Study on Kinetics and Selectivity of Sulfide Bifunctional Catalysts for Hydrocracking of Tetrahydronaphthalene to BTX
DANG Hui,CHEN Shengli,WANG Lei,ZHANG Yanting,JIANG Bo,WU Zhijie
(State Key Laboratory of Heavy Oil Processing,College of Chemical Engineering and Environment,China University of Petroleum,Beijing 102249,China)
Abstract:A study was performed on the selective hydrocracking (HDC)reaction of tetrahydronaphthalene (THN)into benzene,toluene and xylene (BTX)over sulfides bifunctional HDC catalysts with different hydrogenation active centers (Co,Mo,Ni and W)at the reaction temperature of 400 ℃ and reaction pressure of 6 MPa.A nine-lump reaction kinetic model for the THN HDC reaction,and all the reaction rate constants in the reaction network were used to clarify the performance of bifunctional catalysts.The results show that Beta zeolite supported NiW(Ni(5)W(20)/Beta-40)and NiMo(Ni(5)Mo(20)/Beta-40)catalysts exhibit high contents of active metal species and acid sites,respectively,leading to a high BTX selectivity (63% and 56%,molar fraction)at 98% and 96% THN conversion.Based on the reaction results and reaction rate constants,it can be concluded that although the coking rates over Ni(5)W(20)/Beta-40 and Ni(5)Mo(20)/Beta-40 catalysts are relatively high,the ratio of BTX formation reaction rate to the excessive hydrocracking rate of BTX and the ratio of BTX formation reaction rate to the coking rate of catalyst are higher than those of other catalysts,resulting in a high BTX selectivity.
Key words:hydrocracking;bifunctional catalyst;tetrahydronaphthalene;reaction kinetics;benzene,toluene and xylene (BTX)
Tetrahydronaphthalene (THN),monocyclic aromatic hydrocarbon with a naphthenic ring,is one of the main products of hydrotreating (HDT)reaction of light cycle oil (LCO,diesel-boiling range)[1-2].It is highly desirable to upgrade THN to high-value products such as benzene,toluene and xylene (BTX),because these aromatics are increasingly required and the intermediates of THN hydrocracking (HDC)[2-8].Fig.1 shows the possible reaction routes for the THN HDC pathway based on our previous work on 1-methylnaphthalene[9-10],the isomerization reaction of THN,β-scission reaction of methylindane,andβ-scission reaction of C10-alkylbenzene are involved in the tandem reaction over bifunctional catalyst to form BTX[11].To achieve a high BTX selectivity,reaction route Ⅰ and Ⅱ should be reinforced,and reaction route Ⅲ should be suppressed.Thus,the hydrocracking activity (k1andk2)and hydrogenation activity (k3)should be balanced,and a high ratio of formation reaction rate of BTX to the excessive hydrocracking rate of BTX ((k1+k2)/k3,denoted as HDC selectivity)over bifunctional catalyst is required.
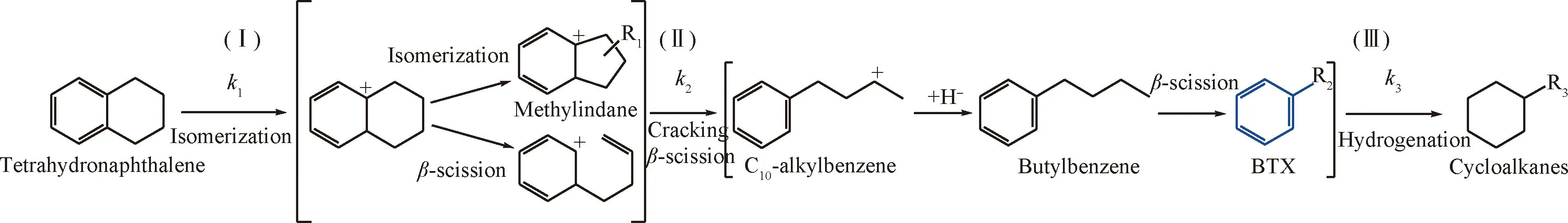
Ⅰ—Isomerization;Ⅱ—Cracking (β-scission);Ⅲ—Hydrogenation R1,R2,R3:AyI.
For bifunctional catalyst for hydrocracking,hydrogen spillover is frequently proposed as an important manner for synchronizing metallic sites and acidic sites.Cao et al.[4]reported that hydrogen spillover originated from metal sites could deeply hydrogenate the naphthalene adsorbed on Brönsted acid sites of zeolite into cycloalkane.They pointed out that a high BTX selectivity can be realized by the appropriate reduction of hydrogen spillover and rational utilization of hydroreaction characteristics for different active metal sites.It should be noted that the as-proposed metal sites for hydrocracking of petroleum-derived oils are generally ascribed to the active sites originated from transitional metal sulfides (i.e.,Ni-Mo(W)-S,Co-Mo(W)-S,MoS2,WS2or RuS2systems),as only metal sulfides possess good activity stability with the sulfur-containing feedstocks.We previously found that[10]the metal-acid interaction was regulated by forming a layer of SiO2on acid sites of Beta zeolite through chemical liquid deposition and the external surface acidity of Beta zeolite was modified,as a result,the high BTX selectivity was obtained.And we found that the dealuminated Beta zeolite supported WS2catalyst possesses a lower coking rate than that of bulk Beta zeolite based catalyst before dealumination treatment[12],suggesting a low acid density can retard the conversion of BTX to coking species.
Even though some studies on THN HDC reaction have been reported,the BTX selectivity and the conversion of reactant cannot directly reflect the HDC selectivity and coking rate of bifunctional catalyst.Kinetic study is useful in calculating the reaction rate constant of each reaction in the reaction network of THN HDC reaction into BTX.Our group previously developed a six-lump reaction model for the HDC of 1-methylnaphthalene into BTX to quantitatively evaluate the selective-hydrogenation activity and metal-acid balance of W/Beta catalyst[9-10].It was shown that the metal-acid ratio within a suitable range is necessary to get a favorable metal-acid balance,and the BTX yield is highly dependent on the selective-hydrogenation activity of metal functions,which is sensitive to the kind and amount of acid sites and their accessibility.Moreover,the interaction between metal sites and zeolite supports is also varied with the acid strength and density of Beta zeolite,in which the acid sites can react with metal species,leading to the difficulty in reduction/sulfidation of metal species,and thus reducing hydrogenation rates of 1-methylnaphthalene and BTX.Again,the presence of strong acid sites also enhances the condensation reaction of aromatics to coking species,leading to the deactivation of hydrocracking catalyst.In our previous work,the as-proposed six-lump reaction model ignored the poly-aromatic components in the product distribution of 1-methylnaphthalene HDC reaction,as 1-methyl-naphthalene can undergo dehydrogenation or condensation reaction at high temperature.In addition,the poly-aromatic components can turn to coke by a series of condensation reaction and the coking rate is also an important parameter to evaluate the performance of catalyst.Here,a nine-lump reaction kinetic model of THN HDC reaction was proposed and discussed to clarify the HDC selectivity and coking rate of bifunctional catalyst.The ratio of BTX formation reaction rate to the excessive hydrocracking rate of BTX and the ratio of BTX formation reaction rate to the coking rate of catalyst were proposed as parameters to evaluate the performance of HDC catalysts.These parameters can be served as the index to comment the BTX selectivity and analyze the differences in the performance of HDC catalysts in THN HDC reaction from the point of view of reaction rates.Also,the catalytic performance of Beta zeolite supported Ni-Mo(W)or Co-Mo bifunctional catalysts were tested by a fixed-bed continuous flow reactor.The physicochemical properties of the HDC catalysts,such as morphology,textural properties,acidity and state of active metals were investigated.The relationship between the properties of catalyst and its catalytic performance was established based on the reaction kinetics data and experimental data.
1 Experiment
1.1 Materials
Nickel nitrate (Ni(NO3)2·6H2O,99%),ammonium molybdate ((NH4)6Mo7O24·4H2O,99%)and cobalt nitrate (Co(NO3)2·6H2O,99%)were purchased from Aladdin Reagent (Shanghai)Co.,Ltd.Ammonium metatungstate ((NH4)6H2W12O40·H2O,99%)was purchased from Qingdao Haiyang Chemical Co.,Ltd.All samples were used as received.
1.2 Preparation of HDC catalysts
H-formed Beta zeolite with SiO2/Al2O3mole ratio of 40 (Beta-40)was received from commercial supplier (Nankai University Catalyst Co.,Ltd.,Tianjin,China),and then calcined at 550 ℃ (at a heating rate of 2 ℃/min)in the air for 6 h before use.NiMo,NiW and CoMo metal species were deposited onto Beta-40 by the wetness co-impregnation method[9-10].The impregnated samples were dried at 80 ℃ for 4 h and calcined at 550 ℃ for 6 h.All the contents of components were calculated based on the amount feedstocks used in the preparation of the catalysts,the composition of the as-prepared catalysts is listed in Table 1.
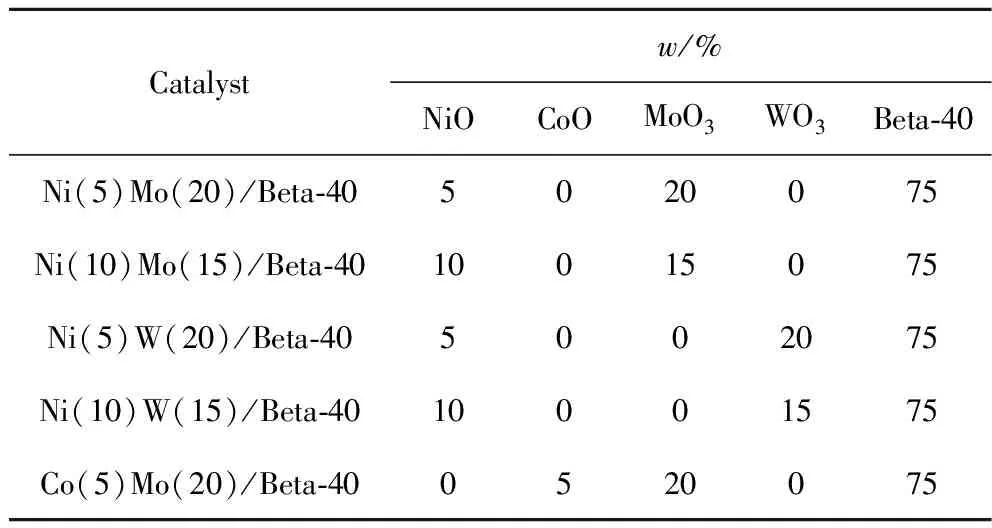
Table 1 The composition of the as-prepared HDC catalysts
1.3 Characterization of HDC catalysts
The structure of catalysts was analyzed by X-ray diffractometer (XRD)(Bruker D8,Bruker Company,Germany).The pore structure of catalysts was analyzed by nitrogen adsorption-desorption (ASAP-2020 M,Micromeritics Company,USA).Temperature-programmed desorption of ammonia (NH3-TPD)(TPD/TPR 5079,Tianjin Xianquan Company,China)was used to characterize the acid amount of sample.X-ray photoelectron spectrometer (XPS)(ESCLAB 250Xi,Thermo Fisher Company,USA)was used to characterize the chemical binding energy of the elements on the surface of the HDC catalyst,the charge correction was carried out according to the binding energy standard of carbon C 1s=284.80 eV.Raman spectra (LabRAM HR,Horiba Scientific Company,France)was used to characterize the chemical states of active metals on Beta zeolite.The test was operated at room temperature with a scanning resolution of 2 cm-1and the He/Cd light source with the wavelength of 532 nm.The procedures of XRD,nitrogen sorption,NH3-TPD and XPS characterized were consistent with our previous work[13].
1.4 Performance of HDC catalysts in THN HDC reaction
The performance of the catalysts in HDC reaction were investigated with THN as feedstock.The HDC reaction was carried out in a high pressure fixed-bed continuous flow reactor.Before THN HDC reaction,it is necessary to convert metal oxides of HDC catalyst into catalytic active metal sulfides[9-10,12].The reaction conditions and reaction procedures were followed with our previous work[13].
After the reaction,the gas-liquid mixture was separated in a pressure separator.The total volume of the gas phase product was measured by gas-collecting method of drainage water,and the total mass of the liquid phase product was measured by the method of weighing.And in the whole reaction,we ensured that all experimental processes can maintain "Feedstock=liquid phase product+gas phase product+carbon deposition",and the carbon balance was determined in the range of 98%—100% for all the catalyst tests.The HDC products include gas-phase and liquid-phase ones,which were analyzed by gas chromatograph the same as our previous work[13].
The conversion of THN (xTHN,%),the yield of BTX (yBTX,%),the selectivity of BTX (sBTX,%)and the yield of gas (ygas,%)were defined as:
(1)
(2)
(3)
(4)
where,WTHN,0andWTHN,trepresent the mass flow rates of THN in the feedstocks and in the effluent,respectively,g/h.WBTX,trepresents the mass flow rate of BTX in the effluent,g/h.NBTX,trepresents the molar flow rate of BTX in the effluent,mol/h.NTHN,0andNTHN,trepresent the molar flow rates of THN in the feedstocks and in the effluent,respectively,mol/h.While,Woutrepresents the mass flow rate of the liquid-phase product,g/h.
2 Results and discussion
2.1 Physical and chemical properties of the HDC catalysts
The HDC catalysts with different metal oxides (NiO,MoO3,WO3and CoO)were characterized by XRD shown in Fig.2.Beta-40 shows the characteristic peaks of Beta zeolite with BEA topology structure.The structure is not changed by the deposition of metal species,but with decrease in crystallinity based on the decrease of peak intensity.In addition,the characteristic peaks due to NiOx,MoOx,WOxand CoOxspecies are not observed,suggesting a high dispersion of these metal species on the Beta-40.
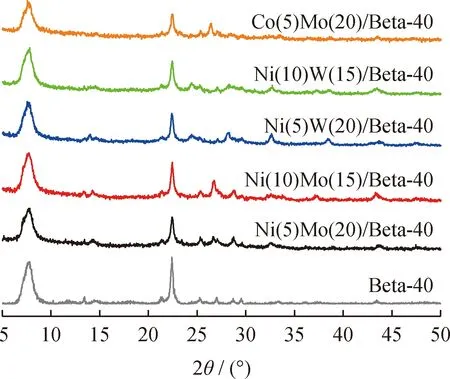
Fig.2 XRD patterns of the different HDC catalysts
The textural properties of HDC catalysts are summarized in Table 2.The BET surface area,micropore surface area and the pore volume of the HDC catalysts decrease to similar values after the deposition rate of 25% (mass fraction)metal oxide species on Beta-40.
Table 3 shows the acidic properties of the HDC catalysts obtained from the temperature-programmed desorption of ammonia (NH3-TPD).According to the ammonia-desorbed temperatures,the desorption peak at the range from 100 ℃ to 300 ℃ is assigned to the weak acid sites,and the range from 300 ℃ to 500 ℃ is due to the strong acid sites.The total acid amounts of HDC catalysts decrease in the order of Ni(5)W(20)/Beta-40>Ni(5)Mo(20)/Beta-40>Ni(10)W(15)/Beta-40>Ni(10)Mo(15)/Beta-40>Co(5)Mo(20)/Beta-40.
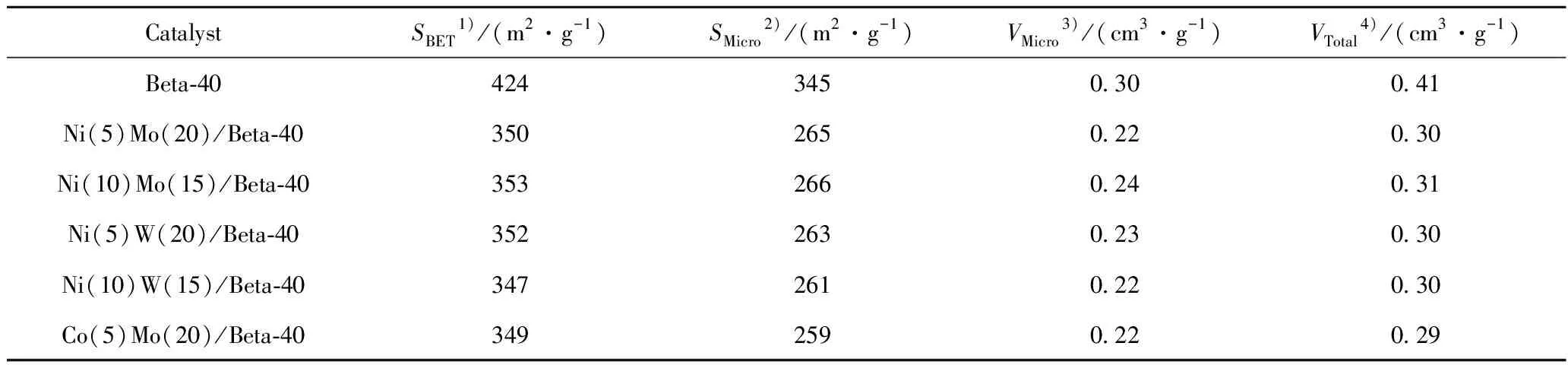
Table 2 Textural properties of the different HDC catalysts
2.2 Chemical state of metal species
XPS spectra were used to analysize chemical valence and sulfidation degree of metal sites[14-15].The Mo 3d and W 4f XPS spectra of the sulfurized HDC catalysts are shown in Fig.3,and the results of the specific peaks of the Mo and W species are summarized in Table 4.Three peaks attributed to Mo6+in an oxidic environment,Mo5+as oxysulfide species and Mo4+sulfurized species are observed in Fig.3(a)and Fig.3(c).Meanwhile,a peak at 226.3 eV corresponding to S2-is also found[4,15-16].The binding energy of Mo species in Ni(5)Mo(20)/Beta-40 and Co(5)Mo(20)/Beta-40 catalysts is different due to the different interaction of Ni-Mo and Co-Mo.For NiW HDC catalysts,Fig.3(b)and Fig.3(d)also show three peaks attributed to W6+in an oxidic environment,W5+as oxysulfide species and W4+sulfurized species[17-19].The interaction of Ni-W increases with the increase of Ni content,resulting in different binding energy of W species in Ni(10)W(15)/Beta-40 and Ni(5)W(20)/Beta-40 catalysts[20-21].Here,the surface sulfide content of catalysts was obtained based on the surface metal loading and surface sulfidation degree shown in Table 4.Clearly,the sulfide content of NiW/Beta and NiMo/Beta catalysts is higher than that of CoMo/Beta catalyst,respectively.
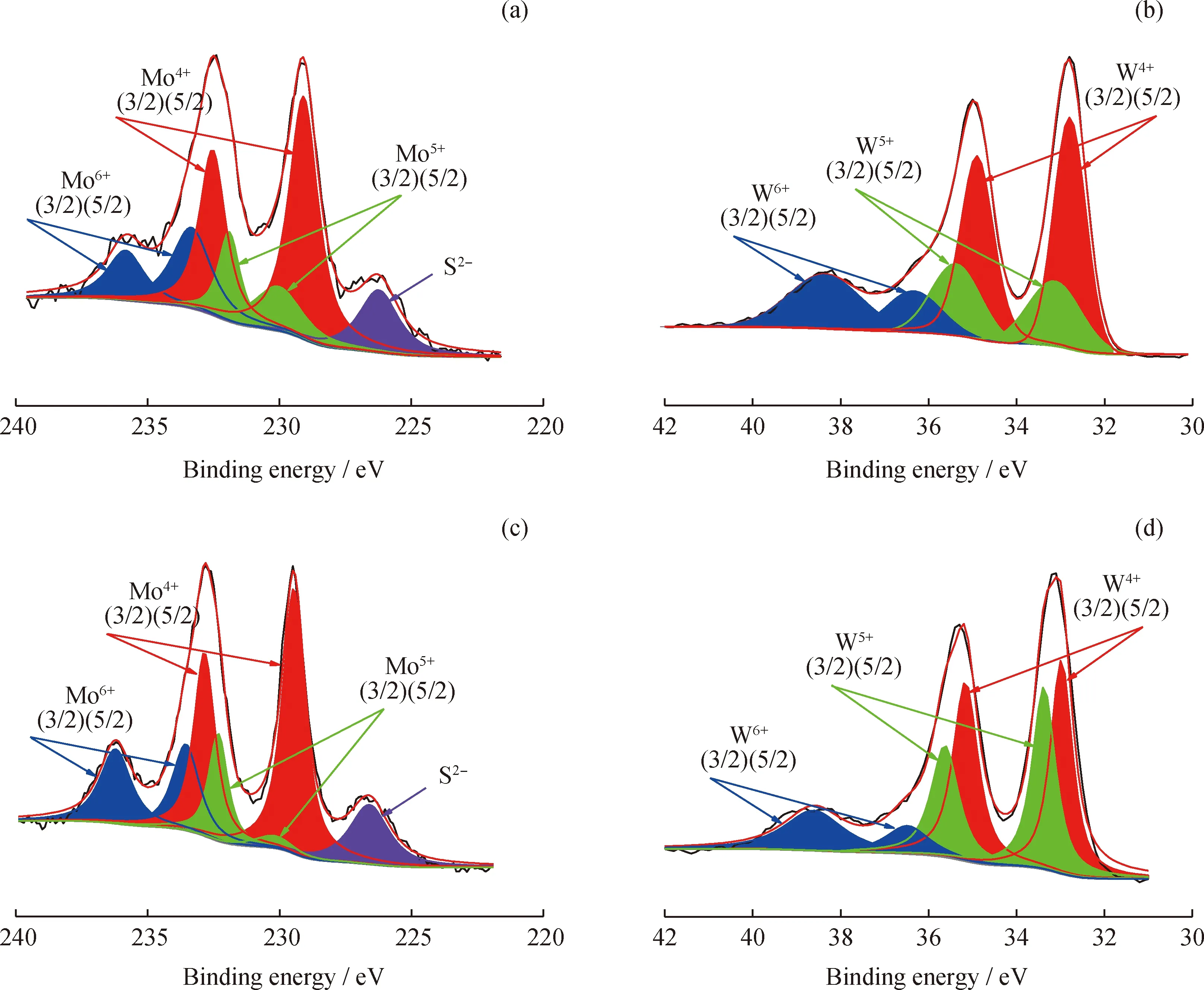
Fig.3 XPS analysis of the Mo 3d and W 4f region of the sulfurized HDC catalysts
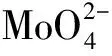
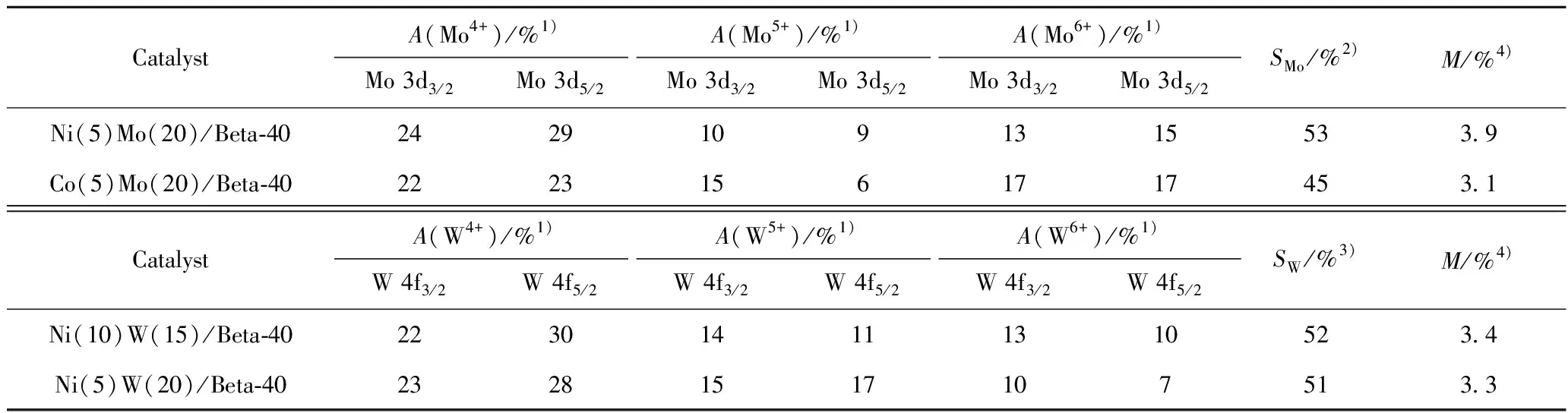
Table 4 The Mo 3d and W 4f XPS analysis results of Mo species and W species over different sulfurized HDC catalysts
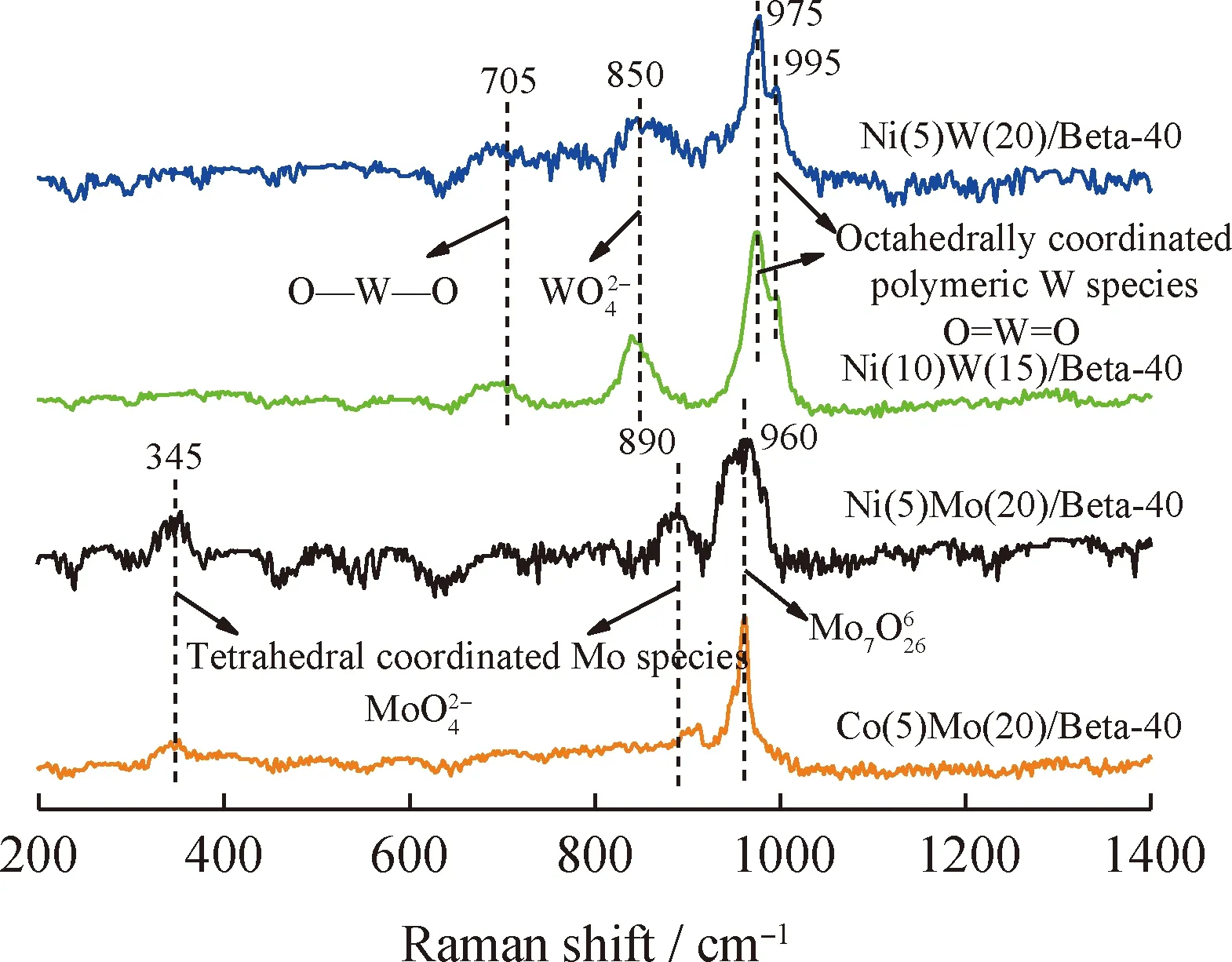
Fig.4 Raman spectra of the different HDC catalysts
2.3 Catalytic performance of the HDC catalysts
The catalytic performance of the HDC catalysts were tested by using THN as probe.All data used here are those which were collected after the yield of all products reached constant (the coking on the catalyst remains almost constant,shown in Fig.5).And the experimental error was established based on the three times experiment and it was no more than 2%.
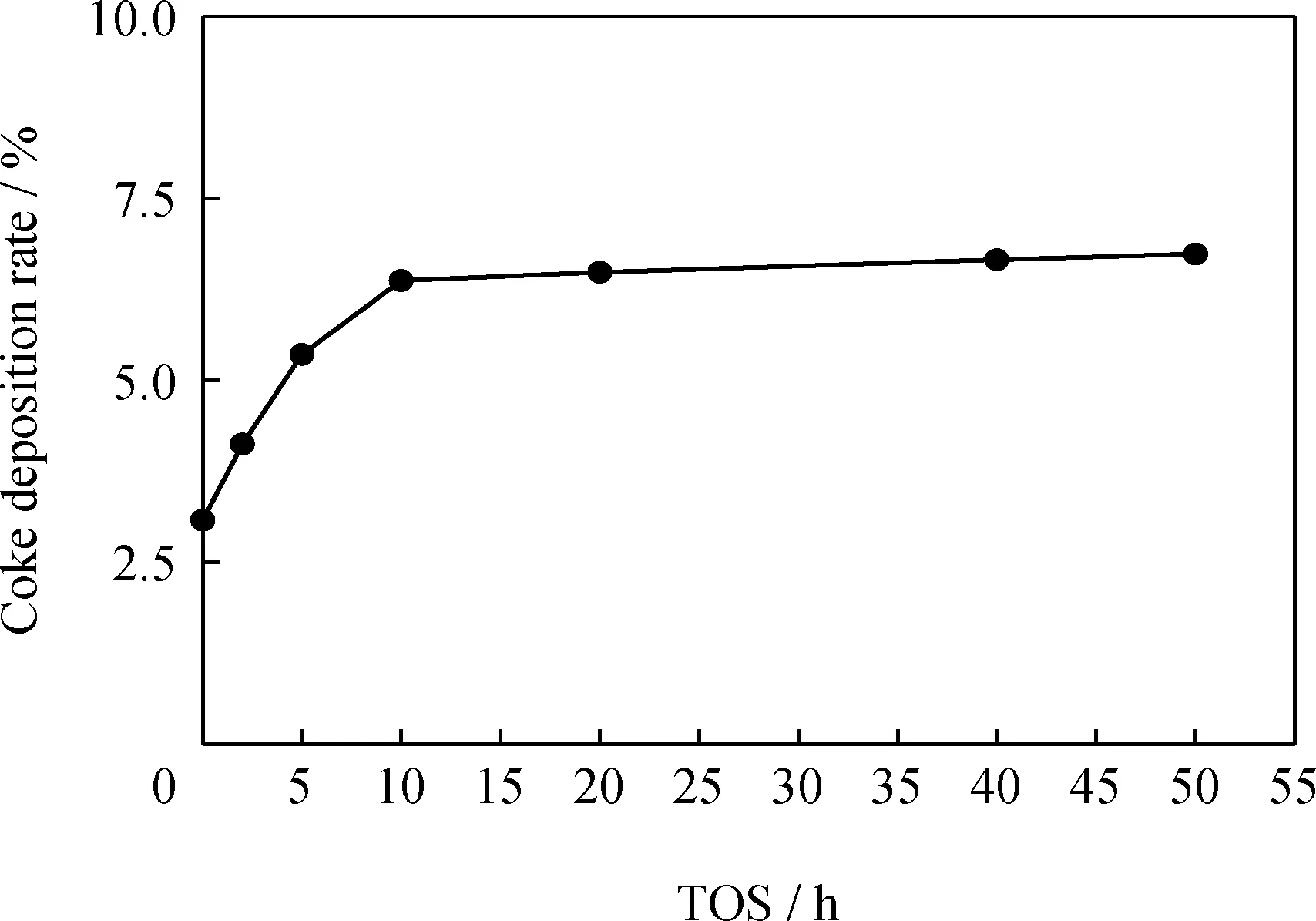
Fig.5 Correlation of coke deposition rate and time on stream (TOS)
Here,the conversion of THN and gas yield are regarded as the index of the activity of the HDC catalysts.The conversion of THN and yield of gas products increase with the growth of space time (Fig.6(a)and Fig.6(b)),and the activity of the HDC catalysts decrease in the order of Ni(5)W(20)/Beta-40>Ni(5)Mo(20)/Beta-40>Ni(10)W(15)/Beta-40>Ni(10)Mo(15)/Beta-40>Co(5)Mo(20)/Beta-40.This is consistent with the larger acid site amount (Table 3)and more active metal species (Fig.4)in NiW/Beta and NiMo/Beta catalysts.The BTX yield increases firstly and then decreases with the increase of space time (Fig.6(c)).The BTX selectivity of NiW/Beta and NiMo/Beta catalysts is higher at low conversion of THN than that of CoMo/Beta catalyst (Fig.6(d)).These results indicate that NiW/Beta and NiMo/Beta catalysts have a high selectivity toward to BTX.The BTX yield increases firstly and then decreases with the increase of gas yield (Fig.6(e)),suggesting excessive HDC of BTX to gas products.
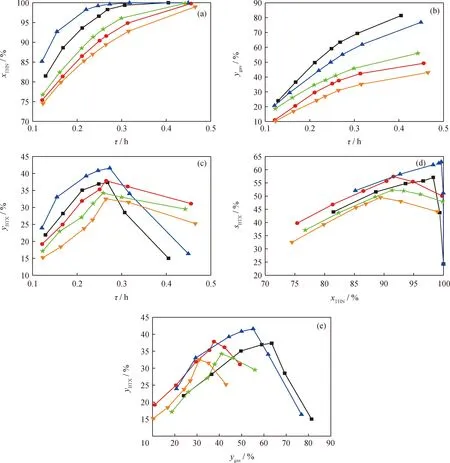
Ni(5)Mo(20)/Beta-40; Ni(10)Mo(15)/Beta-40; Ni(5)W(20)/Beta-40; Ni(10)W(15)/Beta-40; Co(5)Mo(20)/Beta-40
Due to the complicated reaction mechanism,it is difficult to make a clear comment on the HDC selectivity and coking rate of these present bifunctional catalyst from Fig.6.Here,the reaction kinetic model for THN HDC reaction was proposed to calculate the reaction rate constant of each reaction and quantitatively evaluate the activity of acidic sites and metallic sites.Then,we attempted to clarify the performance of catalyst in THN HDC reaction with the point of view of these as-calculated reaction rates.
2.4 Reaction kinetics for THN HDC reaction
We previously developed a six-lump reaction model for the 1-methylnaphthalene HDC reaction into BTX to quantitatively evaluate the selective-hydrogenation activity (the ratio of the hydrogenation reaction rate of 1-methylnaphthalene to the hydrogenation reaction rate of BTX)and metal-acid balance (the ratio of the hydrogenation reaction rate of 1-methylnaphthalene to the cracking rate of alky aromatics (the product of hydrogenation reaction of 1-methylnaphthalene))of W/Beta catalyst[9-10].However,the six-lump reaction model ignored the poly-aromatic components (such as anthracene,phenanthrene and pyrene)in the product distribution of 1-methylnaphthalene HDC reaction,due to the reactant (1-methylnaphthalene)will also undergo dehydrogenation or condensation reaction at high temperature.In addition,the poly-aromatic components can turn to coke by a series of condensation reaction.Thus,the coking rate is also an important parameter to evaluate the performance of catalyst.Here,we modified the previous six-lump reaction model into nine-lump reaction model by including the poly-aromatic components and coke into kinetic model based on the composition of the reaction products for THN HDC reaction,combining with our previously study[9-10]and relevant literature reports[2,27].
Here,the dealkylation reaction (β-scission),transalkylation reaction,isomerization reaction,cracking reaction and hydrogen transfer reaction are involved in the THN HDC reaction.More than 70 kinds of products were detected after THN HDC reaction.The gas-phase products are mainly C1—C5alkanes (Fig.7),and the liquid-phase products are mainly composed of two types (Fig.8):the products with a lower boiling point than that of THN,including paraffins,cycloalkanes,monocyclic aromatic hydrocarbons,BTX and THN;and the other products of poly-aromatic components with a higher boiling point than that of THN,including naphthalene,di-methylindane,methyl-tetrahydrona-phthalene,1-methylnaphthalene,2-methylnaphthalene,ethyl-methylnaphthalene,biphenyl,di-methylnap-hthalene,tri-methylnaphthalene,tetra-methylnap-hthalene,anthracene (phenanthrene),methyl-anthracene (methyl-phenanthrene),pyrene and benzanthracene.

Fig.7 The gas chromatograph spectra of gas-phase product in tetrahydronaphthalene hydrocracking reaction
Fig.8 The gas chromatograph spectra of liquid-phase products in tetrahydronaphthalene hydrocracking reaction
It has been widely accepted that the mechanism of THN HDC reaction was complex at high temperature,consisting of multiple parallel and tandem reactions (Fig.9).THN turns into methylindane by isomerization,or into alkylbenzene by ring-opening reaction,or into naphthalene by dehydrogenation reaction,or into decalin and other by-products by excessive hydrogenation reaction.Then,methylindane turns into C10-alkylbenzene by ring-opening and cracking (β-scission)reaction,or into saturated C10-cycloalkane by excessive hydrogenation reaction (decalin also turns into saturated C10-cycloalkane by isomerization).Subsequently,C10-alkylbenzene turns into BTX by cracking (β-scission)reaction,or into alkyl-cycloalkane by excessive hydrogenation reaction (saturated C10-cycloalkane also turns into alkyl-cycloalkane by cracking reaction).Meanwhile,the BTX turns into alkyl-cycloalkane by excessive hydrocracking reaction.The alkyl-cycloalkane turns into CnH2n+2(gas)by cracking reaction and the pyrene (anthracene or phenanthrene or even to coke)is produced by a series of dehydrogenation reaction of naphthalene.In conclusion,theβ-scission reaction is the key reaction for THN HDC into alkylbenzene or BTX.
Based on Fig.9,the reactants and products of THN HDC reaction can be divided into the following 10 categories:①reactant:THN;② the isomerization products of THN:methylindane or C10-alkylbenzene;③ the cracking (ring-opening)products of methylindane or C10-alkylbenzene:butylbenzene,isopropylbenzene or C9,C10-alkylbenzene;④ light aromatics:benzene,toluene,xylene and ethylbenzene;⑤ the hydrogenation products of light aromatics:C6—C8cycloalkanes;⑥ the products of C6—C8cycloalkanes isomerization:C5—C7cycloalkanes;⑦ the hydrogenation product of THN:decalin;⑧ the dehydrogenation products of THN:naphthalene and poly-aromatic components;⑨ C4—C9alkanes;⑩ gas-phase products:C1—C5alkanes.
In order to evaluate the catalytic activity of catalyst from the conversion of reactant,reactant (THN)was classified as Lump 1.The products of THN isomerization and the cracking (ring-opening)products of methylindane or C10-alkylbenzene were trace in the present work,indicating the fast conversion of these products into BTX,and thus category ② and ③ were classified as Lump 2.In order to evaluate the selectivity and yield of BTX,BTX was classified as Lump 3.The hydrogenation products of BTX and the isomerization products of its hydrogenation product can reflect the ability of excessive hydrogenation reaction for catalyst,which is the key reaction step that directly affects the yield and selectivity of the BTX,then category ⑤ and ⑥were classified as Lump 5.However,the hydrogenation product of THN (decalin)and the dehydrogenation product of THN (naphthalene)reflect the reaction pathway of THN isomerization,and decalin and naphthalene were classified as Lump 4 and Lump 7,respectively.As the naphthalene could condense to poly-aromatic components or even to coke,which reflects the coking rate of catalyst,poly-aromatic components and coke were classified as Lump 8 and Lump 9,respectively.The remaining C4—C9alkanes and gas-phase products were classified as Lump 6.In summary,the THN HDC into BTX was well-represented by the nine-lump reaction network shown in Fig.10.
Base on the nine-lump reaction network of THN HDC reaction (Fig.10),the lump reactions taking part in THN HDC reaction were summarized in Scheme 1.

Scheme 1 Lump reactions occurring in THN HDC reaction
The nine-lump kinetic model of THN HDC reaction,which describes the concentration of reactants and products as a function of space time,was established based on Fig.10 and Scheme 1,and the reaction rate constants were calculated by the following equations.A kinetic model based on the pseudo-first-order dependence on THN has been postulated.The concentration of hydrogen can be considered constant because all of the runs were carried out with a high excess of that reactant (n(H2)/n(THN)=18).
(5)
(6)
(7)
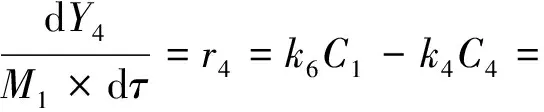

(8)
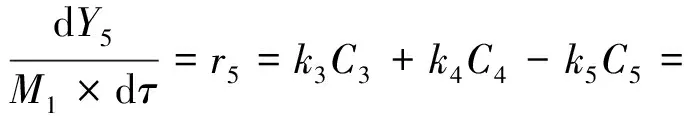
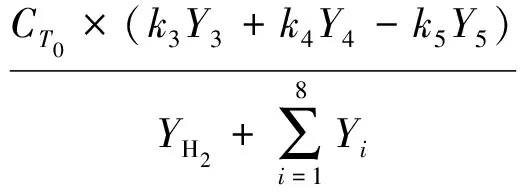
(9)


(10)
(11)
(12)
(13)
The conditions of determining the solution of these difference equations are as follows:
τ=0;Y1=1,Y2=0,Y3=0,Y4=0,Y5=0,Y6=0,Y7=0,Y8=0,YH2=18
Whereki(i=1,2,…,9) is the reaction rate constant for each lump reaction,m3/(g·h);τis the space time,h;M1is the molecular mass of THN,M1=132 g/mol;Yi(i= 1,2,…,8,H2) is defined as the ratio of molar flow rate of lumpior hydrogen to the reactants fed to the reactor;CT0is the concentration of total reactants fed to the reactor,mol/L;Ciis the concentration of lumpi,mol/L;riis the reaction rate of lumpi.
Seven space-times were set to get seven sets of experimental data over each catalyst for evaluation of the catalyst,and these experimental data were used to obtain the nine reaction rate constants for each catalyst through regressing method.As shown in Fig.11,the product distribution predicted by the obtained reaction rate constants are highly consistent with that obtained by experiment,indicating that all obtained reaction rate constants on different catalysts are accurate and reliable (Table 5).
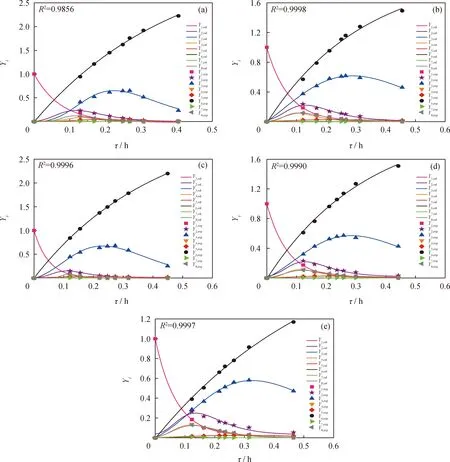
Fig.11 Pseudo-first-order 9-lump kinetic model fitting for THN HDC reaction over different HDC catalysts
Table 5 shows the obtained reaction rate constants of every reaction in the reaction network of THN HDC reaction (Fig.10).Firstly,the hydrogenation (or dehydrogenation)reaction rate constants (k3,k6andk7),which are related to the hydrogenation active sites (MoS2or WS2),are different with the similar loading of active metal.Clearly,the hydrogenation reactivity of NiW/Beta or NiMo/Beta catalysts is higher than that of CoMo/Beta catalyst,which is ascribed to its high content of active metal sulfide species for hydrogenation (Table 4 and Fig.4)in NiW/Beta and NiMo/Beta catalysts and consistent with the reports[9,28-29].On the other hand,the hydrocracking reaction rate constants (k1,k2,k4,andk5)are related to the acidity of HDC catalysts,in which it shows a positive correlation with the total acid site amounts of catalysts (Table 3).
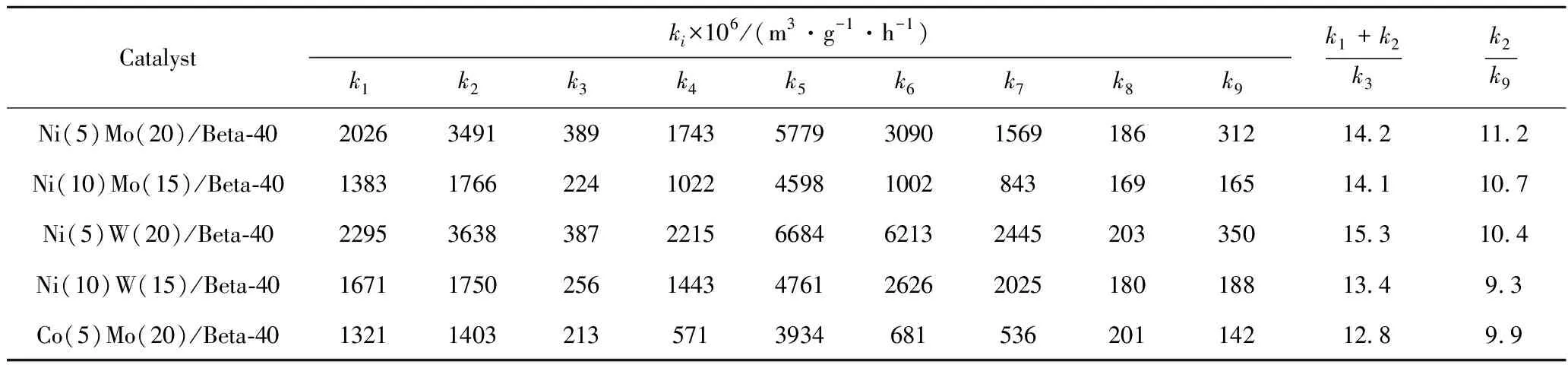
Table 5 Reaction rate constants for THN HDC reaction on different HDC catalysts
For simplicity,the reactions with the reaction rate constant ofkiin the reaction network of THN HDC reaction (Fig.10)are denoted as reactionki.For example,reactionk1and reactionk2represent the isomerization of THN and the hydrocracking of alky aromatics (products of reactionk1)to form BTX,respectively.As is shown in Fig.10,THN undergoes three reaction pathways:①k1,reaction of THN isomerization to form alky aromatics,which could be hydrocracked into BTX through cracking reaction (k2);②k6,reaction of THN hydrogenation to form decalin,which could be further hydrocracked into alkane by-products;③k7,reaction of THN dehydrogenation to form naphthalene,which could be condensed to poly-aromatic components or even to coke.The BTX could be hydrocracked to alkane by-products through secondary hydrogenation reaction (k3).Obviously,reaction pathway ① is the key reaction for forming BTX.A high reaction rate of desired reaction (k1andk2)is required for BTX production.
Here,(k1+k2)/k3represents the ratio of BTX formation reaction rate (k1andk2)to the excessive hydrocracking rate of BTX (k3).Obviously,the higher (k1+k2)/k3,the higher BTX selectivity.Specifically,Table 5 shows that the (k1+k2)of Ni(5)Mo(20)/Beta-40 is 7%,lower than that of Ni(5)W(20)/Beta-40,while itsk3is remains constant,leading to a lower (k1+k2)/k3of Ni(5)Mo(20)/Beta-40 than that of Ni(5)W(20)/Beta-40.Thek3and (k1+k2)of Co(5)Mo(20)/Beta-40 is 45% and 51%,respectively,lower than that of Ni(5)Mo(20)/Beta-40,leading to a lower (k1+k2)/k3of Co(5)Mo(20)/Beta-40 comparing to Ni(5)Mo(20)/Beta-40.As a consequence,the ratio of the isomerization rate of THN (k1)and the hydrocracking rate of alky aromatics (k2)to the excessive hydrocracking reaction rate of BTX (k3)is higher in Ni(5)W(20)/Beta-40 and Ni(5)Mo(20)/Beta-40 than that of Co(5)Mo(20)/Beta-40.These data together explain the high BTX selectivity in Ni(5)W(20)/Beta-40 and Ni(5)Mo(20)/Beta-40 (Fig.6(c)and Fig.6(d)).
k2/k9represents the ratio of BTX formation reaction rate (k2)to the coking rate of catalyst (k9).Specifically,Table 5 shows that thek2andk9of Ni(5)Mo(20)/Beta-40 is 4% and 11%,respectively,lower than that of Ni(5)W(20)/Beta-40,leading to a higherk2/k9of Ni(5)Mo(20)/Beta-40 comparing to Ni(5)W(20)/Beta-40.Thek2andk9of Co(5)Mo(20)/Beta-40 is 61% and 59%,respectively,lower than that of Ni(5)W(20)/Beta-40,leading to a lowerk2/k9of Co(5)Mo(20)/Beta-40 comparing to Ni(5)W(20)/Beta-40.As a consequence,the ratio of the hydrocracking rate of alky aromatics (k2)to the coking rate of catalyst (k9)is higher in Ni(5)W(20)/Beta-40 and Ni(5)Mo(20)/Beta-40 than that of Co(5)Mo(20)/Beta-40.This suggests a high activity of Ni(5)W(20)/Beta-40 and Ni(5)Mo(20)/Beta-40 (Fig.6 (a) and 6 (b)).
In conclusion,although the coking rate (k9)of Ni(5)W(20)/Beta-40 and Ni(5)Mo(20)/Beta-40 is high,the ratio of the isomerization rate of THN (k1)and the hydrocracking rate of alky aromatics (k2)to the excessive hydrocracking reaction rate of BTX (k3),and the ratio of the hydrocracking rate of alky aromatics (k2)to the coking rate of catalyst (k9)are higher than that of Co(5)Mo(20)/Beta-40.This indicates that the BTX selectivity and catalytic activity are high in Ni(5)W(20)/Beta-40 and Ni(5)Mo(20)/Beta-40.
3 Conclusion
Here,a nine-lump reaction kinetic model for the THN HDC to BTX reaction was developed and discussed based on the gas and liquid product distribution.The reaction network is consisted of multiple parallel and tandem reactions,mainly involving the isomerization reaction of THN,the excessive hydrogenation reaction of THN,the dehydrogenation reaction of THN,the hydrocracking reaction of alky aromatics,the hydrogenation reaction of BTX,the cracking reaction of cycloalkane and a series of dehydrogenation reactions of naphthalene.In addition,the ratio of BTX formation reaction rate to the excessive hydrocracking rate of BTX and the ratio of BTX formation reaction rate to the coking rate of catalyst were proposed as parameters to evaluate the performance of HDC catalysts.The kinetic model has been proven to be effective strategy to explain the difference of NiMo,CoMo and NiW based sulfide catalyst.The high BTX selectivity in Ni(5)W(20)/Beta-40 and Ni(5)Mo(20)/Beta-40 is obtained due to the higher ratio of BTX formation reaction rate to the excessive hydrocracking rate of BTX and the higher ratio of BTX formation reaction rate to the coking rate of catalyst than that of Co(5)Mo(20)/Beta-40.

