Efficacy and safety of H1 antihistamines combination therapy for allergic rhinitis
, , , ,
(1.Clinical Pharmacy Teaching and Research Office, School of Pharmacy, Zunyi Medical University, Zunyi Guizhou 563006, China; 2.Department of Pharmacy, Affiliated Hospital of Zunyi Medical University,Zunyi Guizhou 563003, China)
[Abstract]Objective To perform a scoping review to analyze the common treatment regimens of H1 antihistamines (AHs) combination therapy for allergic rhinitis (AR) and evaluate their efficacy and safety. Methods We conducted a comprehensive search of 8 databases from both China and abroad in May 2023, following the methodological framework proposed by Arksey and O’Malley. The quality of the included studies was rigorously evaluated. Results A total of 70 studies, covering 8 combination therapy regimens were included from 8 207 related literatures. Sixty-seven studies reported that 2 AHs combination therapy was more effective than monotherapy, and 3 studies indicated that 2 AHs combination therapy was more effective and safer than 3 AHs combination therapy or monotherapy. Thirty-three studies concluded that 2 AHs combination therapy had a better safety profile than single AHs therapy. Additionally, 1 study found that 3 AHs combination therapy was more effective than monotherapy, but it was associated with a higher incidence of adverse drug reactions (ADRs). Conclusion Based on the current evidence, 2 AHs combination therapy has demonstrated superior efficacy and safety compared to monotherapy or 3 AHs combination therapy for AR. Specifically, the combination of Azelastine nasal spray and Desloratadine tablets has emerged as the most commonly utilized treatment regimen in the review.
[Key words]allergic rhinitis; adverse drug reactions; combination therapy; efficacy; H1 antihistamines
Allergic rhinitis (AR) is a chronic nasal inflammatory disease characterized by symptoms such as sneezing, runny nose, and nasal obstruction. It is primarily caused by the interaction of immunoglobulin E (IgE), allergens, immune cells, and cytokines in susceptible individuals[1-3]. AR is a complex polygenic disorder influenced by both genetic and environmental factors, with environmental factors referring to various allergens present in the human living environment[4]. The prevalence of AR is high, ranging from 10%-25% in adults and 4%-10% in children[5]. Besides the high incidence, AR also has adverse effects on the quality of life (QOL) of patients, including work efficiency, school performance, social life, and mental health[6-7]. Moreover, the management of AR incurs significant direct and indirect costs that burden society[8].
At present, the therapeutic approaches for AR include patient education, avoidance of irritants and allergens, drug therapy, allergen immunotherapy, nasal irrigation and less common measures such as acupuncture, moxibustion and surgery[9-10]. Histamine plays a central role in the pathophysiology of AR, as histamine H1receptors are distributed on vascular endothelial cells, smooth muscle cells, neurons, and immune cells in human skin and mucosa, regulating vasodilation, vascular permeability, blood pressure, sleep, and memory[11]. H1antihistamines (AHs) are the primary treatment for AR, as they inhibit the biological effects of histamine by blocking its binding to H1receptors. Since the development of the first-generation AHs in 1937, 17 AHs have been developed in the form of first-generation, second-generation, and third-generation AHs (new second-generation)[12]. As 20% of rhinitis sufferers do not benefit from standard medication treatments, it is clear that we need to explore alternative treatment options and seek more effective treatment methods[13]. Combination therapy of 2 or more AHs has emerged as a new treatment method, applied by clinical doctors in practice and studied in related clinical trials. However, there are currently no guidelines or expert consensus on this approach, lacking evidence-based support. The scoping review’s purpose is to analyze the common treatment regimens of AHs combination therapy for AR and evaluate their efficacy and safety.
1 Methods
The scoping review was followed the methodology proposed by Arksey and O’Malley[14]and included the following steps: (1) identifying research issues; (2) searching for and identifying relevant studies; (3) selecting studies; (4) charting data;(5) organizing, summarizing and reporting results. The report of the scoping review was based on the PRISMA-ScR (preferred reporting items for the system reviews and meta-analyses extension of scoping reviews) checklist, as described in Appendix 1.
1.1 Inclusion and exclusion criteria Inclusion criteria: (1) Patients diagnosed with AR; (2) Intervention involving 2 or more AHs combination therapy; (3) Study designs as randomized controlled trials (RCTs), non-randomized controlled trials (NRCTs) and systematic reviews/meta-analysis; (4) The languages were Chinese and English.
Exclusion criteria: (1) Duplicates; (2) Patients diagnosed as other diseases or AR combined with other allergic diseases (AD); (3) The control measure without AHs therapy; (4) Study designs as abstracts, cohort studies, retrospective cohort studies, case reports, and observational studies; (5) Studies with unavailable outcomes or missing information.
1.2 Search strategy In conjunction with 2 reviewers, comprehensive research strategies consisted of standard medical topic terms and free text terms such as “allergic rhinitis”, “Chlorpheniramine”, “Diphenhydramine”, “Cycloheptadine”, “Brompheniramine”, “Ketotifen”, “Loratadine” and “Cetirizine”, etc. were performed for studies published in multiple database including PubMed, Cochrane Central Register of Controlled Trials (CENTRAL) database, Web of Science, Embase, China National Knowledge Infrastructure (CNKI) database, Wanfang database, VIP database, and China Biomedical (CBM) database up to May 2023. Precise search strategies can be found in Appendix 2.
1.3 Study selection and data extraction Two independent reviewers used Endnote X9.0 to select eligible studies based on predefined inclusion and exclusion criteria. Disputes were resolved through discussions between the 2 reviewers or with 3 reviewers. A data extraction form was created to collect relevant information from the included studies, including title, first author, year, nationality, sample size, patient characteristics (age, gender, course of disease), treatment regimens, course of treatment, outcomes, and adverse drug reactions (ADRs).
1.4 Quality assessment The quality of the included RCTs was evaluated with the improved Jadad scale, with a score of 1-3 indicating poor quality and≥4 indicating high quality. The quality of included NRCTs was assessed using the MINORS scale, which assigned scores ranging from 0 to 2 for each item and a total score of 24 points. For included meta-analyses, the ANASTAR scale was used, consisting of 11 quality inspection questions answered with ‘yes’ or ‘no’.
1.5 Indicator definition The outcome indicators that will be included are defined as follows: (1) Total effective rate: it is an indicator for evaluating treatment effectiveness, usually obtained by comprehensively considering different levels of therapeutic effects such as cure, significant effect, and improvement. The calculation usually uses the following formula: [Total effective rate = (cure + marked improvement + improvement)/total number of cases × 100%]. (2) Clinical symptom scores: researchers score clinical symptoms of patients before and after treatment, including nasal congestion, itching, sneezing, and runny nose. (3) ADRs: nasal dryness, hoarseness, dizzy, headache, drowsiness, bitter taste, constipation, nasal irritation, nasal tingling, fatigue, rash, malaise, nasal bleeding. (4) Complication: bronchial asthma, sinusitis, nasal polyps, otitis media, and allergic conjunctivitis. (5) Recurrence rate: the rate at which a patient’s symptoms improve or stabilize after treatment, and the disease reappears or symptoms worsen again. The calculation usually uses the following formula: [Recurrence rate = (number of patients with recurrence or worsening/total number of patients × 100%]. ⑥ Total ADR rate: it refers to the overall incidence of ADRs. The calculation usually uses the following formula: [Total ADR rate = number of patients experiencing ADR/total number of patients × 100%].
1.6 Statistical analysis Excel 2010 and SPSS 29.0 were used to analyze the data. Frequency and percentage were used to analyze the treatment regimens of AHs combination therapy for AR and identify the most frequently mentioned regimen. The custom formula proposed by Li et al[15]was utilized to calculate the total ADR rate that was not reported in the included studies. Independent samplet-tests or non-parametric tests were employed to analyze the total effective rate and total ADR rate of certain treatment regimens.P< 0.05 was considered as statistically significant.
2 Results
2.1 Research results and basic characteristics A total of 8 207 literatures were identified, and 3 986 records were selected for further screening after removing duplicates. Following a detailed evaluation of full texts, applying the predefined exclusion criteria, we identified a final set of 70 studies including 52 RCTs, 17 NRCTs, and 1 meta-analysis. These studies originated from China, the United States, and Iran, and involved 8 303 patients with ages around 11 to 80 years old. Please refer to Figure 1 for an overview of the screening process.
2.2 AHs combination therapy regimens for AR Eight treatment regimens of AHs combination therapy were studied: Azelastine nasal spray combined with Desloratadine (n=52, 74.29%); Azelastine nasal spray combined with Loratadine (n=7, 10.00%); Azelastine nasal spray combined with Cetirizine (n=3, 4.29%); Azelastine nasal spray combined with Fexofenadine (n=3, 4.29%); Azelastine nasal spray combined with Rupatadine (n=1, 1.43%); Azelastine nasal spray combined with Desloratadine citrate disodium (n=1, 1.43%); Cetirizine, Desloratadine combined with Chlorpheniramine maleate (n=2, 2.86%); Cetirizine, Loratadine combined with Chlorpheniramine (n=1, 1.43%). Of these regimens, Azelastine nasal spray combined with Desloratadine was the most frequently mentioned.

Figure 1 PRISMA flowchart of study selection and inclusion process
2.3 Efficacy and safety of different AHs combination therapy for AR Among 70 included studies, 51 original studies[16-66]and 1 meta-analysis[67]investigated the efficacy and safety of Azelastine nasal spray combined with Desloratadine for AR. Table 1 provides detailed information on the 51 original studies. Of 51 original studies,47[16-23,25-44,46-58,60-65]revealed that the combination therapy had higher total effective rate compared to Azelastine nasal spray either or Desloratadine monotherapy (P<0.05), furthermore, the total effective rate of combination therapy in each study exceeded 90%. Combination therapy generated lower clinical symptom scores from 28 studies[16-21,25,27,31-33,38-40,42,45,47,49,52,54-55,57-59,63-66]and the remaining 23 studies[22-24,26,28-30,34-37,41,43-44,46,48,50-51,53,56,60-62]didn’t estimate this outcome indicator. Additionally, 3 studies[24,28,41]indicated that clinical symptoms disappeared faster after combination therapy compared to Azelastine nasal spray or Desloratadine monotherapy. With regard to inflammatory factors (IL-4, IL-6, IL-8, IL-10 and IFN-γ) as well as immunocyte (Th1/Th2, CD4+T cells and CD8+T cells), Combination therapy yielded better results than monotherapy from 6[16,18,24,27,31,54]studies. One study[43]reported on the QOL, including physical, psychological, social function, and material life, and demonstrated better QOL in the combination medication. Regarding ADRs, 35 studies[17-19,22-27,29,31-35,37-39,41-42,44-48,50,52,56,58-59,61,63-66]reported their incidence. Out of these, 28 studies[17-18,22,24-25,27,29,32-35,37-38,41-42,44-48,50,52,56,58-59,63-64,66]observed a lower ADR rate in the combination therapy compared to monotherapy. Conversely, 6 studies[19,23,26,31,61,65]reported opposite findings, and 1[39]study reported similar the ADR rate between the 2 regimens (P<0.05). Of 51 included studies,Only 2 studies[19,59]and 6 studies[19,30,36,43,51,62]respectively reported lower recurrence of AR and incidence of complications in combination therapy. The meta-analysis[67]evidenced the combination of Azelastine nasal spray combined with Desloratadine had a better therapeutic effect on AR than the single use of Azelastine nasal spray.

Table 1 Basic features of the combination of Azelastine nasal spray and Desloratadine (n=51)
Seven[68-74]studies evaluated the efficacy and safety of Azelastine nasal spray combined with Loratadine for treating AR. Table 2 provides detailed information on these 7 studies. All 7 studies reported a higher total effective rate in combination therapy compared to Azelastine nasal spray or Loratadine monotherapy (P<0.05), with the highest effective rate up to 100%[73]in combination therapy. The Total Nasal Symptom Scores (TNSS)reported by 3 studies[68-69,73]and the Total Eye Symptom Scores (TOSS)reported by 1 study[68]were lower in the combination therapy compared to Azelastine nasal spray or Loratadine alone. Out of the 3 studies that examined ADRs, 1 study[70]found a lower ADR rate in the combination therapy, while 2 studies[73-74]reported an opposite result (P>0.05). Furthermore, the degree of ADRS was mild to moderate, so that they could be alleviated without treatment.

Table 2 Basic features of the combination of Azelastine nasal spray and Loratadine (n=7)
The efficacy and safety of Azelastine nasal spray combined with Cetirizine for treating AR were evaluated by 3 included studies[75-77]. In these studies (detailed information in Table 3), all reported a higher total effective rate in the combination medication compared to monotherapy (P<0.05). One study[76]showed lower TNSS, and another study reported higher clinical symptom scores and improved QOL scores, as well as lower safety indicator scores in the combination therapy. Two studies[76-77]found a lower total ADR rate in the combination therapy compared to monotherapy. One study[77]reported that patients accepting combination therapy were satisfied with their treatment than accepted monotherapy (94.3% vs 71.4%).

Table 3 Basic features of the combination of Azelastine nasal spray and Cetirizine (n=3)
Two studies[78-79]from China and 1 study[80]from America evaluated the efficacy and safety of Azelastine nasal spray combined with Fexofenadine for treating AR. Detailed information on these studies is provided in Table 4. The combination medication had a higher total effective rate compared to the single medication after treatment[78-79]. In addition, the combination medication had lower TNSS[78,80]. Out of the 3 studies that examined ADRs, 2 studies[78-79]reported a lower incidence in the combination therapy group, while 1 study[80]reported different results (P> 0.05).

Table 4 Basic features of the combination of Azelastine nasal spray and Fexofenadine (n=3)
One[81]study evaluated the efficacy and safety of Azelastine nasal spray combined with Rupatadine in the treatment of AR. Detailed information on the study can be found in Table 5. The study revealed combination therapy brought a higher total effective rate compared to monotherapy after treatment (92% vs 80%,P<0.05). Meanwhile, combination therapy created lower TNSS and better QOL compared to Rupatadine alone after treatment (P<0.05). The study also reported the decrease of IL-17 and IL-1β levels in both regimens after treatment, with a more significant decrease in the combination medication (P<0.05). Moreover, the levels of IL-12 and TGF-β increased in both regimens after treatment, with a more significant increase in the combination medication. No ADRs were reported in the study.

Table 5 Basic features of the combination of Azelastine nasal spray and Rupatadine (n=1)
We included 1 study[82]on evaluating the efficacy and safety of Azelastine nasal spray combined with Desloratadine citrate disodium for treating AR. Detailed information on the study can be found in Table 6. Combination therapy brought a higher total effective rate compared to Azelastine nasal spray alone (97.78% vs 68.89%,P< 0.05). The study also observed a more significant decrease in IL-4 and TGF-α levels in the combination therapy compared to monotherapy, whereas the levels of TNF-γ showed a more significant increase in the combination therapy (P<0.05). Moreover, fewer complications occurred in the combination therapy (n=5, 11.10%) compared to monotherapy (n=13, 28.89%) such as otitis media, sinusitis, nasal polyps, and bronchial asthma (P<0.05).

Table 6 Basic features of the combination of Azelastine nasal spray and Desloratadine citrate disodium (n=1)
Two included studies[83-84]strictly followed stepwise antihistamine therapy of the BSACI guidelines, which evaluated the efficacy and safety of 2 AHs combination therapy (Cetirizine and Desloratadine) compared to 3 AHs combination therapy (Cetirizine, Desloratadine combined with Chlorpheniramine maleate) for treating AR. Detailed information on these 2 studies can be seen in Table 7. Both studies reported a higher total effective rate in the 2 AHs combination therapy compared to the 3 AHs combination therapy after treatment. However, the 3 AHs combination therapy resulted in more ADRs and a higher total ADR rate. Patients receiving 3 AHs combination therapy were more likely to experience headache, sleeping, gastrointestinal discomfort, and dryness of mouth than receiving another treatment plan. In addition, 1 study[84]reported the lever of inflammatory factors such as IL-10, IL-17, IL-25 and TGF-α in 2 AHs combination group were better than in 3 AHs combination (P<0.05).
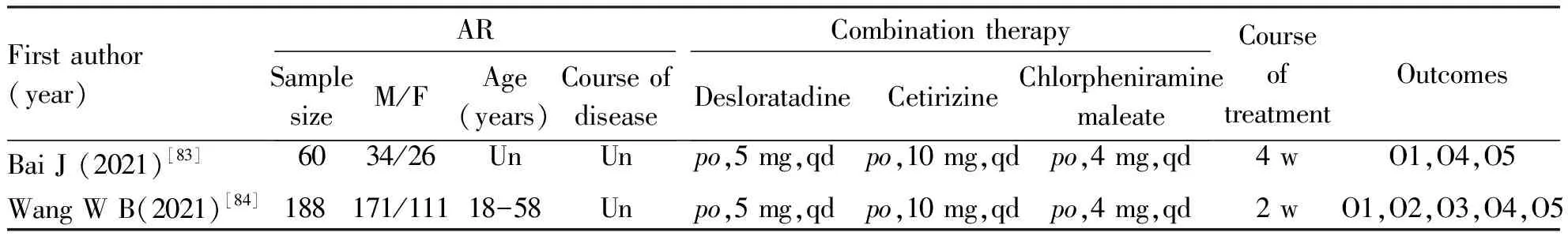
Table 7 Basic features of the combination of Desloratadine Cetirizine, and Chlorpheniramine maleate (n=2)
One study[85]from Iran evaluated the efficacy and safety of intermittent sequential therapy of Apo-Cetirizine and Cetirizine, Loratadine and Chlorpheniramine in the treatment of AR. Detailed information on the study can be found in Table 8. The average post-treatment Major Symptom Complex Scores (MSCS) and Total Symptom Complex Scores (TSCS) showed better improvement in the combination therapy compared to Cetirizine alone (P>0.5). In terms of patients’ treatment satisfaction, 22 patients (78.6%) accepting combination therapy expressed more satisfied with their treatment than 13 patients (54.2%) accepting Apo-Cetirizine (P>0.5). There were more patients experiencing sleepiness, fatigue and dryness of nose in combination therapy compared to the single therapy. Among them, fatigue was the most common side effect.
2.4 Quality assessment Out of the 70 included studies, 52 were RCTs. Among these RCTs, 50 were rated as low-quality with a score of 1-3 points, while only 2 RCTs were rated as high-quality with a score of 4 points. For the 17 NRCTs included, their scores ranged from 16 to 19 points.

Table 8 Basic features of the combination of Loratadine, Cetirizine and Chlorpheniramine (n=1)
It was important to note that the only meta-analysis evaluated in this review did not meet certain criteria. It did not provide a preliminary plan, did not consider the publication status of the literature, and did not report any conflicts of interest.
3 Discussion
In this review, we reviewed the current published studies on AHs combination therapy for treating AR. We found that the most common therapeutic regime is the combination of Azelastine nasal spray with Desloratadine, followed by Azelastine nasal spray with Loratadine. Azelastine nasal spray combined with Cetirizine or Fexofenadine tied for the third place.
All included studies showed that the 2 AHs combination therapy is more effective than monotherapy or the 3 AHs combination therapy. Additionally, the incidence of ADRs was lower in the 2 AHs combination therapy compared to other 2 treatment options.
Azelastine nasal spray was found to be one of the most commonly used combination medications in the review. It is an effective and well-tolerated nasal AHs that improves nasal symptoms[86]. It has been proven to be effective for AR patients who do not respond to one week of oral AHs therapy[87]. Intranasal AHs are recommended as first-line treatment due to their fast onset and targeted administration. However, they need to be administered every 6 to 12 hours[88].
Intranasal AHs have the added benefit of relieving nasal congestion, which is a common symptom of AR. However, they are associated with some local side effects, including epistaxis (nosebleeds), nasal burning, poor taste, sedation, the need for more frequent administration, and increased costs compared to oral formulations[88]. In our review, we found that the most common usage of Azelastine nasal spray was 2 sprays per nostril, 2 daily. This dosage regimen aligns with the recommended guidelines for effective treatment. Furthermore, the most frequently reported ADRs of Azelastine nasal spray in our review were consistent with the previously mentioned side effects, namely bitter taste and nasal irritation. Therefore, our findings corroborate the existing literature regarding the usage and ADRs of Azelastine nasal spray, supporting its effectiveness in relieving nasal congestion while acknowledging the potential local side effects associated with intranasal administration.
Based on the scoping review, it was observed that second-generation or third-generation AHs are commonly used in combination therapy for AR. The first-generation AHs exhibit positive effects in managing allergic conditions. However, their short half-life necessitates frequent dosing. Consequently, with the exception of specific applications, most first-generation AHs have been phased out from the market. In contrast to their predecessors, the second-generation AHs offer notable advantages. Due to their longer half-life, the required dosage of second-generation AHs is generally smaller than that of the first-generation AHs. Importantly, these newer antihistamines demonstrate a reduced likelihood of crossing the blood-brain barrier. They preferentially bind to peripheral H1receptors in a non-competitive manner, resulting in minimal or no central nervous system side effects. The third-generation AHs are derived from active metabolites or optical isomers of earlier second-generation AHs. These third-generation AHs exhibit robust therapeutic effects with fewer ADRs. Notably, there have been no reports of serious ADRs associated with third-generation AHs thus far[12].
Consistent with the findings of this review, combining second-generation AHs, such as Azelastine nasal spray, with third-generation AHs (Fexofenadine, Desloratadine, and Desloratadine citrate disodium) or second-generation AHs (Loratadine, Cetirizine, and Rupatadine), effectively improves nasal symptoms, reduces the duration of nasal symptom relief, and enhances the QOL for patients with AR. The combination therapy approach has demonstrated higher patient satisfaction compared to monotherapy. This could be attributed to the fact that the combination of 2 AHs effectively alleviates nasal symptoms, reduces patient discomfort, and enhances QOL. By combining 2 AHs, patients can experience more comprehensive relief of their nasal symptoms. This synergistic effect may provide superior symptom control, leading to greater overall satisfaction with the treatment method. The combination therapy approach addresses multiple aspects of AR, resulting in improved outcomes and a higher level of patient well-being. The reduction in suffering and improvement in QOL achieved with the combination medication approach further support its effectiveness. Patients report a greater sense of relief and an enhanced ability to engage in daily activities without the burden of persistent nasal symptoms. This positive impact on their daily lives contributes to higher satisfaction levels.
In summary, the combination of 2 AHs in the treatment of AR has shown to effectively alleviate nasal symptoms, reduce patient suffering, and improve overall QOL. As a result, patients in the combination medication group have expressed higher levels of satisfaction compared to those receiving a single medication.
This review addresses a gap in the existing literature by examining the combination of 2 or more AHs in the treatment of AR. It presents a novel treatment approach for clinical management of AR. However, several combination drug regimens had small sample sizes and some included studies were of low quality, highlighting the need for more high-quality studies to be conducted and published. Additionally, this review solely evaluates the efficacy of combination AHs therapy for AR and does not provide specific recommendations regarding usage and duration of treatment. Further evaluation and statistical analysis through systematic evaluation and meta-analysis are required.
Based on current evidence, the combination of 2 AHs has been found to be more effective and safer for AR compared to using single AHs or the combination of 3 AHs. Typically, intranasal antihistamines are administered alongside oral antihistamines. Currently, the most commonly utilized combination treatment regimen consists of Azelastine nasal spray combined with Desloratadine tablets.
Overall, the findings suggest that combining 2 AHs offers advantages over other treatment approaches for AR. Nevertheless, more robust research is essential to further elucidate the benefits and optimize the use of combination therapy in the management of AR.

