p38MAPK抑制剂对大鼠急性坏死性胰腺炎合并肺损伤的保护作用
许永春 刘枫 龚燕芳 金晶 李兆申
·论著·
p38MAPK抑制剂对大鼠急性坏死性胰腺炎合并肺损伤的保护作用
许永春 刘枫 龚燕芳 金晶 李兆申
目的探讨p38MAPK特异性抑制剂SB203580对急性坏死性胰腺炎(ANP)大鼠合并肺损伤的保护作用。方法54只雄性SD大鼠按数字表法随机分为对照组、ANP组和SB203580(干预)组,每组18只。以左旋盐酸精氨酸腹腔注射建立大鼠ANP模型,对照组大鼠腹腔注射等容积生理盐水,干预组在制模前腹腔注射SB203580(用二甲基亚枫溶解配制成10 μmol/L)5 mg/kg体重。术后3、6、12 h分批处死大鼠,取血测淀粉酶、TNF-α和IL-6含量,行胰腺及肺组织病理学检查,计算肺组织湿/干重比,测髓过氧化酶(MPO)含量,RT-PCR法检测中性粒细胞趋化因子(CINC) mRNA表达,蛋白质印迹法检测磷酸化p38MAPK(p-p38MAPK)蛋白表达。结果术后6 h,对照组的血淀粉酶、TNF-α和IL-6含量、肺组织湿/干重比、MPO活性、CINC mRNA及p-p38MAPK蛋白表达量分别为(1035±73)U/L、(0.94±0.16)μg/L、(4.77±0.86)μg/L、3.92±0.29、(0.39±0.02)U/g、0.28±0.04、0.09±0.04;ANP组分别为(5848±656)U/L、(3.84±0.32)μg/L、(103.54±15.32)μg/L、4.97±0.47、(1.03±0.08)U/g、0.62±0.06、0.52±0.14;干预组分别为(4259±286)U/L、(1.64±0.21)μg/L、(76.56±11.46)μg/L、4.32±0.34、(0.78±0.05)U/g、0.37±0.04、0.27±0.08。ANP组的各项指标均显著高于对照组,干预组的各项指标均显著低于ANP组,但仍显著高于对照组(P值均<0.01)。结论SB203580可通过阻断p38MAPK信号通路,在一定程度上减少炎症因子的产生,减轻ANP大鼠胰腺及肺组织损伤。
胰腺炎,急性坏死性; p38丝裂原活化蛋白激酶; SB203580; 急性肺损伤
急性坏死性胰腺炎(ANP)易并发急性肺损伤(acute lung injury,ALT),甚至发展至急性呼吸窘迫综合征(acute respiratory distress syndrome,ARDS),体内大量炎症介质、细胞因子的释放在其发病机制中起重要作用。p38丝裂原活化蛋白激酶(p38MAPK)通路是控制炎症反应最主要的MAPK家族成员之一,它在细胞核内通过磷酸化作用激活许多转录因子,调节炎症介质的产生。本研究应用p38MAPK特异性抑制剂SB203580干预ANP大鼠,观察其对ANP相关肺损伤的保护作用。
材料与方法
一、动物分组及模型制作
健康雄性SD大鼠54只,体重200~250 g,上海第二军医大学实验动物中心提供。按数字表法随机分为对照组、ANP组、干预组。采用腹腔注射15%左旋盐酸精氨酸2.0 mg/g体重2次、间隔1 h的方法制备ANP模型。干预组于制模前腹腔注射p38MAPK特异性抑制剂SB203580 5 mg/kg体重。对照组大鼠腹腔分两次注射等容积无菌生理盐水。术后3、6、12 h分批处死大鼠各6只,腹主动脉取血,离心取血清,-80℃保存;取胰腺、肺组织置4%多聚甲醛固定。
二、方法
1.血淀粉酶、TNF-α、IL-6检测:采用酶法通过HITACHI-7150型自动生化分析仪检测血淀粉酶;采用ELISA法检测血TNF-α、IL-6。
2.肺湿/干重比测定:取部分肺组织,用滤纸拭净血迹后称湿重,再置60℃烤箱内烘烤48 h,称干重,以肺湿/干重比反映肺水肿程度。
3.肺组织髓过氧化酶(MPO)活性测定:应用南京建成生物工程研究所MPO检测试剂盒检测。每克肺组织湿片在37℃的反应体系中H2O2被分解1 μmol为1个酶活力单位。MPO活性=△A460/[11.3×肺重量(g)]。
4.胰腺及肺组织病理学检查:取胰腺和肺组织常规固定,脱水,石蜡包埋,HE染色,由病理医师阅片。
5.肺组织中性粒细胞趋化因子(CINC) mRNA表达检测:以Trizol试剂抽提肺组织总RNA。CINC引物上游5′-GCTCGCTTCTCTGTGCAGC-3′,下游5′-CCATCGGTGCAATCTATCTTC-3′,扩增片段304 bp;内参β-actin引物上游5′-AGGGTGTGATGGTGG-GTATG-3′,下游5′-CATAGCTCTTCTCCAGGGAG-3′, 扩增片段600 bp。RT-PCR条件:37℃ 90 min;95℃ 2 min, 95℃ 15 s、62℃(CINC)或55℃(β-actin)15 s、72℃ 15 s, 40个循环,最后72℃ 10 min。扩增产物经电泳分离,凝胶图像扫描仪扫描,以CINC与β-actin条带灰度比值作为CINC mRNA相对表达量。
6.肺组织磷酸化p38MAPK(p-p38MAPK)表达检测:采用蛋白质印迹法检测。羊抗大鼠p-p38MAPK多抗1∶200稀释。结果以条带的灰度值表示。
三、统计学分析
结 果
一、血淀粉酶、TNF-α、IL-6水平的变化
ANP组大鼠血淀粉酶、TNF-α、IL-6水平均显著高于对照组(P<0.01);干预组较ANP组明显降低(P值均<0.01),但仍显著高于对照组(P值均<0.01,表1)。
二、肺湿/干重比、MPO含量变化
ANP组大鼠肺湿/干重比、MPO含量值均显著高于对照组(P值均<0.01);干预组较ANP组明显降低(P值均<0.01),但仍高于对照组(P值均<0.01,表1)。
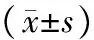
表1 各组大鼠血淀粉酶、TNF-α、IL-6水平及肺湿/干重比、MPO值的变化
注:与对照组同时点比较,q值为4.51~37.58,aP<0.01 ;与ANP组同时点比较,q值为4.32~11.20,bP<0.01
三、胰腺和肺组织学变化
对照组胰腺组织正常;ANP组胰腺间质水肿,小叶间隙增大,炎细胞浸润,腺泡肿胀、小叶结构破坏,胰腺组织片状坏死,血管破裂出血;干预组胰腺间质水肿,伴中等量炎细胞浸润,胰腺腺泡有少量破坏,胰腺组织坏死少见,或胰腺腺泡和导管基本正常,未见血管破裂出血。
对照组肺组织结构清晰,肺泡壁完整,间质无水肿渗出;ANP组肺间质增宽、充血水肿、炎细胞浸润,可见间质、肺泡腔出血;干预组肺间质增宽、充血水肿,见炎细胞浸润,肺泡腔未见出血(图1)。
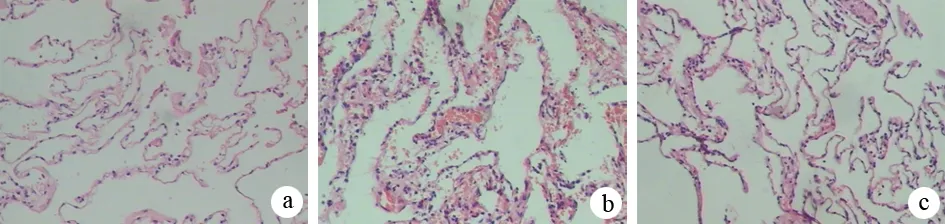
图1对照组(a)、ANP组(b)、干预组(c)大鼠肺组织病理学变化(HE ×100)
四、肺组织CINC mRNA的表达
对照组肺组织CINC mRNA表达量为0.28±0.04;ANP组6 h时的肺组织CINC mRNA表达量为0.62±0.06,较对照组显著增加,差异具有统计学意义(P<0.01);干预组6 h时的肺组织CINC mRNA表达量为0.37±0.04,较ANP组同时间点显著减少(P<0.01),但仍显著高于对照组(P<0.01,图2)。
五、各组大鼠肺组织p-p38 MAPK蛋白的表达
对照组肺组织p-p38 MAPK蛋白的表达量为0.09±0.04;ANP组6 h时为0.52±0.14,较对照组显著增加(P<0.01);干预组6 h时表达量为0.27±0.08,较ANP组同时间点显著减少(P<0.01),但仍显著高于对照组(P<0.01,图3)。
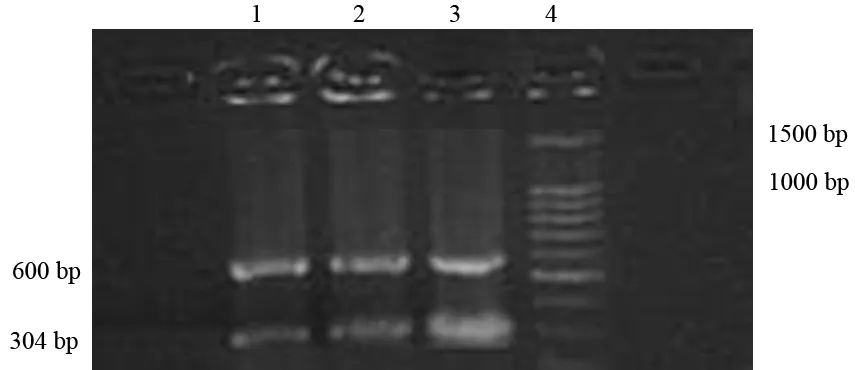
1:对照6 h组;2:干预6 h组;3:ANP 6 h组;4:Marker
图2肺组织中CINC mRNA的表达

图3 各组肺组织p-p38 MAPK蛋白表达
讨 论
在ANP并发急性肺损伤过程中,TNF-α是起始和中轴因子, IL-6是继发于TNF-α和IL-1之后释放的多肽因子,在ANP发生中刺激肝脏合成C反应蛋白,与TNF-α和IL-1协同作用促使全身炎症发应综合征(SIRS)的发生。CINC是一类主要由单核-巨噬细胞产生的中性粒细胞趋化因子,可以导致中性粒细胞的大量激活、迁徙并聚集于肺部,在ANP并发急性肺损伤中起着极为重要的作用。TNF-α和IL-6等是CINC分泌的诱导剂,CINC出现在级联反应的远端,继发于TNF-α和IL-1之后出现峰值[1-8]。
p38MAPK通路是控制炎症反应最主要的MAPK家族成员之一,p38MAPK的激活能促进单核巨噬细胞产生TNF-α、IL-1、IL-6、CINC等炎性因子,介导中性粒细胞的活化,促进中性粒细胞炎性聚集[9-11]。本实验结果显示,诱发ANP后3 h,TNF-α和IL-6水平即迅速升高,而肺组织CINC表达增加较缓慢,峰值在6 h,提示CINC表达增加与TNF-α和IL-1的诱导有关,与文献报道一致[12]。
SB203580通过阻断p38MAPK信号途径,抑制TNF-α、IL-6的分泌和CINC的表达。本实验结果显示,应用p38MAPK特异性抑制剂治疗实验性ANP能显著降低大鼠血TNF-α、IL-6水平,显著下调肺组织CINC的表达,降低肺组织内MPO水平,减少白细胞的浸润,减轻肺组织的病理损伤,对ANP并发的肺损伤有保护作用,提示p38MAPK通路有可能成为临床预防和治疗急性胰腺炎相关肺损伤的新途径。
[1] Ho YP, Chiu CT, Sheen IS, et al. Tumor necrosis factor-α and interleukin-10 contribute to immunoparalysis in patients with acute pancreatitis. Hum Immunol, 2011,72:18-23.
[2] Liang J, Zhou Y, Wang Z, et al. Relationship between liver damage and serum levels of IL-18, TNF-alpha and NO in patients with acute pancreatitis. Nan Fang Yi Ke Da Xue Xue Bao, 2010,30:1912-1914.
[3] Daniel P, Lesniowski B, Mokrowiecka A, et al. Circulating levels of visfatin, resistin and pro-inflammatory cytokine interleukin-8 in acute pancreatitis. Pancreatology, 2010,10:477-482.
[4] Petrella C, Agostini S, Alema′ GS, et al.Cannabinoid agonist WIN55,212 in vitro inhibits interleukin-6 (IL-6) and monocyte chemo-attractant protein-1(MCP-1) release by rat pancreatic acini and in vivo induces dual effects on the course of acute pancreatitis. Neurogastroenterol Motil, 2010,1248-1256.
[5] Gregoric P, Sijacki A, Stankovic D, et al.SIRS score on admission and initial concentration of IL-6 as severe acute pancreatitis outcome predictors. Hepatogastroenterology, 2010,57:349-353.
[6] Dambrauskas Z, Giese N, Gulbinas A, et al.Different profiles of cytokine expression during mild and severe acute pancreatitis. World J Gastroenterol, 2010,16:1845-1853.
[7] Digalakis MK, Katsoulis IE, Biliri K, et al.Serum profiles of C-reactive protein, interleukin-8, and tumor necrosis factor-alpha in patients with acute pancreatitis. HPB Surg, 2009,2009:878490.
[8] Aoun E, Chen J, Reighard D, et al.Diagnostic accuracy of interleukin-6 and interleukin-8 in predicting severe acute pancreatitis: a meta-analysis. Pancreatology, 2009, 9:777-785.
[9] Bhatia M.Acute pancreatitis as a model of SIRS. Front Biosci, 2009,14:2042-2050.
[10] Chen P, Huang L, Zhang Y, et al. SiRNA-mediated PIAS1 silencing promotes inflammatory response and leads to injury of cerulein-stimulated pancreatic acinar cells via regulation of the P38MAPK signaling pathway. Int J Mol Med, 2010,26:619-626.
[11] Yubero S, Ramudo L, Manso MA, et al.The role of redox status on chemokine expression in acute pancreatitis.Biochim Biophys Acta, 2009,1792:148-154.
[12] 刘丽荣,夏时海. p38丝裂原活化蛋白激酶信号通路在急性胰腺炎中的作用.武警医学, 2008, 19: 659-661.
Protectiveeffectsofp38MAPKinhibitoronacutenecrotizingpancreatitisassociatedlunginjuriesinrats
XUYong-chun,LIUFeng,GONGYan-fang,JINJing,LIZhao-shen.
DepartmentofGastroenterology,The94thHospitalofPLA,Nanchang330002,China
Correspondingauthor:LIZhao-shen,Email:lizhaoshen@yahoo.com
ObjectiveTo investigate the protective effects of p38MAPK inhibitor SB203580 on acute necrotizing pancreatitis associated lung injuries in rats.MethodsFifty-four SD male rates were randomly divided into 3 groups, including control group, ANP group, SB203580 group with 18 rats in each group. ANP was induced by intraperitoneal injection of L-arginine solution. Rats in control group were intraperitoneally injected with same amount of saline. Before ANP induction, the rats in SB203580 group
10 μmol/L SB203580 dissolved by dimethyl sulfoxide at a dose of 5mg/kg weight via intraperitoneal injection. The rats were sacrificed at 3, 6, and 12 h after operation, the serum levels of amylase, TNF-α, IL-6 was determined. Pathological changes of pancreas and lung were observed. The wet/dry (W/D) weight ratio of lung and MPO were measured. CINC mRNA of lungs was determined by RT PCR. Expression of phosphated-p38MAPK (p-p38MAPK) protein was evaluated by Western blotting.ResultsThe serum levels of amylase, TNF-α, IL-6 and wet/dry (W/D) weight ratio of lung, MPO activity , CINC mRNA and p-p38MAPK protein expression of lungs were (1035±73)U/L, (0.94±0.16)μg/L, (4.77±0.86)μg/L, 3.92±0.29, (0.39±0.02)U/g, 0.28±0.04,0.09±0.04 in control group at 6 h after operation, and the corresponding values were (5848±656) U/L, (3.84±0.32)μg/L, (103.54±15.32)μg/L,4.97±0.47, (1.03±0.08) U/g, 0.62±0.06, 0.52±0.14 in ANP group, while they were (4259±286) U/L, (1.64±0.21)μg/L, (76.56±11.46)μg/L,4.32±0.34, (0.78±0.05)U/g, 0.37±0.04, 0.27±0.08 in SB203580 group. The values in ANP group were significantly higher than those in control group, and the values in SB203580 group were significantly lower than those in ANP group, but they were still significantly higher than those in control group (P<0.01).ConclusionsSB203580 may attenuate injury of lung and pancreas in ANP by blocking p38MAPK signal transduction pathway, and decreasing the production of inflammatory cytokines.
Pancreatitis,acute necrotizing; p38 mitogen-activatal protein kinase; SB203580; Acute lung injury
10.3760/cma.j.issn.1674-1935.2012.04.012
全军医药卫生科研基金资助(08MA064)
330002 南昌,解放军第九四医院消化内科(许永春);第二军医大学长海医院消化科(刘枫、龚燕芳、金晶、李兆申)
李兆申,Email:lizhaoshen@yahoo.com
2012-02-21)
(本文编辑:屠振兴)
本刊2012(2)期作者杨立新的论文“抑癌基因ppENK甲基化在胰腺癌发病机制中的作用”做如下修正:
1.作者单位为北京协和医院消化科(第一作者现在首都医科大学附属北京朝阳医院消化科)
2.该课题由国家自然科学基金(81072055)和卫生部临床学科重点项目资助

