原发性胰腺小细胞癌的荟萃分析
彭春艳 吕瑛 姚仁玲 徐肇敏 邹晓平
·论著·
原发性胰腺小细胞癌的荟萃分析
彭春艳 吕瑛 姚仁玲 徐肇敏 邹晓平
目的探讨原发性胰腺小细胞癌的临床病理特点、治疗及预后情况。方法联机检索中国期刊全文数据库、维普中文科技期刊数据库、Medline/Pubmed及OVID全文数据库,检索截止至2012年4月,并结合南京鼓楼医院诊治的1例患者,进行系统评价。结果纳入28篇文献46例诊断明确的病例,加上鼓楼医院1例,共47例胰腺小细胞癌患者。该病以男性多见,中位年龄62岁,临床表现以腹痛、黄疸及消瘦为主,较少产生副肿瘤综合征;多见血清神经元特异性烯醇化酶升高;CT检查示病灶多呈不均匀强化,确诊依靠病理检查;63.8%(30/47)的患者确诊时已发生转移,患者总的中位生存期为28周。目前无统一治疗模式,推荐以化疗为主的系统治疗。结论胰腺小细胞癌是一种发生率低、恶性程度高、侵袭性强、预后差的神经内分泌肿瘤。
神经内分泌瘤; 癌,小细胞; 胰腺; Meta分析
小细胞癌是一种恶性程度高、侵袭性强及预后极差的神经内分泌肿瘤,多见于肺脏,约占所有支气管起源肿瘤的20%~25%[1],肺外部位少见,仅占所有小细胞癌的2.5%~4%[2],可发生于膀胱、前列腺、子宫、食管、胆囊、胰腺及结肠等部位。原发性胰腺小细胞癌最早报道于1973年[3],其占所有胰腺恶性肿瘤的1%[4]。目前研究多以个案报道为主,故对其临床表现、生物学特征及治疗方案的系统了解非常有限。此外,临床实践显示此类患者早期确诊困难,就诊时多属中晚期,预后差。因此,如何正确认识和处理好该病,对于改善患者预后有重要意义。本研究回顾性分析国内外文献,并结合我院诊治的1例原发性胰腺小细胞癌资料,以期提高对该病的认识。
材料与方法
一、文献检索及入选、排除标准
联机检索中英文数据库,包括Medline/Pubmed(1960.1-2012.4)及OVID(1978.1-2012.4)全文数据库、中国期刊全文数据库(Chinese Journal Full-text Database, 1979.1-2012.4)及维普中文科技期刊数据库(VIP Database for Chinese Technical Periodicals, 1989.1-2012.4),手工检索所获文献的参考文献。关键词:neuroendocrine carcinoma pancreas,small cell cancer pancreas,胰腺小细胞癌,胰腺神经内分泌癌。
入选标准:(1)通过活检穿刺或手术病理确诊的病例;(2)患者资料至少包括年龄、性别、临床表现、实验室检查、影像学资料、病理表现、免疫组化、治疗方法及预后情况等项目中的5项;(3)重复报道病例仅纳入最近发表的一篇文章。排除标准:(1)临床资料不全,仅达上述纳入标准5项以下者;(2)重复病例;(3)除外其他部位转移的继发性胰腺小细胞癌。
二、统计学分析
结 果
一、纳入文献
共检索出1057篇文献,经仔细阅读标题和摘要,初步排除不符合研究目的的文献1020篇,获得可能符合纳入标准的英文文献27篇,中文文献10篇。仔细阅读全文,9篇文献因未能提供患者详细信息而被排除。最终纳入22篇英文文献[3-24]和6篇中文文献[25-30]。
二、一般资料及临床特征
28篇文献共报道46例胰腺小细胞癌患者,加上我院1例,共47例。其中男性26例,女性21例,男女比为1.2∶1,发病年龄23~75岁,中位年龄62岁。最常见临床表现为腹痛(15/47,31.9%)、黄疸(9/47,19.1%)、体重下降(8/47,17.0%)。个例表现为副肿瘤综合征,例如异位促肾上腺皮质激素增多症[3]和高钙血症[14]。
胰腺小细胞癌可发生于胰腺任何部位,本组胰头部26例(55.3%),胰尾部10例(21.3%),胰体部6例(12.8%),全胰弥漫性病变2例(4.1%),胰头体部1例(2.1%),头尾部1例(2.1%),体尾部1例(2.1%);肿瘤长径2~15 cm,平均(5.5±2.7)cm;单发45例(95.7%),多发2例。确诊时30例(63.8%)发生转移,常见转移部位为胰周淋巴结(18/47,38.3%)、肝脏(11/47,23.4%),少见的转移部位有肾上腺、骨髓、脾脏、邻近血管及皮肤等。
三、肿瘤标记物及激素分泌
18例检测血清CEA和CA19-9,各有2例轻度升高[16-17,19-20]。10例检测血清神经元特异性烯醇化酶(neuron-specific enolase,NSE),5例显著升高[11,16-17, 20]。5例测定血胰岛素、促胃液素及胰高血糖素水平,结果均在正常范围[9, 11, 17-18,20]。3例行血乳酸脱氢酶检查,结果均显著升高[8-9,11]。1例血促肾上腺皮质激素升高[3],1例高钙血症[14],1例血胃泌素释放肽前体升高,1例(术后检查)血泌乳素升高[28],1例检测血肠血管活性多肽及尿5-羟吲哚乙酸,结果正常[17]。
四、影像学特征
共35例有详细的影像学特征记录。20例患者行上腹部CT平扫+增强[7, 9-14, 18-21, 24,27-29],其中18例平扫时显示为低密度软组织占位,增强扫描示肿块边界清晰,11例[7, 11, 13, 18, 27-29]内部呈不均匀强化,7例呈低密度灶[9-10, 12, 16-17, 21, 24];2例胰腺弥漫性轻度强化[10,19]。8例行腹部B超,其中6例呈低回声占位[7, 16-18, 27-28],2例胰腺弥漫性增大,回声轻度增强[10,19]。5例行内镜超声(EUS),显示肿块轮廓清晰,内部呈低回声,边缘呈高回声[13,18]。4例行上腹部磁共振扫描,仅2例详细描述特征,病灶在T1加权呈低信号,T2加权呈高信号。2例行选择性血管造影,提示病灶血管丰富或肿瘤浓染[17-18]。2例行ERCP,显示肝内外胆管及胆总管扩张[12,17]。2例行正电子发射断层显像-计算机断层扫描(PET)检查,显示高代谢病灶[13,24]。1例采用生长抑素受体闪烁成像检测转移灶[20]。
五、组织病理学及免疫组化特征
26例通过手术、9例通过B超及CT或EUS引导下穿刺活检[4, 9, 19,13, 24]、1例通过腹腔镜下胰体和淋巴结活检、2例通过ERCP活检[11-12]、9例通过尸检[3-4, 10, 14]获得组织标本。光镜下见癌细胞体积较小,胞质稀少或呈裸核状,核呈圆形或短梭形,深染,核仁不明显,细胞界限不清,弥漫排列成片或呈巢团状,并可见大片坏死,部分可见菊形团样结构。44例为单一小细胞型(图1a),3例为小细胞癌-腺癌混合型[8,15]。
18例有详细的免疫组化结果[13,15-22,24,28-29],突触融素(synaptophysin, Syn)阳性率100%(8/8,图1b),嗜铬蛋白A (chromogranin A, CgA)阳性率90%(9/10),NSE阳性率77.8%(7/9),角蛋白阳性率100%(5/5),CA19-9阳性率10% (1/10),CEA阳性率20% (2/10),CD20阴性(0/10)。
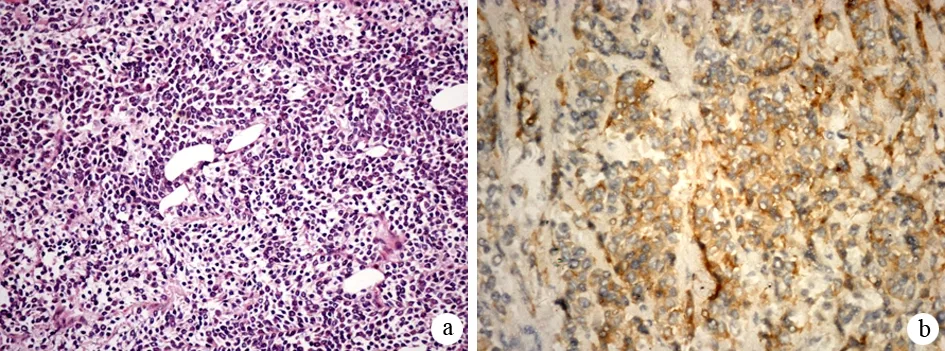
图1原发性胰腺小细胞癌的病理学改变(a HE×100)及Syn蛋白表达(b 免疫组化×100)
六、治疗方式及预后
单纯手术治疗6例,单纯化疗9例,手术联合化疗16例,手术联合放疗1例,放疗联合化疗3例,手术联合放化疗3例,对症处理9例。常用化疗方案为铂类+吉西他滨,其他如5-FU、表阿霉素、干扰素及生长抑素等也有应用。
43例患者记录了详细的生存时间。截止文献报道之日,有5例患者存活。患者生存2~692周,中位生存期28周(图2a),局限期和进展期患者的中位生存期分别为60周和16周(图2b),两组差异有统计学意义(χ2=5.90,P=0.015)。单纯手术组的中位生存期为24周,单纯化疗组为200周,手术联合化疗组为24周,手术联合放疗组为224周,化疗联合放疗组为16周,手术联合放化疗的1例生存期为692周,对症处理组为4周。
讨 论
原发性胰腺小细胞癌又称低分化内分泌癌、高度恶性神经内分泌癌,是胰腺神经内分泌癌的一种。目前对其组织来源及发生机制尚未明了。传统认为胰腺神经内分泌癌起源于胰岛细胞,而目前学者则倾向于多能干细胞学说,即多极分化能力的胰腺导管细胞在致癌因素作用下既可转变为小细胞癌,也可转变为腺癌[31]。本研究中3例混合型癌的免疫组化显示角蛋白高表达,似乎支持多能干细胞分化学说。近期有学者已建立胰腺小细胞癌的稳定细胞株[32],这将有助于对其分子生物学和遗传学特性的深入研究。

图2原发性胰腺小细胞癌患者总的生存曲线(a)及不同分期患者的生存曲线(b)
原发性胰腺小细胞癌好发于中老年男性,病灶多为单发,可发生于胰腺各个部位,以胰头部多见。临床常表现为腹痛、黄疸及体重下降,而反映神经内分泌功能的临床特征较为少见,且多为无功能性,故早期诊断极为困难。CEA、CA19-9等胰腺肿瘤标记物多为正常,而反映神经内分泌功能的标记物例如NSE表达率较高,可作为疗效观察的指标之一[11, 17]。因此,对临床上CA19-9正常的胰腺占位需考虑该症可能,联合血清NSE检查可协助诊断。
CT是最常用的诊断原发性胰腺小细胞癌的手段之一。CT征象包括:(1)平扫时肿瘤密度比较均匀,亦可发生囊变坏死;(2)肿瘤强化程度高于胰腺导管腺癌;(3)发生肝转移的概率相对较高,且亦具有富血供特点。但CT征象无法与其他富血供肿瘤例如胰岛细胞瘤、类癌、腺泡细胞癌等鉴别。EUS的优势在于增加胰腺小病灶检出率及进行病灶细针穿刺获取组织有助于定性诊断。近年来,生长抑素受体闪烁成像是根据内分泌肿瘤的生物学特性开发的成像技术,可显示其他常规影像学检查无法检测的小病灶和转移灶[33],可作为胰腺小细胞癌分期及预后评估的首选方法[20]。其他一些检查手段例如选择性血管造影、PET及MRI也可用于胰腺小细胞癌的定位诊断。
原发性胰腺小细胞癌确诊主要依靠病理检查。神经内分泌肿瘤标记物Syn、CgA及NSE阳性率较高,而CA19-9、CEA以及CD20等多为阴性。值得注意的是,原发性胰腺小细胞癌发生率低,故确诊该病首先须排除其他部位的小细胞癌的转移灶。
原发性胰腺小细胞癌局限期患者的中位生存期显著长于进展期患者,不同治疗方案组患者中位生存期也有差别。本研究纳入的均属个案报道,存在选择性偏倚,故不能得出最佳的治疗模式。有学者认为肺外小细胞癌的组织学表现、基因改变和高度侵袭性的生物学特点与小细胞肺癌相似,故强调了化疗在胰腺小细胞癌治疗中的地位[34]。目前常用的化疗方案以铂类和健择为主。部分学者认为手术不仅可切除病灶,而且可延长患者的无瘤生存时间[35]。部分学者则认为因局限期患者存在微小转移,手术可导致癌细胞的扩散,加速肿瘤进展,从而强调以化疗为基础的全身系统治疗[36]。本组采用手术联合放化疗的1例生存时间长达10余年。因此,三者联合的综合治疗不失为一种可选择的疗法。其他如采用生长抑素受体介导的靶向治疗也有一定疗效。
[1] Percy C,Sobin L.Surveillance,epidemiology,and end results lung cancer data applied to the World Health Organization′s classifications of lung tumors.J Natl Cancer Inst,1983,70:663-666.
[2] Van der Heijden HF, Heijdra YF. Extrapulmonary small cell carcinoma. South Med J, 2005, 98: 345-349.
[3] Corrin B,Gilby ED,Jones NF,et al.Oat cell carcinoma of the pancreas with ectopic ACTH secretion.Cancer,1973,31:1523-1527.
[4] Reyes CV, Wang T. Undifferentiated small cell carcinoma of the pancreas: a report of five cases. Cancer, 1981, 47: 2500-2502.
[5] Ordónez NG,Cleary KR,Mackay B.Small cell undifferentiated carcinoma of the pancreas.Ultrastruct Pathol,1997, 21:467-474.
[6] Morikawa T, Kobayashi S, Yamadori I, et al. Three cases of extrapulmonary small cell carcinoma occurring in the prostate, stomach, and pancreas. Indian J Cancer,1994, 31: 268-273.
[7] Kinoshita K, Minami T, Ohmori Y, et al. Curative resection of a small cell carcinoma of the pancreas: report of a case of long survival without chemotherapy. J Gastroenterol Hepatol, 2004, 19: 1087-1091.
[8] Wahid NA, Neugut AI, Hibshoosh H, et al. Response of small cell carcinoma of pancreas to a small cell lung cancer regimen: a case report. Cancer Invest, 1996, 14: 335-339.
[9] Morant R, Bruckner HW. Complete remission of refractory small cell carcinoma of the pancreas with cisplatin and etoposide. Cancer, 1989, 64:2007-2009.
[10] Chetty R, Clark SP, Pitson GA. Primary small cell carcinoma of the pancreas. Pathology, 1993, 25: 240-242.
[11] O′Connor TP, Wade TP, Sunwoo YC, et al. Small cell undifferentiated carcinoma of the pancreas. Report of a patient with tumor marker studies. Cancer, 1992, 70: 1514-1519.
[12] Chung MS, Ha TK, Lee KG, et al. A case of long survival in poorly differentiated small cell carcinoma of the pancreas. World J Gastroenterol, 2008, 14: 4964-4967.
[13] Sakamoto H, Kitano M, Komaki T, et al. Small cell carcinoma of the pancreas: role of EUS-FNA and subsequent effective chemotherapy using carboplatin and etoposide. J Gastroenterol, 2009, 44: 432-438.
[14] Hobbs RD,Stewart AF,Ravin ND,et al.Hypercalcemia in small cell carcinoma of the pancreas.Cancer,1984,53:1552-1554.
[15] Motojima K, Furui J, Terada M, et al. Small cell carcinoma of the pancreas and biliary tract. J Surg Oncol,1990, 45: 164-168.
[16] Matsubayashi H, Fujiwara S, Kobayashi Y, et al. A small cell carcinoma of the pancreas with a high level of serum ProGRP. J Clin Gastroenterol, 2004, 38: 834-835.
[17] Nakamura Y, Tajiri T, Uchida E, et al. Changes to levels of serum neuron-specific enolase in a patient with small cell carcinoma of the pancreas. J Hepatobiliary Pancreat Surg, 2005, 12: 93-98.
[18] Namieno T, Koito K, Nagakawa T, et al. Diagnostic features on images in primary small cell carcinoma of the pancreas. Am J Gastroenterol, 1997, 92: 319-322.
[19] Wang RF, Chou YH, Hwang JI, et al. Primary small cell carcinoma of the pancreas with an unusual sonographic appearance. J Clin Ultrasound, 2007, 35: 82-84.
[20] Berkel S, Hummel F, Gaa J, et al. Poorly differentiated small cell carcinoma of the pancreas. A case report and review of the literature. Pancreatology, 2004, 4:521-526.
[21] Wang D, Rong Y, Wu W, et al. Primary small cell carcinoma of the pancreas: rare type of pancreatic cancer and review of the literatures. World J Surg Oncol, 2012, 10: 32.
[22] Winter JM, Narang AK, Mansfield AS, et al. Resectable pancreatic small cell carcinoma. Rare Tumors, 2011, 3: e5.
[23] Yachida S, Vakiani E, White CM, et al. Small cell and large cell neuroendocrine carcinomas of the pancreas are genetically similar and distinct from well-differentiated pancreatic neuroendocrine tumors. Am J Surg Pathol, 2012, 36: 173-184.
[24] Choi EH, Coyle W. A pancreatic mass in a patient with nausea and vomiting. Extrapulmonary small cell carcinoma of the pancreas. Gastroenterology, 2011, 140: e5-e6.
[25] 戎叶飞, 楼文晖, 靳大勇. 胰腺神经内分泌癌3例报道. 中华临床医药杂志,2004,5: 97-98.
[26] 周莉,侯安继. 胰腺小细胞癌术后长期存活一例. 临床内科杂志,2011,28: 351.
[27] 冀子中,邬万新, 朱文君. 无功能胰腺神经内分泌癌. 中华消化杂志,2009,29: 669.
[28] 吕叶,王宁菊, 马涛. 胰腺神经内分泌癌2例并文献复习. 宁夏医科大学学报,2009,31: 833-835.
[29] 刘骞,黄帅,刘剑坡,等. 胰腺神经内分泌癌的诊断及外科治疗(附10例临床报告). 实用肿瘤杂志,2011,26: 262-265.
[30] 李荣强,高春妮,张晓康,等. 胰尾部胰腺小细胞癌误诊1例. 山东医药,2001,41: 68-69.
[31] Park JY, Hong SM, Klimstra DS, et al. Pdx1 expression in pancreatic precursor lesions and neoplasms. Appl Immunohistochem Mol Morphol, 2011, 19: 444-449.
[32] Yachida S, Zhong Y, Patrascu R, et al. Establishment and characterization of a new cell line, A99, from a primary small cell carcinoma of the pancreas. Pancreas, 2011, 40: 905-910.
[33] Lebtahi R,Cadiot G,Mignon M,et al.Somatostatin receptor scintigraphy:a first-line imaging modality for gastroenteropan-creatic neuroendocrine tumors.Gastroenterology,1998,115:1025-1027.
[34] Van Der Gaast A, Verwey J, Prins E, et al. Chemotherapy as treatment of choice in extrapulmonary undifferentiated small cell carcinomas. Cancer, 1990, 65: 422-424.
[35] Mitani M, Kuwabara Y, Shinoda N, et al. Long-term survivors after the resection of limited esophageal small cell carcinoma. Dis Esophagus, 2000, 13: 259-261.
[36] Casas F, Ferrer F, Farrús B, et al. Primary small cell carcinoma of the esophagus: a review of the literature with emphasis on therapy and prognosis. Cancer, 1997, 80: 1366-1372.
Systematicreviewof47casesofprimarysmallcellcarcinomaofthepancreas
PENGChun-yan,LÜYing,YAORen-ling,XUZhao-min,ZOUXiao-ping.
DepartmentofGastroenterology,DrumTowerHospital,MedicalSchool,NanjingUniversity,Nanjing210008,China
Correspondingauthor:ZOUXiao-ping,Email:zouxiaoping795@hotmail.com
ObjectiveTo investigate the clinicopathologic features, therapy, and prognosis of primary small cell carcinoma of the pancreas.MethodsDatabases including Chinese Journal Full-text Database, VIP Database for Chinese Technical Periodicals, Medline/Pubmed, and OVID were searched electronically up to April 2012. A systematic review was performed together with one case in our hospital.ResultsTwenty-eight articles fulfilling the criteria consisting of 46 patients with pathologically confirmed diagnosis of primary small cell carcinoma of the pancreas were studied, together with 1 patient in our Drum Tower Hospital, finally 47 patients were included. The results of this systematic review showed: (1)Primary small cell carcinoma of the pancreas was more common in men with a median age of 62. The most common clinical presentations were abdominal pain, jaundice and weight loss. Para-neoplastic syndrome was rarely observed. (2)Most cases were found to have abnormally elevated serum levels of neuron-specific enolase. CT displayed heterogeneous, and marked enhancing masses in most cases. The conclusive diagnosis depended on histological confirmation. (3)63.8% of the cases were found to be associated with metastasis at the time of diagnosis. The overall median survival time was 28 weeks. (4) There was no consensus on the treatment of primary small cell carcinoma of the pancreas. Chemotherapy was currently considered as the treatment of choice among the systematic management for these patients.ConclusionsPrimary small cell carcinoma of the pancreas was a rare and aggressive neuroendocrine tumor with a poor prognosis.
Neuroendorine tumors; Carcinoma, small cell; Pancreas; Meta-analysis
10.3760/cma.j.issn.1674-1935.2012.04.003
210008 南京,南京大学医学院附属鼓楼医院消化科
邹晓平,Email:zouxiaoping795@hotmail.com
2012-04-27)
(本文编辑:吕芳萍)

