solid line and dotted line show two different conditions:(1)effusive beam of OCS molecules in the gas phase;(2)a single OCS molecule embedded in helium droplets,respectively.For case(1),the spectral features related to CO(v=0)and CO(v=1)all exhibit high-J and bimodal distributions.17,23Previous studies confirmed that the bimodal distribution is mainly due to the different dissociation pathways on the 21A'(1Δ)potential energy surface,named direct dissociation and internal conversion to the ground state.12,24,25For case(2),however,rotational distributions for both CO(v=0)and CO(v=1)all go to low-J's while the high-J components disappear,as shown in the superimposed assignment in Fig.1.This indicates that in the helium droplets environment rotational cooling is much more efficient than vibrational cooling,as also observed in other molecular systems.

Fig.1 (2+1)REMPI spectra(Q branch of the B1Σ+←←X1Σ+transition)of CO from photodissociation of OCS embeded helium droplets at~230 nm
In order to obtain the insight into the dissociation dynamics,we chose the intense band-heads of CO(v=0,1)and recorded the images from the photodissociation of OCS doped helium droplets.The typical image recorded at 2.8 nm droplets size is shown in Fig.2(a).Due to the sensitive REMPI probe of CO molecule,the CO ions from the effusive OCS beam photodissociation and thermal background in the vacuum chamber were also accumulated by the imaging detection.However,benefited from the velocity mapping technique,26the background signal was separated very well spatially and could be subtracted accordingly.As shown in Fig.2(a)the region in the circle is the interested CO signal from the photodissociation of OCS doped helium droplets.The speed and angular distributions can be derived from the raw image with the BASEX method27and be corrected for the homogenous image background noise using the procedure described previously.28We accumulated several images with different droplets sizes.All angular distributions from resulting images are nearly isotropic.However,some intriguing speed distributions for CO(v=0)are observed,as shown in Fig.2(b).Obviously,the speed distributions(peaking around 240 m·s−1)for the photodissociation of doped helium droplets are much lower than those from the isolated gaseous OCS molecule dissociation(peaking at 1350 m·s−1).Previous studies on alkyl iodides doped helium droplets dissociation6−8imply that the“direct”mechanism could dominate the process.
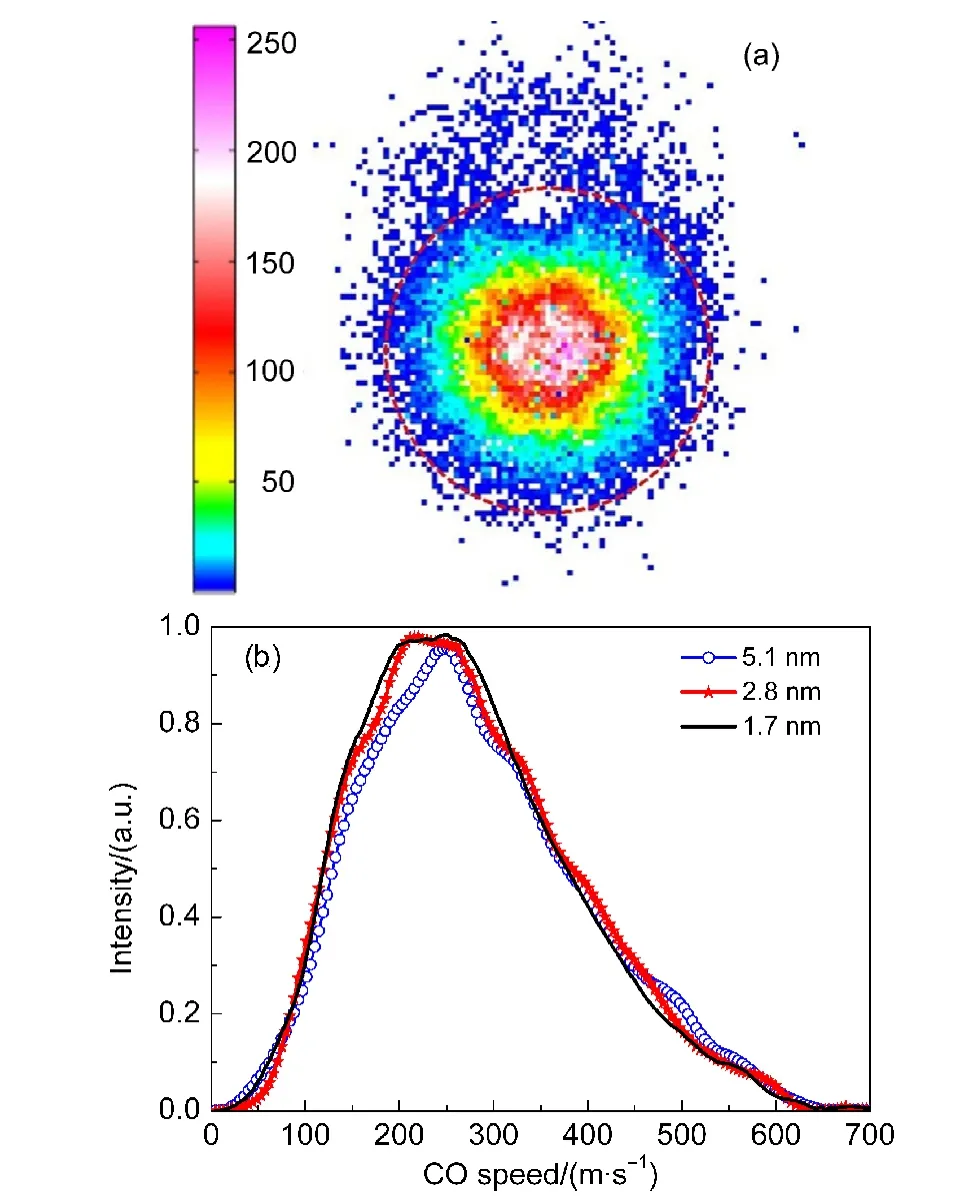
Fig.2 (a)Typical velocity map image of CO(v=0)resulting from the photodissociation of OCS embeded helium droplets at an average size of 2.8 nm;(b)CO(v=0)speed distributions for the selected different sizes of helium droplets
The photofragments may push away helium atoms,travel through the confined superfluid system,and depart into the gas phase.Due to the high speed of the photofragements,which is largely exceed the Landau critical velocity(58 m·s−1)in He II,the escaping process should overcome the friction and lose the kinetic energy to superfluid environment.The results show that the relaxation of the speed and angular distributions is strongly mass dependent.Briefly,the lighter photofragments lose more kinetic energy and have the larger reduction of the anisotropy.More than 95%of the kinetic energies are relaxed for CH3and C2H5products.But the value shifts to 70%for escaping iodine atoms.For the CO products probed in the present studies,de-tailed survey shows that average~97%of the kinetic energies transfer to the helium environment,which agrees very well with the previous studies.The average kinetic energies for the dissociation of the different sizes of the doped helium droplets are listed in Table 1.The dependence of the average kinetic energies with helium droplet sizes shows somehow contrary of the previous observations.Previous direct mechanism implies that the average kinetic energy distributions shift to lower with increasing the droplet sizes.However,the kinetic energy distributions derived from ground state of CO images show that the average kinetic energies are increased with the increase of the droplets sizes.We proposed a mechanism for the intriguing findings as follows.The OCS parent molecules embedded in helium droplets are photodissociated into CO and S fragments.The CO fragments lose kinetic as well as rotational energies by colliding with the surrounding helium atoms,and subsequently the rotationally cooled CO fragments are ionized by REMPI laser inside the helium droplets.The charged CO+fragment in droplets will transfer energy to the helium atoms to form a local high-pressure gas bubble,29and the bubble will inevitably expand to the surface of droplet.The external electric field will accelerate such an expansion.30When the bubble moves to the surface of droplet,CO+escapes from the droplet to vacuum as a bare ion.According to the law of momentum/energy conservation,the CO+fragment will acquire higher speed distribution with increasing the droplet size(i.e.,decreasing the mass ratio of CO over helium droplet),as the available energy is kept constant.Besides the electron bubble,the dimple generated on droplets surface may contribute to the trend of the average kinetic energy distributions.As described previously,the alkali atoms and clusters interact weakly with the helium and reside in a dimple on the helium droplets surface.31−34Considering the photodissociation of OCS doped helium droplets,the CO fragments move to the droplet surface and stay in the dimple within several picoseconds.7This exit time of photofragment is much shorter than the laser pulse width(8 ns).Therefore considerable amount of the CO fragments reside in the dimples and are ionized on the surface which produces the two-body alike dissociation phenomenon.
We also extended studies to the vibrationally excited product probe.The images of CO(v=1)were recorded with droplet sizes of 1.7,2.8,and 5.1 nm,respectively.Again,all these images show nearly isotropic angular distributions.The speed distributions of CO(v=1)fragments are shown in Fig.3.Obviously,the average kinetic energies for products(v=1)are larger thanthose for fragments(v=0),as show in Fig.3 and Table 1.The results are contrary to the gaseous phase observations.Considering the same available energy,products(v=1)take more energy for internal excitation.The less kinetic energy releases are expected for the vibrationally excited products.We refer to assign this to the difference of the interaction of the finite-sized superfluid helium with the vibrational ground state and vibrationally excited state products.As discussed above,the“direct”nonthermal process dominates the generation process of vibrational ground state products.However,the vibrationally excited products may be governed by the ion ejection model.More related to this issue,we recall the IR spectra of cations embedded in superfluid helium droplets.Drabbels et al.35,36have developed a spectroscopic method to study the IR spectra of the cold cations inside the helium droplets.The experimental evidence indicates that after excitation the embedded ions are ejected from the droplets which are governed by a nonevaporative process.The kinetic energy of the departing ions is molecular specific and independence of the excitation energy.Their studies on 1,1-diphenylethylene(DPE)cations show that the different vibrational excitations of the embedded cations have the identical speed distributions.Qualitatively,the present CO(v=1)results agree with the previous DPE studies.Both results provide a new insight of cation ejection from embedded helium droplets,the understanding of which invokes further ongoing experiments and theoretical calculations.

Table 1 Average translational energies of CO products from photodissociation of OCS doped helium droplets with various mean droplet sizes
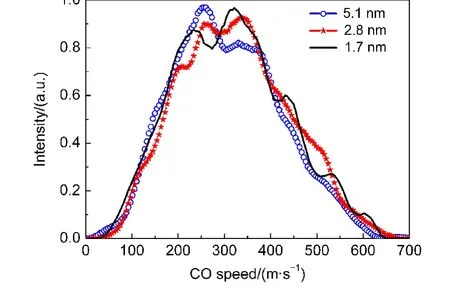
Fig.3 CO(v=1)speed distributions for the photodissociation of OCS doped helium droplets at selected average sizes
4 Conclusions
To summarize,we have studied photodissociation dynamics of OCS molecules embedded in helium droplets using REMPI combined with velocity map imaging techniques.Rotational cooling is found to be much more efficient than vibrational cooling in helium droplets.Nearly isotropic angular distributions were observed in the velocity map images for both CO(v=0)and CO(v=1).The slightly modified“direct”mechanism is proposed for the CO(v=0)fragments departing from the droplets.For the case of CO(v=1),the non-evaporative ion ejection process is confirmed.
(1) Toennies,J.P.;Vilesov,A.F.Angew.Chem.Int.Edit.2004,43,2622.
(2) Grebenev,S.;Hartmann,M.;Havenith,M.;Sartakov,B.;Toennies,J.P.;Vilesov,A.F.J.Chem.Phys.2000,112,4485.doi:10.1063/1.481011
(3) Hartmann,M.;Pörtner,N.;Sartakov,B.;Toennies,J.P.;Vilesov,A.F.J.Chem.Phys.1999,110,5109.doi:10.1063/1.479111
(4)Choi,M.Y.;Douberly,G.E.;Falconer,T.M.;Lewis,W.K.;Lindsay,C.M.;Merritt,J.M.;Stiles,P.L.;Miller,R.E.Int.Rev.Phys.Chem.2006,25,15.doi:10.1080/01442350600625092
(5) Toennies,J.P.;Vilesov,A.F.Annu.Rev.Phys.Chem.1998,49,1.doi:10.1146/annurev.physchem.49.1.1
(6) Braun,A.;Drabbels,M.J.Chem.Phys.2007,127,114305.doi:10.1063/1.2767263
(7) Braun,A.;Drabbels,M.J.Chem.Phys.2007,127,114303.doi:10.1063/1.2767261
(8) Braun,A.;Drabbels,M.J.Chem.Phys.2007,127,114304.doi:10.1063/1.2767262
(9) Pentlehner,D.;Nielsen,J.H.;Slenczka,A.;Mølmer,K.;Stapelfeldt,H.Phys.Rev.Lett.2013,110,093002.doi:10.1103/PhysRevLett.110.093002
(10) Sato,Y.;Matsumi,Y.;Kawasaki,M.;Tsukiyama,K.;Bersohn,R.J.Phys.Chem.1995,99,16307.doi:10.1021/j100044a017
(11) Brouard,M.;Quadrini,F.;Vallance,C.J.Chem.Phys.2007,127,084305.doi:10.1063/1.2757619
(12) Suzuki,T.;Katayanagi,H.;Nanbu,S.;Aoyagi,M.J.Chem.Phys.1998,109,5778.doi:10.1063/1.477200
(13) Sugita,A.;Mashino,M.;Kawasaki,M.;Matsumi,Y.;Bersohn,R.;Trott-Kriegeskorte,G.;Gericke,K.H.J.Chem.Phys.2000,112,7095.doi:10.1063/1.481324
(14) Kim,M.H.;Li,W.;Lee,S.K.;Suits,A.G.Can.J.Chem.2004,82,880.doi:10.1139/v04-072
(15) Rakitzis,T.P.Science 2004,303,1852.doi:10.1126/science.1094186
(16) Brouard,M.;Green,A.V.;Quadrini,F.;Vallance,C.J.Chem.Phys.2007,127,084304.doi:10.1063/1.2757618
(17) Rijs,A.M.;Backus,E.H.G.;de Lange,C.A.;Janssen,M.H.M.;Westwood,N.P.C.;Wang,K.;McKoy,V.J.Chem.Phys.2002,116,2776.doi:10.1063/1.1434993
(18) Katayanagi,H.;Suzuki,T.Chem.Phys.Lett.2002,360,104.doi:10.1016/S0009-2614(02)00788-1
(19) Grebenev,S.;Havenith,M.;Madeja,F.;Toennies,J.P.;Vilesov,A.F.J.Chem.Phys.2000,113,9060.doi:10.1063/1.1286243
(20) Kunze,M.;Markwick,P.R.L.;Pörtner,N.;Reuss,J.;Havenith,M.J.Chem.Phys.2002,116,7473.doi:10.1063/1.1467330
(21) Grebenev,S.Science 1998,279,2083.doi:10.1126/science.279.5359.2083
(22) Zhang,C.;Zhang,Z.;Huang,C.;Zhang,Q.;Chen,Y.Chin.J.Chem.Phys.2013,26,270.doi:10.1063/1674-0068/26103/270-276
(23) van den Brom,A.J.;Rakitzis,T.P.;van Heyst,J.;Kitsopoulos,T.N.;Jezowski,S.R.;Janssen,M.H.M.J.Chem.Phys.2002,117,4255.doi:10.1063/1.1496464
(24) Mo,Y.X.;Katayanagi,H.;Heaven,M.C.;Suzuki,T.Phys.Rev.Lett.1996,77,830.doi:10.1103/PhysRevLett.77.830
(25) Katayanagi,H.;Mo,Y.X.;Suzuki,T.Chem.Phys.Lett.1995,247,571.doi:10.1016/S0009-2614(95)01253-2
(26) Eppink,A.T.J.B.;Parker,D.H.Rev.Sci.Instrum.1997,68,3477.doi:10.1063/1.1148310
(27) Dribinski,V.;Ossadtchi,A.;Mandelshtam,V.A.;Reisler,H.Rev.Sci.Instrum.2002,73,2634.doi:10.1063/1.1482156
(28) Braun,A.;Drabbels,M.Rev.Sci.Instrum.2005,76,113103.doi:10.1063/1.2130941
(29) Lewis,W.K.;Applegate,B.E.;Sztáray,J.;Sztáray,B.;Baer,T.;Bemish,R.J.;Miller,R.E.J.Am.Chem.Soc.2004,126,11283.doi:10.1021/ja030653q
(30) Fárnšk,M.;Henne,U.;Samelin,B.;Toennies,J.P.Phys.Rev.Lett.1998,81,3892.doi:10.1103/PhysRevLett.81.3892
(31) Loginov,E.;Callegari,C.;Ancilotto,F.;Drabbels,M.J.Phys.Chem.A 2011,115,6779.doi:10.1021/jp111146n
(32) Stienkemeier,F.;Higgins,J.;Ernst,W.;Scoles,G.Phys.Rev.Lett.1995,74,3592.doi:10.1103/PhysRevLett.74.3592
(33) Ancilotto,F.;Lerner,P.B.;Cole,M.W.J.Low Temp.Phys.1995,101,1123.doi:10.1007/BF00754527
(34) Stienkemeier,F.;Higgins,J.;Ernst,W.E.;Scoles,G.Z Physica B 1995,98,413.doi:10.1007/BF01338416
(35) Smolarek,S.;Brauer,N.B.;Buma,W.J.;Drabbels,M.J.Am.Chem.Soc.2010,132,14086.doi:10.1021/ja1034655
(36) Zhang,X.;Brauer,N.B.;Berden,G.;Rijs,A.M.;Drabbels,M.J.Chem.Phys.2012,136,044305.doi:10.1063/1.3678011

