12-苯基取代四氢-β-咔啉二酮哌嗪的合成
李延超, 马养民, 郭 会, 闫倩茹
(陕西科技大学 教育部轻化工助剂化学与技术重点实验室,陕西 西安 710021)
作为吲哚生物碱,四氢-β-咔啉二酮哌嗪对于乳腺癌细胞(BCRP/ABCG2)是一种选择性的抑制剂,能够使细胞周期停留在G2/M转化阶段[1~3],因其特有的临床耐药性而成为化疗药物。因此,合成具有四氢-β-咔啉二酮哌嗪结构的化合物引起人们广泛的兴趣。
目前,该类化合物的合成主要是通过Pictet-Spengler反应,Schotten-Baumann反应,酰胺键形成等过程[4~8]。本文在文献[8]方法的基础上,选用L-色氨酸甲酯盐酸盐(1)为起始原料,经Pictet-Spengler反应,Schotten-Baumann反应,酰胺键形成及脱保护4步反应合成了12-苯基取代四氢-β-咔啉二酮哌嗪(6, Scheme 1),其结构经1H NMR,13C NMR, IR和元素分析表征。并对各步反应进行了工艺改进,结果表明,改进工艺具有反应时间短、条件温和、产品纯化简单、易于分离等优点。
1 实验部分
1.1 仪器与试剂
X-T5显微熔点仪(温度未校正);WZZ-1S型自动旋光仪;Bruker 400 MHz型核磁共振仪(DMSO为溶剂,TMS为内标);VECTOR-22型傅立叶红外光谱仪(KBr压片);Vario EL Ⅲ型元素分析仪。
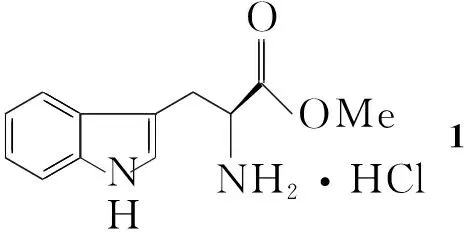

Scheme 1
1,自制;二氯亚砜,分析纯,使用前重蒸;其余所用试剂均为分析纯。
1.2 合成
(1) 1-苯基-四氢-β-咔啉环(4)的合成
在三口烧瓶中加入无水甲醇50 mL,搅拌下依次加入1 3.0 g(12 mmol)和三乙胺1.19 g(12 mmol),搅拌1 h;加入苯甲醛1.5 g(14 mmol),滴入6滴冰乙酸,反应2 h[TLC跟踪,展开剂:V(氯仿) ∶V(甲醇)=4 ∶1,下同]。减压蒸除溶剂得淡黄色黏稠液体。加入重蒸二氯甲烷50 mL,降温至0 ℃以下,分三次滴加三氟乙酸(溶液颜色逐渐加深,最后呈黄色乳浊液状态),滴毕,反应1.5 h;自然升至室温,反应6 h(TLC跟踪)。倾入分液漏斗中,用饱和Na2CO3溶液洗涤,无水MgSO4干燥,浓缩后用混合溶剂[V(二氯甲烷) ∶V(正己烷)=1 ∶1]重结晶得白色粉末4,收率77%, m.p.223 ℃~224 ℃, [α]-14.2°(c1.5, CHCl3),立体构型由1H-1H NOESY确定[9];1H NMR(CDCl3)δ: 7.56~7.52(m, 1H, ArH), 7.45(s, 1H, NH in indole), 7.40~7.34(m, 5H, PhH), 7.22~7.18(m, 1H, ArH), 7.17~7.10(m, 2H, ArH), 5.23(s, 1H, 1-H), 3.98(dd,J=11.2 Hz, 4.2 Hz, 1H, 3-H), 3.81(s, 3H, OCH3), 3.23(m, 1H, 4-H), 3.05~2.97(m, 1H, 4-H), 2.45(s, 1H, NH);13C NMRδ: 173.23, 140.72, 136.14,134.70, 129.01, 128.66, 127.11, 121.99, 119.66, 118.24, 110.96, 108.93, 58.71, 56.91, 52.32,25.73; IRν: 3 394, 3 332, 2 952, 2 785, 1 740, 1 452, 1 437, 1 357, 1 325, 1 205, 746, 690 cm-1; Anal.calcd for C19H18N2O2: C 74.49, H 5.92, N 9.14; found C 74.56, H 6.03, N 9.15。
(2) 6的合成
在三口烧瓶中加入二氯甲烷30 mL,搅拌下加入Fmoc-L-Pro 2.02 g(6 mmol),搅拌使其充分溶解;滴加氯化亚砜10 mL,滴毕,回流反应2 h;减压蒸除溶剂得橘黄色黏稠液体Fmoc-L-Pro-Cl粗品。将其溶于二氯甲烷中,搅拌下滴加41.5 g(5 mmol)的二氯甲烷(15 mL)溶液,滴毕,加入饱和K2CO3溶液使反应液呈两相反应体系,于40 ℃反应4 h。静置分层,水相用二氯甲烷(3×20 mL)萃取,合并有机相,用无水MgSO4干燥,减压蒸除溶剂,残余物用二氯甲烷(30 mL)溶解,加入吗啡啉10 mL,于室温搅拌40 min;减压蒸除溶剂后经硅胶柱层析[洗脱剂:V(二氯甲烷) ∶V(甲醇)=10 ∶1]分离得白色粉末6, 收率92%, m.p.329 ℃~330 ℃;1H NMRδ: 11.25(s, 1H, ArH), 7.58(d,J=7.7 Hz, 1H, ArH), 7.35(d,J=8.0 Hz, 1H, ArH), 7.29(dt,J=15.1 Hz, 7.6 Hz, 4H, PhH), 7.17(t,J=7.0 Hz, 1H, PhH), 7.08(t,J=7.4 Hz, 1H, ArH), 7.02(t,J=7.4 Hz, 1H, ArH), 6.36(s, 1H, 12-H), 4.56(dd,J=11.5 Hz, 5.0 Hz, 1H, 5a-H), 4.36(t,J=7.8 Hz, 1H, 14a-H), 3.59~3.48(m, 2H, 3-H), 3.46(d,J=5.4 Hz, 1H, 6-H), 3.03(dd,J=15.7 Hz, 11.8 Hz, 1H, 6-H), 2.26~2.14(m, 1H, 1-H), 2.00~1.90(m, 1H, 1-H), 1.90~1.79(m, 2H, 2-H);13C NMRδ: 170.40,165.86,143.25, 136.52, 134.51, 128.94, 127.41, 126.22, 126.19, 121.72, 119.40, 118.62, 111.85, 104.96,58.88, 56.74, 55.46, 45.35, 28.50, 23.11, 22.04; IRν: 3 285, 1 663, 1 456, 1 396 cm-1; Anal.calcd for C23H21N3O2: C 74.37, H 5.70, N 11.31; found C 74.56, H 6.03, N 11.25。
2 结果与讨论
4的合成采用一锅法。将1溶于无水甲醇中,加入三乙胺脱去盐酸,再加入苯甲醛,将三步反应一步完成,不仅避免了文献[4~8]方法用Na2CO3溶液脱去盐酸时的繁琐操作,且造成产品损失等缺陷,而且将多步反应合并一步完成,中间过程无需处理,避免产品浪费,即节省了反应时间,又提高了反应收率。
6的合成是Schotten-Baumann反应,用饱和K2CO3溶液和二氯甲烷提供两相溶剂体系,维持体系温度约40 ℃,反应仅需2 h可完全。在最后酰胺键形成及脱保护基过程中,文献[4~8]方法采用哌啶提供碱性环境,本文采用价格便宜的吗啡啉替代哌啶,结果发现,反应时间较短,产品易于纯化,收率较高。
[1] Rabindran S K, He H, Singh M,etal. Reversal of a novel multidrug resistance mechanism in human colon carcinoma cells by fumitremorgin C[J].Cancer Res,1998,58:5850-5858.
[2] Hazlehurst L A, Foley N E, Gleason-Guzman M C,etal. Multiple mechanisms confer drug resistance to mitoxantrone in the human 8226 myeloma cell line[J].Cancer Res,1999,59:1021-1028.
[3] Cui C B, Kakeya H, Osada H. “Novel mammalian cell cycle inhibitors, cyclotryprostatins A-D,produced by Aspergillus fumigatus,which inhibit mammalian cell cycle at G2/M phase”[J].Tetrahedron,1997,53:59-72.
[4] Wang H S, Ganesan A. Concise synthesis of the cell cycle inhibitor demethoxy-fumitremorgin C[J].Tetrahedron Letters,1997,38(24):4327-4328.
[5] Wu G F, Liu J W, Lanrong B,etal. Toward breast cancer resistance protein(BCRP) inhibitors:Design,synthesis of a series of new simplified fumitremorgin C analogues[J].Tetrahedron,2007,63:5510-5528.
[6] Yen Y H, Chu Y H. Synthesis of tetrahydro-β-carbolinediketopiperazines in [bdmim][PF6] ionic liquid accelerated by controlled microwave heating[J].Tetrahedron Letters,2004,45:8137-8140.
[7] Steven A, Boyd W, Thompson J. Stereoselective synthesis of desmethoxy-(+)-verruculogen TR-2[J].J Org Chem,1987,52:1790-1794.
[8] Wang H S, Usui T, Osada H,etal. Synthesis and evaluation of tryprostatin B and demethoxyfumitremorgin C analogues[J].J Med Chem,2000,43:1577-1585.
[9] Xiao S, Lu X, Shi X X,etal. Syntheses of chiral 1,3-disubstituted tetrahydro-β-carbolines via CIAT process:Highly stereoselective Pictet-Spengler reaction of D-tryptophan ester hydrochlorides with various aldehydes[J].Tetrahedron:Asymmetry,2009,20:430-439.

