聚苯胺-石墨烯-Co3O4纳米复合材料的制备及其表征
刘攀博,黄英
(西北工业大学理学院应用化学系空间应用物理与化学教育部重点实验室,陕西西安 710129)
聚苯胺-石墨烯-Co3O4纳米复合材料的制备及其表征
刘攀博,黄英
(西北工业大学理学院应用化学系空间应用物理与化学教育部重点实验室,陕西西安 710129)
首次以三步法制备了聚苯胺-石墨烯-Co3O4(PANI-RGO-Co3O4)纳米复合材料。利用FT-IR,XRD,XPS和TEM对所制备的纳米复合材料进行表征,结果表明:PANI-RGO-Co3O4纳米复合材料中氧化石墨(GO)的含氧官能团数量大幅降低,GO已被还原成石墨烯(RGO);PANI和RGO之间具有较强的相互作用,且形成的Co3O4纳米粒子分布在PANI-RGO表面,其粒径在5~15nm之间,该纳米复合材料有望在超级电容器材料、电极材料和吸波材料等领域有广泛的应用前景。
聚苯胺;石墨烯;纳米粒子;复合材料
前言
石墨烯是一种具有二维蜂窝纳米结构、由单一碳原子紧密排列组成的的新型碳材料[1],它具有较大的比表面积、良好的电导率、机械稳定性和热稳定性,因此在电子设备、电容器、复合物增强等方面都有广泛的应用[2~4]。石墨烯的制备方法主要有机械剥离法、外延生长法、化学气相沉积法和化学还原法等[5~9]。其中,化学还原法简单易行、成本低、可大量制备,因此被认为是一种制备石墨烯的有效方法。化学还原法制备的石墨烯(RGO)与其它物质(如导电聚合物或纳米粒子)掺杂之后可赋予其特殊的用途。如RGO与导电聚合物掺杂之后所制备的复合材料(石墨烯-聚苯胺(RGO-PANI)、石墨烯-聚吡咯(RGO-PPy)或石墨烯-聚3,4-乙烯二氧噻吩(RGO-PEDOT))可用来制备超级电容器材料[10~13]或吸波材料[14];与Co3O4纳米粒子掺杂之后所制备的RGO-Co3O4复合材料可用来制备电极材料[15,16]或电容器材料[17];与Fe3O4纳米粒子掺杂之后所制备的RGO-Fe3O4复合材料可用来制备吸波材料[18,19]等。但这些复合材料的制备仅限于二元复合(RGO-导电聚合物或RGO-纳米粒子),而对于三元复合材料的研究报道较少(如RGO-导电聚合物-纳米粒子)。
本文首次以三步法制备了聚苯胺-石墨烯-Co3O4(PANI-RGO-Co3O4)纳米复合材料。利用FT-IR,XRD和XPS研究了RGO中含氧官能团的变化及PANI和RGO之间的相互作用,并利用TEM对所制备的PANI-RGO-Co3O4纳米复合材料中的纳米粒子进行了研究。
1 实验部分
1.1 制备
氧化石墨(GO)的制备:以天然鳞片石墨作为前驱体,采用Hummers法合成GO[20]。
PANI-RGO-Co3O4纳米复合材料的制备:第一步,将0.2mL苯胺单体和2mL浓硫酸溶液加入100 mL的GO溶液(1mg/mL)中超声2h,然后加入0.95 g(NH4)2S2O8,冰浴中搅拌24h后用去离子水洗涤数次并配成100mL溶液;第二步,将1.4g CoCl2·6H2O加入上述溶液,搅拌2h后倒入聚四氟乙烯内衬的高压釜中,然后加入0.94g NaOH和4mL H2O2(30 wt%)置于160℃烘箱中反应24h,室温冷却后将所得的产物用去离子水洗涤数次并配成100mL溶液;第三步,将0.1mL水合肼溶液(80 wt%)加入上述溶液,在95℃中反应24h后用乙醇和去离子水洗涤数次,然后将所得产物在真空干燥箱中60℃放置24h。
PANI-RGO的制备:依照上述第一步和第三步过程,可制备PANI-RGO。
PANI的制备:将0.2mL苯胺单体和2mL浓硫酸溶液混合超声2h,加入0.95g(NH4)2S2O8,冰浴中搅拌24h后用去离子水洗涤数次,然后将所得产物在真空干燥箱中60℃放置24h。
RGO的制备:将0.1mL水合肼溶液(80wt%)加入100mL的GO溶液(1mg/mL)中,在95℃中反应24h后用乙醇和去离子水洗涤数次,然后将所得产物在真空干燥箱中60℃放置24h。
1.2 结构表征
采用美国necolet公司型号为iS10的傅里叶变换红外光谱仪对样品的化学结构进行分析。采用日本SHI-MADZU公司型号为XRD-7000X的X射线衍射仪测试样品的结构。测试条件:采用Cu靶Kα辐射,入射波长λ=0.154060nm,电压40.0kV,电流40.0mA,扫描速度0.5°/min,扫描步长0.002°。采用型号为Phoibos 100的X射线光电子能谱仪对样品进行元素分析。采用Tecnai F30 G2场发射透射电镜对样品的形貌进行分析,加速电压200kV。
2 结果与讨论
2.1 FT-IR分析
由图1可知,GO在1730cm-1处有一个明显的吸收峰,对应于C=O的伸缩振动,而在1224cm-1和1065cm-1处的强吸收峰则归属于C-O的伸缩振动,说明GO中存在大量的含氧官能团[21,22]。还原之后,所得的RGO在1730cm-1处的吸收峰明显的降低,同时,1224cm-1和1065cm-1处的吸收峰在一定程度上也有所降低,说明还原之后GO中的含氧官能团明显降低[23]。RGO在1635cm-1处出现的较强的吸收峰则归属于C=C的伸缩振动,说明还原之后所得的RGO的石墨化程度有所提高[24]。PANI-RGO-Co3O4在1585cm-1,1161cm-1和1495cm-1处出现较强的吸收峰,分别对应于PANI中醌环和苯环的C=C伸缩振动[10,25],而在1297cm-1和1238cm-1处的吸收峰则主要归属于PANI中C-N和C=N的伸缩振动[13],这说明PANI已成功的覆盖在RGO上且与RGO之间具有较强的相互作用。同时,PANI-RGO-Co3O4在597cm-1和660cm-1处也出现两个明显的吸收峰,归属于Co-O的伸缩振动,说明所制备的复合材料中存在Co3O4纳米粒子[26]。

图1 GO,RGO和PANI-RGO-Co3O4的FT-IR谱图Fig.1FT-IR spectra of GO,RGO and PANI-RGO-Co3O4
2.2 XRD分析

图2 RGO,PANI和PANI-RGO-Co3O4的XRD谱图Fig.2XRD patterns of RGO,PANI and PANI-RGO-Co3O4
由图2可知,RGO在2θ=24.5°出现一个较为明显的衍射峰,根据布拉格公式2dsinθ=nλ(n=1)(式中d为晶面距离,θ为衍射角,n为衍射级数, λ为X射线波长),其晶面距离为0.36nm,与石墨的晶面距离(0.34nm)十分相近,说明RGO已被基本还原且具有石墨的结构。PANI-RGO在15.3°,20.7°和25.2°处出现明显的衍射峰,分别对应于PANI中(011),(020)和(200)的晶面,说明PANI已成功的制备[27]。而PANI-RGO-Co3O4在19.2°,31.7°, 37.0°,38.3°,45.1°,56.1°,59.6°和65.6°处出现八个较为明显的衍射峰,分别对应于Co3O4中(111),(220),(311),(222),(400),(422),(511)和(440)的晶面,说明所制备的复合材料中含有Co3O4纳米粒子,同时,这些衍射峰的强度都相对较弱,说明形成的Co3O4纳米粒子的尺寸较小。
2.3 XPS分析

图3 GO和PANI-RGO-Co3O4的C 1s谱图(a)和XPS谱图(b);PANI-RGO-Co3O4的Co 2p谱图(c)Fig.3C 1s spectra(a)and XPS spectra(b)of GO and PANI-RGOCo3O4,Co 2p spectra(c)of PANI-RGO-Co3O4
由图3a可知,GO的C1s谱图上有四种类型的碳键,C=C/C-C键的结合能在284.5eV,C-O键的结合能在286.4eV,C=O键的结合能在287.8eV,O=C-OH键的结合能在289.0eV[28],且这四种碳键的结合能所对应的峰的强度均相对较强,说明GO中含氧官能团的含量较多。PANI-RGO-Co3O4中含氧官能团的结合能对应的峰的强度有所降低,尤其是C-O键的结合能所对应的峰的强度降低很大,说明还原之后GO中含氧官能团的数量急剧下降,GO已大部分被还原成RGO[8]。而PANI-RGO-Co3O4在285.6eV出现的峰则对应于PANI中C-N键的结合能。由图3b可知,GO中仅含有C和O元素,而PANI-RGO-Co3O4中除了C和O元素之外,还存在N和Co元素,且其C和O元素的比值有所提高,说明GO被还原。由图3c可知,PANI-RGO-Co3O4在780.2 eV和795.6eV出现的峰分别对应于Co 2p3/2和Co 2p1/2的结合能,说明所形成的粒子为Co3O4纳米粒子[29]。由以上分析可以,Co3O4成功地附着在PANI-RGO,这与XRD所得的结果一致。
2.4 TEM分析
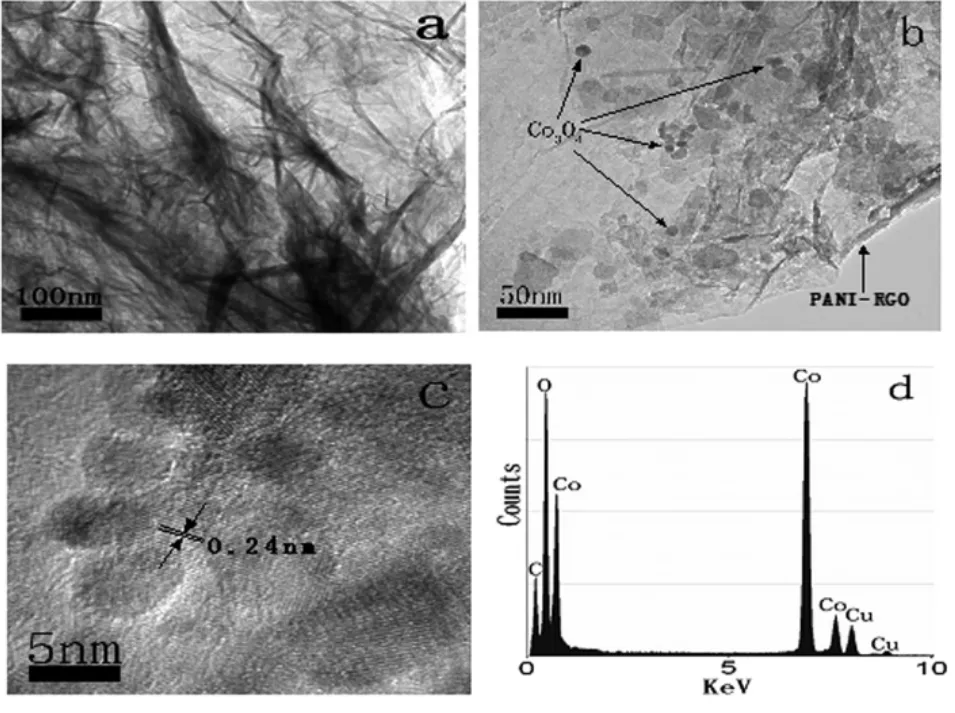
图4 PANI-RGO(a)的TEM图;PANI-RGO-Co3O4的TEM图(b),HRTEM图(c)和能谱图(d)Fig.4TEM image of PANI-RGO(a),TEM(b),HRTEM(c)and EDS image(d)of PANI-RGO-Co3O4
由图4a可知,PANI-RGO具有纸张状形态,且其表面上具有较多的褶皱,这主要是因为PANI均匀地分布在RGO的表面上[30],且与RGO之间具有较强的相互作用。由图4b可知,许多Co3O4纳米粒子分布在PANI-RGO表面,其粒径在5~15nm之间,粒径较小,这与XRD所得的结果一致。由HRTEM可知(图4c),Co3O4纳米粒子的晶格距离为0.24nm,与Co3O4中(311)晶面对应的晶格距离相同。由图4d可知所制备的复合材料中含有C,O和Co元素(其中Cu元素来源于铜网),这与XPS所得的结果一致。
3 结论
(1)首次以三步法制备了PANI-RGO-Co3O4纳米复合材料。
(2)PANI-RGO-Co3O4纳米复合材料中GO的含氧官能团数量急剧下降,合成的PANI附着在RGO表面且与RGO之间由较强的相互作用。
(3)生成的Co3O4纳米粒子的粒径在5~15nm之间。通过结构测试结果,我们推测该纳米复合材料有望在超级电容器、电极和吸波材料等领域有广泛的应用前景。
[1]NOVOSELOV K S,GEIM A K,MOROZOV S V,et al.Electric Field Effect in Atomically Thin Carbon Films[J].Science,2004, 306(22):666~669.
[2]BALANDIN A A,GHOSH S,BAO W,et al.Superior Thermal Conductivity of Single-Layer Graphene[J].Nano Lett,2008,8 (3):902~907.
[3]RAO C N R,SOOD A K,SUBRAHMANYAM K S,et al.Graphene:The New Two-Dimensional Nanomaterial[J].Angew.Chem.Int.Ed,2009,48(42):7752~7778.
[4]PARK S,SUK J W,AN J,et al.The effect of concentration of graphene nanoplatelets on mechanical and electrical properties of reducedgrapheneoxidepapers[J].Carbon,2012,50(12):4573~4578.
[5]BERGER C,SONG Z M,LI X B,et al.Electronic Confinement and Coherence in Patterned Epitaxial Graphene[J].Science,2006,312 (5777):1191~1196.
[6]KIM K S,ZHAO Y,JANG H,et al.Large-scale pattern growth of graphene films for stretchable transparent electrodes[J].Nature,2009,457:706~710.
[7]ZHANGY,GOMEZL,ISHIKAWAFN,etal.ComparisonofGraphene Growth on Single-Crystalline and Polycrystalline Ni by Chemica l VaporDeposition[J].J.Phys.Chem.Lett,2010,1:3101~3107.
[8]STANKOVICH S,DIKIN D A,PINER R D,et al.Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide[J].Carbon,2007,45(7):1558~1565.
[9]ZHANG S,SHAO Y Y,LIAO H G,et al.Polyelectrolyte-Induced Reduction of Exfoliated Graphite Oxide:A Facile Route to Synthesis of Soluble Graphene Nanosheets[J].ACS Nano,2011, 5(3):1785~1791.
[10]ZHANG K,ZHANG L L,ZHAO X S,et al.Graphene/Polyaniline Nanofiber Composites as Supercapacitor Electrodes[J].Chem.Mater,2010,22(4):1392~1401.
[11]LIU A R,LI C,BAI H,et al.Electrochemical Deposition of Polypyrrole/Sulfonated Graphene Composite Films[J].J.Phys.Chem.C,2010,114(51):22783~22789.
[12]XU Y F,WANG Y,LIANG J J,et al.A Hybrid Material of Graphene and Poly(3,4-ethyldioxythiophene)with High Conductivity,Flexibility,and Transparency[J].Nano Res,2009,2 (4):343~348.
[13]ZHANG J T AND ZHAO X S.Conducting Polymers Directly Coated on Reduced Graphene Oxide Sheets as High-Performance Supercapacitor Electrodes[J].J.Phys.Chem.C,2012,116 (9):5420~5426.
[14]YU H L,WANG T S,WEN B,et al.Graphene/polyaniline nanorod arrays:synthesis and excellent electromagnetic absorption properties[J].J.Mater.Chem,2012,22:21679~21685.
[15]LI B J,CAO H Q,SHAO J,et al.Co3O4@graphene Composites as Anode Materials for High-PerformanceLithium Ion Batteries[J].Inorg.Chem,2011,50(5):1628~1632.
[16]WU Z S,RENW C,WEN L,et al.Graphene Anchored with Co3O4Nanoparticles as Anode of Lithium Ion Batteries with Enhanced Reversible Capacity and Cyclic Performance[J].ACS Nano, 2010,4(6):3187~3194.
[17]WANG X W,LIU S Q,WANG H Y,et al.Facile and green synthesis of Co3O4nanoplates/graphene nanosheets composite for supercapacitor[J].JSolidStateElectrochem,2012,16(11):3593~3602.
[18]XU H L,BI H,YANG R-B.Enhanced microwave absorption property of bowl-like Fe3O4hollow spheres/reduced graphene oxide composites[J].J.Appl.Phys,2012,111:07A522.
[19]HE H K AND GAO C.Preparation of reduced graphene oxide/ Fe3O4nanocomposite and its microwave electromagnetic properties[J].ACS Appl.Mater.Interfaces,2010,2(11):3201~3210.
[20]HUMMERS W S,OFFEMAN R E.Preparation of Graphitic Oxide[J].J.Am.Chem.Soc,1958,80(6):1339~1339.
[21]ZHANG J,YANG H.SHEN G,et al.Reduction of graphene oxide viaL-ascorbic acid[J].Chem.Commun,2010,46:1112~1114.
[22]PARK S,AN J,PINER R D,et al.Aqueous Suspension and Characterization of Chemically Modified Graphene Sheets[J].Chem.Mater,2008,20(21):6592~6594.
[23]SONG P,ZHANG X Y,SUN M X.Synthesis of graphene nanosheets via oxalic acid-induced chemical reduction of exfoliated graphite oxide[J].RSC Adv,2012,2:1168~1173.
[24]BOSE S,KUILA T,MISHRA A K,et al.Dual role of glycine as a chemical functionalizer and a reducing agent in the preparation of graphene:an environmentally friendly method[J].J.Mater.Chem,2012,22:9696~9703.
[25]PANDEY R K,LAKSHMINARAYANAN V.Electro-Oxidation of Formic Acid,Methanol,and Ethanol on Electrodeposited Pd-Polyaniline Nanofiber Films in Acidic and Alkaline Medium[J].J.Phys.Chem.C,2009,113(52):21596~21603.
[26]DONG Z,FU Y Y,HAN Q,et al.Synthesis and Physical Properties of Co3O4Nanowires[J].J.Phys.Chem.C,2007,111(50):18475~18478.
[27]YAN J,WEI T,SHAO B,et al.Preparation of a graphene nanosheet/polyaniline composite with high specific capacitance[J].Carbon,2010,48(2):487~493.
[28]STANKOVICH S,PINER R D,CHEN X,et al.Stable Aqueous Dispersions of Graphitic Nanoplatelets via the Reduction of Exfoliated Graphite Oxide in the Presence of Poly(Sodium 4-styrenesulfonate)[J].J.Mater.Chem,2006,16:155~158.
[29]WU Z S,REN W C,WENL,et al.Graphene Anchored with Co3O4Nanoparticles as Anode of Lithium Ion Batteries with Enhanced Reversible Capacity and Cyclic Performance[J].ACS Nano, 2010,4(6):3187~3194.
[30]LI Y,PENG H R,LI G C,et al.Synthesis and electrochemical performance of sandwich-like polyaniline/graphene composite nanosheets[J].Eur.Polym.J,2012,48(8):1406~1412.
Preparation and Characterization of Polyaniline-Reduced Graphene Oxide-Co3O4Nano-composites
LIU Pan-bo and HUANG Ying
(Key Laboratory of Space Applied Physics and Chemistry,Ministry of Education,College of Science,Northwestern Polytechnical University,Xi'an 710129,China)
The polyaniline-reduced graphene oxide-Co3O4(PANI-RGO-Co3O4)nanocomposites were synthesized through a three-step approach for the first time.The nanocomposites were characterized by FT-IR,XRD,XPS and TEM.The results indicated that the oxygen-containing functional groups of GO decreased to a great extent;the GO had been reduced into RGO.The PANI greatly interacted with RGO and the formed Co3O4nanoparticles,with a particle size of 5~15 nm,were closely anchored on the surface of PANI-RGO.The nanocomposites were expected to have wide applications in super capacitor,electrode and wave-absorbing materials.
Polyaniline;reduced graphene oxide;nanoparticles;composites
TQ322.95
A
1001-0017(2013)04-0017-04
2013-04-26
刘攀博(1986-),男,陕西西安人,博士,主要从事石墨烯的研究。

