外源γ-氨基丁酸对蛇龙珠葡萄叶片碳氮代谢的影响
王春恒 韩爱民 张立梅 李斗 王宇航 金鑫 冯丽丹 杨江山


摘 要:【目的】碳氮代謝是植物体内重要的生理过程,探究γ-氨基丁酸(GABA)对葡萄碳氮代谢的影响,并筛选最适调控浓度。【方法】以10年生酿酒葡萄蛇龙珠为试材,于开花期、坐果期、膨大期、转色期对长势一致的植株进行叶面喷施,研究了不同浓度γ-氨基丁酸5、10、15、20 mmol·L-1处理对叶片碳、氮代谢及其关键酶活性的影响。【结果】与对照相比,喷施外源GABA提高了葡萄叶片碳代谢相关蔗糖合酶(SuSy)、蔗糖磷酸合成酶(SPS)、酸性转化酶(AI)和中性转化酶(NI)活性,增加淀粉、可溶性糖、果糖、葡萄糖和蔗糖含量;GABA处理也增强了氮代谢相关硝酸还原酶(NR)、谷氨酰胺合成酶(GS)、谷氨酸合成酶(GOGAT)、谷氨酸脱氢酶(GDH)、谷氨酸草酰乙酸转氨酶(GOT)、谷氨酸丙酮酸转氨酶(GPT)活性,增加硝态氮(NO3-·N)含量,增加了内源GABA含量。【结论】外源GABA增加葡萄叶片内源GABA含量,从而增强碳代谢和氮代谢相关酶活性,增加淀粉、可溶性糖的积累,促进硝态氮(NO3-·N)吸收和铵态氮(NH4+·N)的转化,其中以10 mmol·L-1外源γ-氨基丁酸效果最佳。
关键词:葡萄;γ-氨基丁酸;碳代谢;氮代谢
中图分类号:S663.1 文献标志码:A 文章编号:1009-9980(2023)07-1386-13
Effects of γ-aminobutyric acid on carbon and nitrogen metabolism in leaves of Cabernet Gernischt grape
WANG Chunheng1, HAN Aimin1, ZHANG Limei1, LI Dou1, WANG Yuhang1, JIN Xin1, FENG Lidan2, YANG Jiangshan1*
(1College of Horticulture, Gansu Agricultural University, Lanzhou 730070, Gansu, China; 2Gansu Wine Industry Technology Research and Development Center, Lanzhou 730070, Gansu, China)
Abstract: 【Objective】 Carbon metabolism and nitrogen metabolism are the most basic and important physiological processes in plant. The ability of carbon and nitrogen metabolism can directly affect the quality and yield of crops. It has been shown that regulating carbon and nitrogen metabolism is an important measure to improve plant yield and quality. The aim of this study was to investigate the effect of γ-aminobutyric acid (GABA) on carbon and nitrogen metabolism in grape and to screen the optimal regulation concentration. 【Methods】 Using 10-year-old wine grape Cabernet Gernischt as test material, the effects of different concentrations of γ-aminobutyric acid (GABA) on the carbon and nitrogen metabolism and key enzyme activities in the leaves of grape were studied. A total of 4 GABA concentration treatments were set up: 5 mmol·L-1 (T1), 10 mmol·L-1 (T2), 15 mmol·L-1 (T3), 20 mmol·L-1 (T4), and distilled water treatment was used as the control (CK). The leaves of grape plants with the same growth and without diseases and pests were sprayed at the flowering stage, fruit setting stage, fruit expansion stage and fruit color conversion stage. Each treatment had 3 replicates and each replicate had 5 plants. The amount of spray was controlled by the appearance of initial drip from the leaves. The leaf sampling time was 8:00 am on the third day after treatment, and the leaves were sampled again at mature stage. After freezing in liquid nitrogen, the samples were placed in an ultra-low temperature refrigerator at -80 ℃ for later use. 【Results】Compared with CK, the exogenous GABA treatments increased the activities of sucrose synthase (SuSy), sucrose phosphate synthase (SPS), acid invertase (AI) and neutral invertase (NI) related to the carbon metabolism in the grape leaves, and increased the contents of starch, soluble sugar, fructose, glucose and sucrose. The GABA treatments also enhanced the activities of nitrate reductase (NR), glutamine synthetase (GS), glutamate synthase (GOGAT), glutamate dehydrogenase (GDH), glutamate oxaloacetate transaminase (GOT) and glutamate pyruvate transaminase (GPT), and also increased the content of nitrate nitrogen (NO3-·N) and the endogenous GABA content. The exogenous GABA treatments significantly increased the contents of starch, soluble sugar, NO3-·N and the activities of SuSy-s, NR, GS, GDH, GOT and GPT in the leaves of Cabernet Gernischt during the growth period, and significantly increased the contents of fructose, glucose, sucrose, NH4+·N and the activities of SuSy-c, SPS, AI, NI and GOGAT, and also increased the content of endogenous GABA. Specifically, the endogenous GABA content in the grape leaves increased significantly at flowering stage, fruit setting stage, veraison stage and maturity stage after the exogenous 10 mmol·L-1 GABA treatment. The starch content in the leaves increased significantly in the growth period, the fructose content increased significantly at the fruit setting stage, the expanding stage and the mature stage, the glucose content increased significantly at the expanding stage and the turning stage, the sucrose content increased significantly at the expanding stage and the mature stage, and the total soluble sugar increased significantly from the flowering stage to the mature stage. The activity of carbon metabolism-related enzyme AI increased significantly at the expansion stage, the turning stage and the mature stage, and the activity of NI increased significantly at the fruit setting stage and the mature stage. At the same time, the GABA treatments significantly increased the SPS activity at the fruit setting stage, the expansion stage and the mature stage, respectively. The activity of SuSy-s increased significantly at the fruit setting stage, the turning stage and the mature stage, and the activity of SuSy-c increased significantly at the expansion stage, the turning stage and the mature stage. Similarly, after the exogenous GABA treatments, the content of NO3-·N in grape leaves increased significantly from flowering stage to maturity stage, and maintained a higher level of NO3-·N at the late stage of grape growth compared with the control, while the content of NH4+·N had no significant difference with those of the CK except for that at maturity stage. The activity of GOGAT, GS and NR increased significantly from fruit setting stage to maturity stage, the activity of GDH increased significantly from flowering stage to maturity stage, and the activity of GOT and GPT increased significantly except for maturity stage. 【Conclusion】 During the growth period, the GABA treatments increased the carbon metabolism and nitrogen metabolism related substances and enzyme activities in the grape leaves. It was speculated that the exogenous GABA treatments increased the carbon and nitrogen metabolism activity of the grape leaves, and affected the carbon metabolism of the leaves as a nitrogen source, which strengthened the relationship between nitrogen metabolism and carbon metabolism to some extent. The exogenous GABA increased the endogenous GABA in the grape leaves, thereby enhanced the activities of enzymes related to carbon metabolism and nitrogen metabolism, increased the accumulation of starch and soluble sugar, and promoted the absorption of nitrate nitrogen (NO3-·N) and the transformation of ammonium nitrogen (NH4+·N). 10 mmol·L-1 exogenous GABA had the best effect.
Key words: Grape; γ-aminobutyric acid; Carbon metabolism; Nitrogen metabolism
碳代谢和氮代谢是植物生命活动中最基础的代谢和重要生理过程[1],植物碳氮代谢能力直接影响作物的品质和产量[2]。为提高作物碳氮代谢能力,降低干旱、盐碱、低温、水涝、病虫害等不利条件对产量和品质的影响,以及在正常生长条件下增强植物碳氮代谢能力,前人已做大量研究。研究表明,在盐胁迫条件下,可通过褪黑素提高水稻幼苗碳、氮代谢能力来提升抗性抵御环境胁迫,恢复生长力[3]。吕腾飞等[4]利用缓释氮肥与尿素配施显著提高杂交籼稻幼穗和剑叶细胞碳、氮代谢关键酶活性,进一步提高杂交稻产量。李健忠等[5]通过打顶后喷施油菜素内酯和生长素增强烟草碳氮代谢从而提高烟叶质量和产量。金正勋等[6]对水稻喷施6-BA和ABA,调控籽粒碳氮代谢相关酶基因表达从而提高稻米品质。因此,调控碳氮代谢是提高植物产量和品质的重要措施。
γ-氨基丁酸(GABA)是动植物体内一种天然存在的非蛋白组成氨基酸,是一种重要的抑制性神经递质,多数研究侧重于医药研究,能有效抑制谷氨酸的脱羧反应,提高葡萄糖磷酸酯酶的活性,具有镇静、催眠、抗惊厥、降血压的生理作用;在植物界的研究中发现,GABA是植物生长所必需的,它与碳和氮代谢密切相关,是桥接碳和氮代谢的关键因素[7-8]。植物细胞遭受胁迫时,可诱导体内谷氨酸脱羧酶(GAD)催化谷氨酸脱羧产生高于正常水平的GABA,同时消耗H+,因此认为GABA的生成可维持细胞pH的稳定[9]。王泳超[10]和于立尧[11]的研究证明适量的外源GABA能恢复盐胁迫和干旱条件下玉米和甜瓜幼苗的株高、根系发育以及各种生长指标,对植物形态建成具有调控作用。闫妮等[12]利用GABA浸种提高了番茄出苗率以及促进植株生长缓解盐胁迫;适度喷施GABA可恢复NaCl胁迫下西伯利亚白刺叶肉细胞光合活性[13];降低高温、干旱胁迫下高羊茅叶绿素含量下降速度[14];增强硝酸钙胁迫下甜瓜幼苗对NO3-·N的同化能力[15]。目前,通过施用含氮肥料来提高作物产量和品质一直是绿色革命的一个重要因素[16],然而,在生态方面,过度施用化肥会造成灾难性的影响,例如富营养化[17]。更好地了解植物氮代谢对于提高植物产量和减少肥料过度使用至关重要[18]。GABA可作为氮源被植株直接吸收[19],且外源GABA在葡萄生长发育过程中对叶片碳氮代谢的影响鲜有报道。笔者在本试验中通过对葡萄叶面GABA的喷施处理,探究外源GABA对葡萄碳氮代谢的影响,以期为葡萄生产施用γ-氨基丁酸类肥料提供理论依据和技术参数。
1 材料和方法
1.1 植物材料和试验地概况
试验以10年生酿酒葡萄蛇龙珠(Cabernet Gernischt)为试材,对葡萄叶片进行不同浓度GABA处理,于2021年4—11月在甘肃农业大学葡萄园进行,行株距0.75 m×1.5 m,单干双臂Y形整形,南北走向。
1.2 试验设计
试验共设4个GABA浓度处理:5 mmol·L-1(T1)、10 mmol·L-1(T2)、15 mmol·L-1(T3)、20 mmol·L-1(T4),以蒸馏水处理为对照,于开花期、坐果期、膨大期、转色期对长势一致、无病虫害的植株进行叶面喷施,以叶片开始滴液为准,每个处理设3个重复,每个重复5株。叶片采样时间为处理后第3天上午8:00,成熟期再取样1次,液氮冷冻后放入-80 ℃超低温冰箱备用。
1.3 测定项目与方法
1.3.1 叶片碳代谢指标测定 可溶性总糖含量采用蒽酮-硫酸法测定[20],使用高效液相色谱仪(美国Waters Acquity Arc)测定蔗糖、葡萄糖和果糖含量,参照贺雅娟等[21]的方法,色谱条件:XBridge BEH Amide色谱柱(4.6 mm×150.0 mm、2.5 μm),柱温40 ℃,流动相为75%乙腈、0.2%乙胺以及24.8%超纯水,流速0.8 mL·min-1,进样量 20 μL,检测波长为254 nm。
蔗糖合酶合成方向(SuSy-s)、蔗糖合酶分解方向(SuSy-c)、蔗糖磷酸合成酶(SPS)、酸性转化酶(AI)、中性转化酶(NI)酶液提取参考张弦[22]的方法,酶活性测定参考潘俨[23]的方法。
1.3.2 叶片氮代谢指标测定 硝态氮含量通过水杨酸-硫酸溶液比色法测定[24]。铵态氮含量采用靛酚蓝-分光光度法测定[25]。硝酸还原酶(NR)活性采用磺胺比色法测定[26]。谷氨酰胺合成酶(GS)活性采用FeCl3络合显色比色法测定[27]。谷氨酸合成酶(GOGAT)活性参照赵鹏等[28]的方法测定。谷氨酸脱氢酶(GDH)活性参考王小纯等[29]的方法测定。谷氨酸草酰乙酸转氨酶(GOT)和谷氨酸丙酮酸转氨酶(GPT)活性参考吴良欢等[30]的方法测定。
1.3.3 数据分析 用 Excel 2016进行数据处理及作图,用SPSS 23.0对数据进行统计分析。
2 结果与分析
2.1 GABA对蛇龙珠葡萄叶片碳代谢的影响
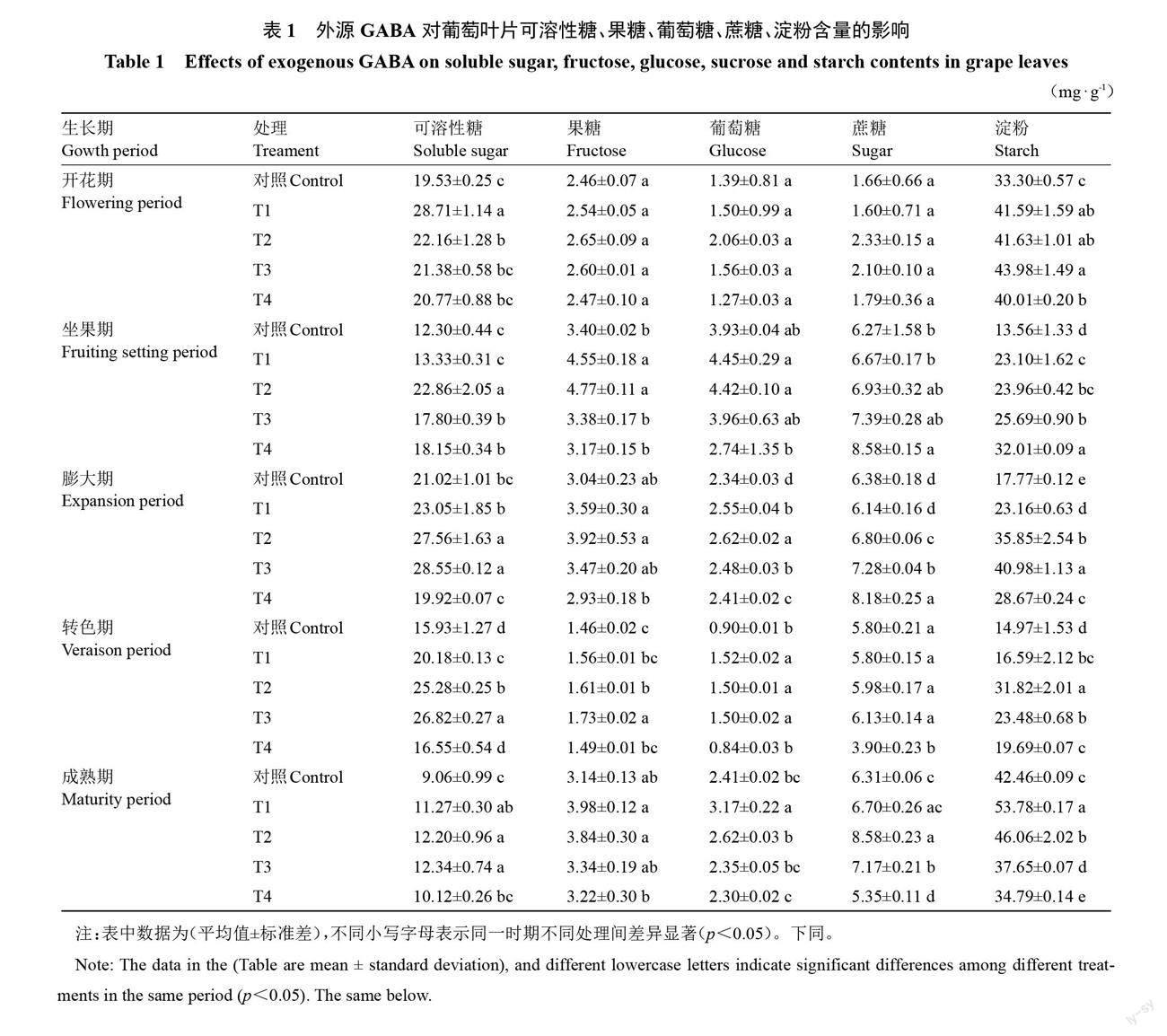
2.1.1 GABA对叶片可溶性糖、果糖、葡萄糖、蔗糖以及淀粉含量的影响 不同浓度GABA对蛇龙珠葡萄叶片可溶性糖、果糖、葡萄糖、蔗糖和淀粉含量的影响如表1所示,生育期内叶片可溶性糖含量呈“下降-上升-下降”的变化趋势,果糖、葡萄糖和蔗糖含量均呈先上升的波动变化趋势,淀粉含量呈先降后升的趋势。与对照相比,GABA处理于坐果期、膨大期、转色期明显提高可溶性糖含量;于开花期至成熟期提高果糖、葡萄糖和蔗糖含量;开花期、坐果期、膨大期、转色期明显增加淀粉含量,并随GABA浓度增大均呈现出先升后降的变化趋势。在不同浓度GABA处理中,可溶性糖含量在T2、T3处理后各时期较对照显著增加(p<0.05),T2处理提升效果最佳,开花期至成熟期较对照提高了13.48%、85.94%、31.09%、58.72%、34.65%。T1、T2处理使果糖含量于坐果期、膨大期和成熟期显著增加(p<0.05)及葡萄糖含量在膨大期、转色期显著增加(p<0.05),而T2和T3处理使蔗糖含量在膨大期、成熟期顯著增加(p<0.05)。淀粉含量在T3、T4处理后于开花期至转色期提升效果较好,在成熟期反而显著降低其含量(p<0.05),而T1、T2处理使其含量各时期显著增加(p<0.05),T2处理提高效果最佳,开花期至成熟期分别较对照提高25.00%、76.63%、101.74%、112.6%、8.47%。
2.1.2 GABA对叶片蔗糖合酶(SuSy)和蔗糖磷酸合成酶(SPS)活性的影响 不同浓度GABA对葡萄叶片蔗糖合酶合成方向(SuSy-s)活性影响如图1-A所示,随着果实的生长发育,叶片SuSy-s活性呈“上升-下降-上升”的变化趋势。与对照相比,GABA处理明显增强坐果期、膨大期、成熟期SuSy-s活性,尤其在成熟期急剧增强,其中,T4处理增强效果最佳,分别较对照提高了40.95%、95.50%、138.16%(p<0.05);开花期、转色期T1处理显著提高其活性(p<0.05),较对照提升33.33%、56.07%,而T4处理降低了其活性。
生育期叶片SPS活性逐渐升高(图1-B)。与对照相比,GABA处理后SPS活性于坐果期、膨大期和成熟期明显增强。T3、T4处理于坐果期、膨大期提升效果较好,在转色期、成熟期降低其活性。而T2处理于坐果期、膨大期、成熟期显著增强其活性(p<0.05),较对照提升21.57%、30.44%、15.82%。
叶片蔗糖合酶分解方向(SuSy-c)活性呈逐渐上升趋势(图1-C)。与对照相比,GABA处理使SuSy-c活性于坐果期、膨大期、转色期、成熟期明显增强;其中,坐果期、成熟期T4处理提升SuSy-c活性效果突出,于膨大期、转色期、成熟期T2、T3处理使其活性显著上升(p<0.05),T2处理增强效果最佳,较对照提升86.63%、32.27%、35.72%。
2.1.3 GABA对叶片酸性转化酶(AI)、中性转化酶(NI)活性的影响 不同浓度GABA对葡萄叶片AI活性影响如图1-D所示,AI活性总体呈逐渐上升趋势。与对照相比,GABA处理使AI活性在膨大期、转色期、成熟期明显增强,尤其在成熟期T2、T3、T4处理使其活性急剧增强;其中,T4处理于坐果期、膨大期、成熟期明显增强AI活性,但在转色期显著降低其活性(p<0.05);而T2处理使AI活性在膨大期和成熟期显著增强(p<0.05);T3处理使其活性在膨大期、转色期、成熟期显著增强(p<0.05),较对照提升94.19%,20.21%,95.90%。
叶片NI活性呈先上升的波动变化趋势(图1-E)。与对照相比,GABA处理使NI活性于坐果期、膨大期、转色期明显增强及成熟期急剧增强,并随GABA浓度增加呈先升后降的变化趋势;其中,NI活性在T3、T4处理后,成熟期显著升高、膨大期和转色期反而明显降低;而T1处理于坐果期、膨大期、转色期、成熟期提升效果显著(p<0.05),较对照提升40.61%、14.84%、39.10%、55.20%。
2.2 GABA对蛇龙珠葡萄叶片氮代谢的影响
2.2.1 GABA对叶片硝态氮(NO3-·N)和铵态氮(NH4+·N)及GABA含量的影响 不同浓度GABA对葡萄叶片NO3-·N含量影响如图2-A所示,NO3-·N含量在生育期内呈先升后降的变化趋势,与对照相比,GABA处理使其含量在转色期、成熟期上升并趋于稳定,并随GABA处理浓度增加呈先升后降的变化趋势,其中,T2、T3处理明显提升其含量,T2处理显著提升NO3-·N含量(p<0.05),开花期至成熟期分别较对照提升51.80%、17.75%、5.86%、64.97%、89.53%。
叶片NH4+·N含量呈先升后降的变化趋势(图2-B),与对照相比,GABA处理后NH4+·N含量于膨大期、转色期、成熟期明显上升,其中,T4处理提升效果显著(p<0.05),分别较对照升高16.31%、14.42%、18.10%;而在成熟期T2、T3处理显著提升NH4+·N含量(p<0.05),其他时期T1、T2、T3处理与对照基本无显著差异。
不同浓度GABA对葡萄叶片内源GABA含量的影响如图2-C所示,内源GABA含量从开花期至成熟期呈逐渐上升的变化趋势。与对照相比,外源GABA处理使得叶片内源GABA含量在开花期、坐果期、转色期、成熟期增加,尤其成熟期T2、T3处理明显增加其含量,并随外源GABA浓度增加其含量呈先升后降的变化趋势,其中,T2处理显著增加其含量(p<0.05),开花期、坐果期、转色期、成熟期分别较对照提高73.55%、10.46%、18.41%、30.91%。
2.2.2 GABA对叶片硝酸还原酶(NR)活性的影响 不同浓度GABA对葡萄叶片NR活性的影响如图3-A所示,NR活性呈先升后降的变化趋势。与对照相比,GABA处理在各时期明显增强NR活性,并随GABA处理浓度增加呈先升后降的变化趋势,其中,坐果期T1、T2、T3处理急剧增强其活性。T2处理NR活性顯著增强(p<0.05),开花期至成熟期分别较对照提高了58.93%、57.30%、72.43%、38.36%、63.83%。
2.2.3 GABA对叶片谷氨酰胺合成酶(GS)、谷氨酸合成酶(GOGAT)以及谷氨酸脱氢酶(GDH)活性的影响 不同浓度GABA对葡萄叶片GS活性的影响如图3-B所示,GS活性总体呈逐渐增强的趋势。与对照相比,GABA处理在各物候期均提高GS活性,并随GABA浓度增加其活性呈先升后降的变化趋势,其中,除膨大期T3、T4处理急剧增强GS活性外,其他时期T2处理增强效果最佳,GS活性显著增强(p<0.05),开花期至成熟期分别较对照升高53.40%、28.72%、25.85%、25.18%、15.05%。
叶片GOGAT(图3-C)和GDH活性(图3-D)均呈先升后降的变化趋势,GOGAT和GDH活性随GABA浓度增加均呈先升后降的变化趋势,GOGAT活性除开花期外其他时期明显增强,GDH活性在各时期均明显增强。其中,T2、T3处理提升二者活性效果显著(p<0.05),GOGAT活性于坐果期至成熟期T2处理较对照升高46.78%、33.41%、86.55%、58.53%,以及T3处理较对照提升48.53%、65.54%、103.11%、46.92%;GDH活性于开花期至成熟期T2处理较对照升高12.63%、46.78%、33.41%、86.55%、58.53及T3处理较对照提高5.96%、48.53%、65.53%、103.11%、46.92%。
2.2.4 GABA对叶片谷氨酸草酰乙酸转氨酶(GOT)和谷氨酸丙酮酸转氨酶(GPT)活性的影响 不同浓度GABA对葡萄叶片GOT和GPT活性影响如图3-E和图3-F所示,GOT和GPT活性均呈先升后降的变化趋势。与对照相比,GABA处理在各时期明显增强二者活性,并随GABA浓度增加二者活性呈先升后降的趋势;其中,在成熟期T1、T2处理使GOT和GPT活性显著增强(p<0.05),于开花期、坐果期、膨大期和转色期T2、T3处理显著增强二者活性(p<0.05),T2处理提升效果最优,GOT活性于开花期至成熟期T2处理较对照升高47.75%、31.84%、10.89%、16.83%、8.01%,GPT活性在开花期至成熟期较对照升高63.10%、11.80%、16.22%、48.28%、5.56%。
3 讨 论
植物生长过程中碳氮代谢在植株体内的动态变化直接影响着光合产物的合成、转化以及矿质营养的吸收、蛋白质的合成等[1],氮代谢为碳代谢提供酶和光合色素,碳代谢为氮代谢提供碳源和能量,且二者需要共同的还原力和ATP、碳骨架等[31]。植物碳代谢产物主要是淀粉、可溶性糖、蔗糖、葡萄糖、果糖等,SPS、SuSy、AI、NI等酶参与碳代谢物质的形成和转化[32]。韩丽娜等[33]对葡萄的试验研究中,通过控制施氮量增强碳代谢关键酶SPS、SS活性,进一步促进蔗糖、葡萄糖和果糖的积累,从而提高葡萄产量和品质。植物氮代谢主要包括NO3-·N、NH4+·N的合成和固定,NR、GOGAT、GS、GDH、GOT、GPT等酶参与二者的吸收及固定[34]。吴薇等[35]的研究表明,通过控制施氮量增强NR活性和氮代谢物含量从而促进烤烟生长发育提高品质。高松等[36]的研究通过增加大葱叶片蔗糖、还原糖、NO3-·N、NH4+·N含量和提高SPS、SS、NR、GS、GDH、GOGAT等的活性,增强大葱生长力。因此,研究植物碳氮代谢规律,探索有效调控途径和方法,对提高作物产量和品质具有重要的作用[5,9,34]。笔者课题组在GABA对果实品质影响的研究结果中显示,GABA处理显著提高了果实可溶性糖、蔗糖、果糖、葡萄糖和有机酸含量[37],改善了果实品质。本研究中外源GABA处理在生育期显著增加了蛇龙珠葡萄叶片淀粉、可溶性糖、NO3-·N含量及提高SuSy-s、NR、GS、GDH、GOT、GPT活性,明显增加了果糖、葡萄糖、蔗糖、NH4+·N含量及提高SuSy-c、SPS、AI、NI、GOGAT活性,增加了内源GABA含量,与对照相比,在各处理中10 mmol·L-1 GABA提升效果最佳。
试验研究表明,外源10 mmol·L-1 GABA处理在生育期显著增加了蛇龙珠葡萄叶片淀粉含量,与宋锁玲[34]的研究结论相似,GABA增加了甜瓜幼苗叶片淀粉含量。Chen等[38]研究发现GABA显著增加杨树茎中蔗糖、果糖和非结构性碳水化合物含量。GABA喷施后果糖含量于坐果期、膨大期和成熟期显著增加,葡萄糖含量在膨大期和转色期显著提升,蔗糖含量在膨大期和成熟期显著增加,可溶性糖总量于开花期至成熟期显著增强。GABA处理使AI活性在膨大期、转色期和成熟期显著增强,NI活性于坐果期和成熟期显著增加。同时GABA处理使得SPS活性分别于坐果期、膨大期和成熟期显著增强,SuSy-s活性于坐果期、转色期和成熟期显著增强,SuSy-c活性在膨大期、转色期和成熟期显著增强,与刘金平[39]的研究结果一致,GABA增强了不结球白菜幼苗SPS、SuSy活性。GABA通过提高GAD、GABA-T活性和CmGAD基因表达量,促进GABA的生物合成[40-41],外源GABA处理后开花期、坐果期、转色期和成熟期葡萄叶片内源GABA含量显著上升。研究表明,给杨树幼苗施用GABA后树体茎中蔗糖含量增加并伴随蔗糖代谢相关基因SUS和SPS表达上调[38,42]。在植物碳代谢中,SPS活性强弱直接影响植株体内蔗糖和淀粉的分配,其活性越低蔗糖积累越少[32];SuSy在植株体内对蔗糖的转化方向存在催化合成(SuSy-s)和催化分解(SuSy-c),两个催化方向的转换与自身是否被磷酸化有关[43],通常SuSy被认为主要起分解蔗糖作用,也有研究者认为其在光合器官中具有较强的催化蔗糖合成能力,还有研究者提出SuSy的作用在不同植物中存在较大差异[44]。表明外源GABA处理增加葡萄叶片蔗糖含量与SPS、SuSy活性的增强有关。蔗糖在叶片内的贮存和转化还与AI、NI活性变化相关,可被AI、NI不可逆分解为果糖和葡萄糖[45]。蔗糖可作为信号分子调控基因的表达,从而影响酶催化活性[46]。推测蔗糖含量变化影响了蔗糖催化分解反应活性,增强AI、NI活性促进果糖、葡萄糖的生成。外源GABA也可提高正常条件和低温条件下番茄叶片叶绿素含量,提高抗氧化酶活性和叶片净光合速率,从而增加可溶性糖、还原糖及非还原糖含量[47]。推断淀粉、可溶性糖含量的增加也与GABA影响叶片光合作用相关。表明外源GABA增加蛇龙珠葡萄叶片内源GABA含量,进一步影响碳代谢相关酶活性,从而调控淀粉、可溶性糖含量增加。
本试验结果表明,外源10 mmol·L-1 GABA处理在生育期使得蛇龙珠葡萄叶片NO3-·N含量在开花期至成熟期显著增加,且在葡萄生长后期与对照相比保持较高NO3-·N水平,与任文奇[48]的研究结果一致,GABA增加了甜瓜幼苗叶片NO3-·N含量。而GABA处理后叶片NH4+·N含量除成熟期外其他时期与对照无显著差异,可能与GS/GOGAT途径活性和GDH活性增强影响NH4+转化有关。劉金平[39]和弓瑞娟[9]的研究发现,GABA增强了不结球白菜和生菜GS、GOGAT和NR活性。本试验结果显示,GABA处理使得GOGAT活性于坐果期至成熟期显著增强,GS和NR活性在开花期至成熟期均显著增强。GABA处理使GDH活性在开花期至成熟期显著增强,与燕博文[49]的研究结论相似,GABA增强了玉米幼苗GDH活性。GABA处理后GOT、GPT活性除成熟期外其他时期显著增强,与谷海涛等[50]的研究结果一致,GABA增强了粳稻叶片GOT、GPT活性。研究表明,外源GABA使生菜叶片硝酸还原酶基因表达上调,从而显著增强NR活性[51]。GABA可有效促进甜瓜根系对NO3-·N的吸收及其向地上部分的运输[15]。表明GABA处理增加葡萄叶片NO3-·N的积累与NR活性增强有关。植株吸收NO3-被NR还原为NH4+,NH4+在植物体内必须及时被同化来消除其对细胞的毒性,再通过主要途径GS/GOGAT循环催化下进一步被固定为酰胺态氮[15]。前人研究发现,GABA也可诱导植株叶片中GS2/GOGAT基因表达上调进一步增强GS/GOGAT途径活性从而提升氮的通量[52]。推测内源GABA含量的增加影响葡萄叶片氮代谢关键酶活性,促进NO3-·N的吸收及向NH4+·N的转化固定。GDH是除GS/GOGAT途径以外的另一种NH4+同化途径,GDH既能催化NH4+与a-酮戊二酸合成谷氨酸,又能催化谷氨酸氧化脱氨释放出NH4+,在氮代谢中起着重要的作用[53]。葡萄叶片GDH活性增强加速了NH4+的转化,同时促进谷氨酸氧化脱氨释放出NH4+。而GOT和GPT活性增强会消耗GDH催化NH4+反应的合成物谷氨酸,可催化谷氨酸与其他底物的反应生成天冬氨酸和丙氨酸[54]。使NH4+的消耗大于累积,与本试验中叶片NH4+·N含量变化不显著相契合,内源GABA含量的增加进一步促进NH4+·N的转化。
在生育期内,GABA处理不同程度提高了葡萄叶片碳代谢和氮代谢有关物质的含量和酶的活性。研究发现,GABA参与氮的储存与运输,其代谢途径被认为能够调节碳、氮营养平衡,维持植物正常的生长和发育[19]。氮素是植物必需的元素,给小麦施氮增强旗叶SS活性、蔗糖和淀粉含量[55]。拟南芥在GABA作为唯一氮源的培养基上可正常生长[56]。推测外源GABA处理激发了葡萄叶片碳、氮代谢活力,同时作为氮源影响叶片碳代谢,在一定程度加强了氮代谢与碳代谢的联系。
4 结 论
综上所述,10 mmol·L-1外源GABA增加葡萄叶片内源GABA含量,从而诱导碳、氮代谢相关酶SPS、SuSy、AI、NI、NR、GS、GOGAT、GDH、GOT、GPT活性增强,增加淀粉、可溶性糖、蔗糖、果糖、葡萄糖的积累和促进了氮素的吸收转化,提升葡萄叶片碳氮代谢水平,促进植株生长。
参考文献 References:
[1] 宁宇,邓惠惠,李清明,米庆华,韩宾,艾希珍. 红蓝光质对芹菜碳氮代谢及其关键酶活性的影响[J]. 植物生理学报,2015,51(1):112-118.
NING Yu,DENG Huihui,LI Qingming,MI Qinghua,HAN Bin,AI Xizhen. Effects of red and blue light quality on the metabolites and key enzyme activities of carbon-nitrogen metabolism in celery[J]. Plant Physiology Journal,2015,51(1):112-118.
[2] GANGWAR S,SINGH V P. Indole acetic acid differently changes growth and nitrogen metabolism in Pisum sativum L. seedlings under chromium (Ⅵ) phytotoxicity:Implication of oxidative stress[J]. Scientia Horticulturae,2011,129(2):321-328.
[3] 陳丽珊,周红艳,林伟伟. 外源褪黑素对盐胁迫下水稻苗期碳氮代谢的影响[J/OL]. 生态学杂志,2022:1-11. http://kns.cnki.net/kcms/detail/21.1148.Q.20220917.1053.004.html.
CHEN Lishan,ZHOU Yanhong,LIN Weiwei. Effects of exogenous melatonin on carbon and nitrogen metabolism of rice seedlings under salt stress[J/OL]. Chinese Journal of Ecology,2022:1-11. http://kns.cnki.net/kcms/detail/21.1148.Q.20220917.1053.004.html.
[4] 吕腾飞,谌洁,代邹,马鹏,杨志远,郑传刚,马均. 缓释氮肥与尿素配施对机插杂交籼稻碳氮积累的影响[J]. 作物学报,2021,47(10):1966-1977.
L? Tengfei,SHEN Jie,DAI Zou,MA Peng,YANG Zhiyuan,ZHENG Chuangang,MA Jun. Effects of combined application of slow release nitrogen fertilizer and urea on carbon and nitrogen accumulation in mechanical transplanted hybrid rice[J]. Acta Agronomica Sinica,2021,47(10):1966-1977.
[5] 李健忠,薛立新,朱金峰,许自成,许仪,金磊,郝浩浩,苏谦. 打顶后喷施油菜素内酯和生长素对烤烟田间生长、碳氮代谢及烟叶品质的影响[J]. 中国生态农业学报,2015,23(11):1404-1412.
LI Jianzhong,XUE Lixin,ZHU Jinfeng,XU Zicheng,XU Yi,JIN Lei,HAO Haohao,SU Qian. Effects of brassinolide and auxin on growth,carbon and nitrogen metabolism and tobacco quality of flue-cured tobacco leaves after topping[J]. Chinese Journal of Eco-Agriculture,2015,23(11):1404-1412.
[6] 金正勋,王思宇,王珊,王剑,张忠臣,李钢夑,朴钟泽. 外源激素对水稻籽粒碳氮代谢相关酶基因表达影响[J]. 东北农业大学学报,2020,51(7):1-9.
JIN Zhengxun,WANG Siyu,WANG Shan,WANG Jian,ZHANG Zhongchen,LEE Gangseob,PIAO Zhongze. Effect of exogenous hormones on gene expression of carbon and nitrogen metabolism-related enzymes in rice grains[J]. Journal of Northeast Agricultural University,2020,51(7):1-9.
[7] BATUSHANSKY A,KIRMA M,GRILLICH N,TOUBIANA D,PHAM P A,BALBO I,FROMM H,GALILI G,FERNIE A R,FAIT A. Combined transcriptomics and metabolomics of Arabidopsis thaliana seedlings exposed to exogenous GABA suggest its role in plants is predominantly metabolic[J]. Molecular Plant,2014,7(6):1065-1068.
[8] FAIT A,FROMM H,WALTER D,GALILI G,FERNIE A R. Highway or byway:The metabolic role of the GABA shunt in plants[J]. Trends in Plant Science,2008,13(1):14-19.
[9] 弓瑞娟. γ-氨基丁酸对生菜氮代谢及营养品质的影响[D]. 保定:河北农业大学,2012.
GONG Ruijuan. Effect of exogenous aminobutyric acid on nitrogen metabolism and nutrition quality of lettuce[D]. Baoding:Hebei Agricultural University,2012.
[10] 王泳超. γ-氨基丁酸(GABA)调控盐胁迫下玉米种子萌发和幼苗生长的机制[D]. 哈尔滨:东北农业大学,2016.
WANG Yongchao. Mechanism of aminobutyric acid (GABA) regulating maize seed germination and seedling growth under salt stress[D]. Harbin:Northeast Agricultural University,2016.
[11] 于立尧. 外源γ-氨基丁酸对甜瓜幼苗生长、抗干旱胁迫的影响[D]. 上海:上海交通大学,2018.
YU Liyao. Effects of exogenous γ- aminobutyric acid on growth,drought stress resistance of melon seedlings in greenhouse[D]. Shanghai:Shanghai Jiao Tong University,2018.
[12] 闫妮,冯棣,杨凤娟,张敬敏,桑茂鹏,祝海燕. γ-氨基丁酸浸种对盐分胁迫下番茄出苗及幼苗生长的影响[J]. 中国瓜菜,2022,35(10):58-63.
YAN Ni,FENG Di,YANG Fengjuan,ZHANG Jingmin,SANG Maopeng,ZHU Haiyan. GABA soaking affects tomato emergence and seedling growth under salt stress[J]. China Cucurbits and Vegetables,2022,35(10):58-63.
[13] 王馨,閆永庆,殷媛,刘威,王贺,季绍旭. 外源γ-氨基丁酸(GABA)对盐胁迫下西伯利亚白刺光合特性的影响[J]. 江苏农业学报,2019,35(5):1032-1039.
WANG Xin,YAN Yongqing,YIN Yuan,LIU Wei,WANG He,JI Shaoxu. Effect of exogenous γ-aminobutyric acid (GABA) on photosynthetic characteristics of Nitraria sibirica pall under salt stress[J]. Jiangsu Journal of Agricultural Sciences,2019,35(5):1032-1039.
[14] 陈梓健. 外源GABA对高温、干旱胁迫下高羊茅的生理影响[D]. 广州:仲恺农业工程学院,2017.
CHEN Zijian. Physiological effects of exogenous GABA on Festuca arundinacea under high temperature and drought stress[D]. Guangzhou:Zhongkai University of Agriculture and Engineering,2017.
[15] 甄爱,胡晓辉,任文奇,苏春杰,靳晓青,孙先鹏. 外源γ-氨基丁酸对Ca(NO3)2胁迫下甜瓜幼苗NO3--N同化的影响[J]. 应用生态学报,2016,27(12):3987-3995.
ZHEN Ai,HU Xiaohui,REN Wenqi,SU Chunjie,JIN Xiaoqing,SUN Xianpeng. Effect of exogenous γ-aminobutyric acid on NO3--N assimilation in muskmelon under Ca(NO3)2 stress[J]. Chinese Journal of Applied Ecology,2016,27(12):3987-3995.
[16] TILMAN D,CASSMAN K G,MATSON P A,NAYLOR R,POLASKY S. Agricultural sustainability and intensive production practices[J]. Nature,2002,418(6898):671-677.
[17] SINHA E,MICHALAK A M,BALAJI V. Eutrophication will increase during the 21st century as a result of precipitation changes[J]. Science,2017,357(6349):405-408.
[18] GAUDINIER A,RODRIGUEZ-MEDINA J,ZHANG L F,OLSON A,LISERON-MONFILS C,B?GMAN A M,FORET J,ABBITT S,TANG M,LI B H,RUNCIE D E,KLIEBENSTEIN D J,SHEN B,FRANK M J,WARE D,BRADY S M. Transcriptional regulation of nitrogen-associated metabolism and growth[J]. Nature,2018,563(7730):259-264.
[19] 宋红苗,陶跃之,王慧中,徐祥彬. GABA在植物体内的合成代谢及生物学功能[J]. 浙江农业科学,2010,51(2):225-229.
SONG Hongmiao,TAO Yuezhi,WANG Huizhong,XU Xiangbin. The anabolism and biological function of GABA in plants[J]. Journal of Zhejiang Agricultural Sciences,2010,51(2):225-229.
[20] 刘晓涵,陈永刚,林励,庄满贤,方晓娟. 蒽酮硫酸法与苯酚硫酸法测定枸杞子中多糖含量的比较[J]. 食品科技,2009,34(9):270-272.
LIU Xiaohan,CHEN Yonggang,LIN Li,ZHUANG Manxian,FANG Xiaojuan. Comparison of methods in determination of polysaccharide in Lycium barbarum L.[J]. Food Science and Technology,2009,34(9):270-272.
[21] 贺雅娟,马宗桓,韦霞霞,李玉梅,李彦彪,马维峰,丁孙磊,毛娟,陈佰鸿. 黄土高原旱塬区不同品种苹果果实糖及有机酸含量比较分析[J]. 食品工业科技,2021,42(10):248-254.
HE Yajuan,MA Zonghuan,WEI Xiaxia,LI Yumei,LI Yanbiao,MA Weifeng,DING Sunlei,MAO Juan,CHEN Baihong. Comparative analysis of sugar and organic acid contents of different apple cultivars in dryland of loess plateau[J]. Science and Technology of Food Industry,2021,42(10):248-254.
[22] 張弦. 不同施钾水平对‘嘎拉苹果果实糖、酸生理代谢的影响[D]. 杨凌:西北农林科技大学,2016.
ZHANG Xian. Effects of different potassium level on sugar and acid metabolism in‘Gala apple fruit[D]. Yangling:Northwest A & F University,2016.
[23] 潘俨. 库尔勒香梨果实发育及采后糖代谢与呼吸代谢关系的研究[D]. 乌鲁木齐:新疆农业大学,2016.
PAN Yan. The relationship between sugar metabolism and respiratory metabolism throughout fruit development and postharvest of Korla fragrant pear (Pyrus sinkiangensis Yu)[D]. Urumqi:Xinjiang Agricultural University,2016.
[24] 黎冰. 氮素形态对赤霞珠葡萄氮代谢和蔗糖代谢调控机制的研究[D]. 杨凌:西北农林科技大学,2017.
LI Bing. Study on regulation mechanism of nitrogen forms on nitrogen and sucrose metabolism in Cabernet Sauvignon grape[D]. Yangling:Northwest A & F University,2017.
[25] 梁剑光,朱玲,徐正军. 靛酚蓝-分光光度法测定发酵液中氨态氮含量研究[J]. 食品与发酵工业,2006,32(9):134-137.
LIANG Jianguang,ZHU Ling,XU Zhengjun. Study on the determination of NH4+-N content in microbial fermentation liquor by indophenol blue spectrophotometric method[J]. Food and Fermentation Industries,2006,32(9):134-137.
[26] 李慧,丛郁,常有宏,蔺经,盛宝龙. 豆梨NADH型硝酸还原酶基因克隆、表达及酶活性分析[J]. 果树学报,2014,31(5):760-768.
LI Hui,CONG Yu,CHANG Youhong,LIN Jing,SHENG Baolong. Cloning,expression and enzyme activity analysis of nitrite reductase gene from Pyrus calleryana[J]. Journal of Fruit Science,2014,31(5):760-768.
[27] 马宗桓,陈佰鸿,毛娟,胡紫璟,李文芳. 施氮时期对酿酒葡萄叶片氮代谢酶及相关基因表达的影响[J]. 西北植物学报,2018,38(2):298-306.
MA Zonghuan,CHEN Baihong,MAO Juan,HU Zijing,LI Wenfang. Effects of nitrogen metabolism enzymes and related gene expression in leaves of Vitis vinifera during nitrogen application period[J]. Acta Botanica Boreali-Occidentalia Sinica,2018,38(2):298-306.
[28] 趙鹏,何建国,熊淑萍,马新明. 氮素形态对专用小麦旗叶酶活性及籽粒蛋白质和产量的影响[J]. 中国农业大学学报,2010,15(3):29-34.
ZHAO Peng,HE Jianguo,XIONG Shuping,MA Xinming. Studies on the effects of different nitrogen forms on enzyme activity in flag leaves in wheat and protein and yield of grain for specialized end-uses[J]. Journal of China Agricultural University,2010,15(3):29-34.
[29] 王小纯,熊淑萍,马新明,张娟娟,王志强. 不同形态氮素对专用型小麦花后氮代谢关键酶活性及籽粒蛋白质含量的影响[J]. 生态学报,2005,25(4):802-807.
WANG Xiaochun,XIONG Shuping,MA Xinming,ZHANG Juanjuan,WANG Zhiqiang. Effects of different nitrogen forms on key enzyme activity involved in nitrogen metabolism and grain protein content in speciality wheat cultivars[J]. Acta Ecologica Sinica,2005,25(4):802-807.
[30] 吴良欢,蒋式洪,陶勤南. 植物转氨酶(GOT和GPT)活度比色测定方法及其应用[J]. 土壤通报,1998,29(3):136-138.
WU Lianghuan,JIANG Shihong,TAO Qinnan. Colorimetric determination method of plant transaminase (GOT and GPT) activity and its application[J]. Chinese Journal of Soil Science,1998,29(3):136-138.
[31] 申丽霞,王璞. 玉米穗位叶碳氮代谢的关键指标测定[J]. 中国农学通报,2009,25(24):155-157.
SHEN Lixia,WANG Pu. Determination of C-N metabolism indices in ear-leaf of maize (Zea mays L.)[J]. Chinese Agricultural Science Bulletin,2009,25(24):155-157.
[32] 苏丽英,吴勇,於新建,夏叔芳. 水稻叶片蔗糖磷酸合成酶的一些特性[J]. 植物生理学报,1989,15(2):117-123.
SU Liying,WU Yong,YU Xinjian,XIA Shufang. Some properties of rice leaf sucrose phosphate synthetase[J]. Physiology and Molecular Biology of Plants,1989,15(2):117-123.
[33] 韩丽娜,马宗桓,王颖,胡紫璟,史星雲,毛娟,陈佰鸿. 荒漠区滴灌施氮量对葡萄叶绿素荧光特性及碳代谢的影响[J]. 华北农学报,2020,35(2):170-177.
HAN Lina,MA Zonghuan,WANG Ying,HU Zijing,SHI Xingyun,MAO Juan,CHEN Baihong. Effects of nitrogen application rate in drip irrigation on chlorophyll fluorescence characteristics and carbon metabolism of grape in desert area[J]. Acta Agriculturae Boreali-Sinica,2020,35(2):170-177.
[34] 宋锁玲. 低氧胁迫下γ-氨基丁酸对甜瓜幼苗无机氮代谢、糖代谢及矿质元素含量的影响[D]. 保定:河北农业大学,2012.
SONG Suoling. Effects of γ-aminobutyric acid on inorganic nitrogen metabolism,sugar metabolism and mineral elements contents of melon seedling under hypoxia stress[D]. Baoding:Hebei Agricultural University,2012.
[35] 吴薇,韩相龙,郑璞帆,韦成才,袁帅,张立新. 移栽方式与施氮量对烤烟生长发育和产质量的影响[J]. 植物营养与肥料学报,2018,24(2):535-543.
WU Wei,HAN Xianglong,ZHENG Pufan,WEI Chengcai,YUAN Shuai,ZHANG Lixin. Effects of transplanting mode and nitrogen application rate on growth,development and yield of flue-cured tobacco[J]. Journal of Plant Nutrition and Fertilizers,2018,24(2):535-543.
[36] 高松,刘颖,刘学娜,曹逼力,陈子敬,徐坤. 光质对大葱叶片碳氮代谢的影响[J]. 植物生理学报,2020,56(3):565-572.
GAO Song,LIU Ying,LIU Xuena,CAO Bili,CHEN Zijing,XU Kun. Effects of light quality on carbon and nitrogen metabolism in leaves of Welsh onion (Allium fistulosum)[J]. Plant Physiology Journal,2020,56(3):565-572.
[37] 王宇航,韩爱民,张立梅,李斗,金鑫,王春恒,馮丽丹,杨江山. 外源γ-氨基丁酸对蛇龙珠葡萄果实糖酸代谢的影响[J].果树学报,2023,40(4):699-711.
WANG Yuhang,HAN Aimin,ZHANG Limei,LI Dou,JIN Xin,WANG Chunheng,FENG Lidan,YANG Jiangshan. Effects of exogenous GABA on sugar and acid metabolism of Cabernet Gernischet [J]. Journal of Fruit Science,2023,40(4):699-711.
[38] CHEN W,MENG C,JI J,LI M H,ZHANG X M,WU Y Y,XIE T T,DU C J,SUN J C,JIANG Z P,SHI S Q. Exogenous GABA promotes adaptation and growth by altering the carbon and nitrogen metabolic flux in poplar seedlings under low nitrogen conditions[J]. Tree Physiology,2020,40(12):1744-1761.
[39] 刘金平. γ-氨基丁酸对淹水胁迫下不结球白菜幼苗碳氮代谢相关指标的影响[D]. 南京:南京农业大学,2016.
LIU Jinping. Effects of exogenous γ-ambutyric acid on relevant indicators of carbon and nitrogen metabolism of non-heading cabbage under waterlogging stress[D]. Nanjing:Nanjing Agricultural University,2016.
[40] LI Y X,LIU B Y,PENG Y X,LIU C L,ZHANG X Z,ZHANG Z J,LIANG W,MA F W,LI C Y. Exogenous GABA alleviates alkaline stress in Malus hupehensis by regulating the accumulation of organic acids[J]. Scientia Horticulturae,2020,261:108982.
[41] 梁静宜,郭凡,赵科,王鸿飞,许凤. 外源γ-氨基丁酸对鲜切南瓜品质和γ-氨基丁酸代谢的影响[J]. 食品工业科技,2022,43(19):385-392.
LIANG Jingyi,GUO Fan,ZHAO Ke,WANG Hongfei,XU Feng. Effect of exogenous γ-aminobutyric acid on the quality and γ-aminobutyric acid metabolism of fresh-cut pumpkins[J]. Science and Technology of Food Industry,2022,43(19):385-392.
[42] WANG Y,YUAN B,JI Y C,LI H. Hydrolysis of hemicellulose to produce fermentable monosaccharides by plasma acid[J]. Carbohydrate Polymers,2013,97(2):518-522.
[43] TANASE K,SHIRATAKE K,MORI H,YAMAKI S. Changes in the phosphorylation state of sucrose synthase during development of Japanese pear fruit[J]. Physiologia Plantarum,2002,114(1):21-26.
[44] MORIGUCHI T,ABE K,SANADA T,YAMAKI S. Levels and role of sucrose synthase,sucrose-phosphate synthase,and acid invertase in sucrose accumulation in fruit of Asian pear[J]. Journal of the American Society for Horticultural Science,1992,117(2):274-278.
[45] VERMA A K,UPADHYAY S K,VERMA P C,SOLOMON S,SINGH S B. Functional analysis of sucrose phosphate synthase (SPS) and sucrose synthase (SS) in sugarcane (Saccharum) cultivars[J]. Plant Biology,2011,13(2):325-332.
[46] WIND J,SMEEKENS S,HANSON J. Sucrose:Metabolite and signaling molecule[J]. Phytochemistry,2010,71(14/15):1610-1614.
[47] ABD ELBAR O H,ELKELISH A,NIEDBA?A G,FARAG R,WOJCIECHOWSKI T,MUKHERJEE S,ABOU-HADID A F,EL-HENNAWY H M,ABOU EL-YAZIED A,ABD EL-GAWAD H G,AZAB E,GOBOURI A A,EL-SAWY A M,BONDOK A,IBRAHIM M F M. Protective effect of γ-aminobutyric acid against chilling stress during reproductive stage in tomato plants through modulation of sugar metabolism,chloroplast integrity,and antioxidative defense systems[J]. Frontiers in Plant Science,2021,12:663750.
[48] 任文奇. 外源γ-氨基丁酸對Ca(NO3)2胁迫下甜瓜幼苗氮代谢和光合作用的调控[D]. 西北农林科技大学, 2016.
REN Wenqi. Regulation of exogenous γ-aminobutyric acid on nitrogen metabolism and photosynthesis of melon seedlings under Ca(NO3)2 stress [D]. Northwest A & F University, 2016.
[49] 燕博文. 低氮胁迫下γ-氨基丁酸对玉米幼苗氮代谢调控机制研究[D]. 郑州:河南农业大学,2022.
YAN Bowen. Regulation mechanism of γ-aminobutyric acid on nitrogen metabolism in maize seedlings under low nitrogen stress[D]. Zhengzhou:Henan Agricultural University,2022.
[50] 谷海涛,贾琰,张博,孙斌,王卓茜,赵宏伟. 孕穗期干旱胁迫下外源γ-氨基丁酸对寒地粳稻籽粒氮素形成及产量的影响[J]. 华北农学报,2018,33(5):209-217.
GU Haitao,JIA Yan,ZHANG Bo,SUN Bin,WANG Zhuoqian,ZHAO Hongwei. Effects of exogenous γ-aminobutyric acid on grain nitrogen formation and yield in cold-region Japonica rice under drought stress at booting stage[J]. Acta Agriculturae Boreali-Sinica,2018,33(5):209-217.
[51] 田真,李敬蕊,王祥,吴晓蕾,宫彬彬,高洪波. 生菜硝酸还原酶基因的克隆及高氮水平下外源γ-氨基丁酸对其表达和叶片硝酸盐含量的影响[J]. 西北植物学报,2015,35(6):1098-1105.
TIAN Zhen,LI Jingrui,WANG Xiang,WU Xiaolei,GONG Binbin,GAO Hongbo. Cloning of nitrate reductase gene of lettuce and effect of exogenous γ-aminobutyric acid on gene expression and nitrate content in leaves under high nitrogen level[J]. Acta Botanica Boreali-Occidentalia Sinica,2015,35(6):1098-1105.
[52] CAMARGO E L O,NASCIMENTO L C,SOLER M,SALAZAR M M,LEPIKSON-NETO J,MARQUES W L,ALVES A,TEIXEIRA P J P L,MIECZKOWSKI P,CARAZZOLLE M F,MARTINEZ Y,DECKMANN A C,RODRIGUES J C,GRIMA-PETTENATI J,PEREIRA G A G. Contrasting nitrogen fertilization treatments impact xylem gene expression and secondary cell wall lignification in Eucalyptus[J]. BMC Plant Biology,2014,14:256.
[53] 李冰,張照贵,王佳佳,李斯深. 小麦GDH1基因克隆及其功能标记开发[J]. 山东农业科学,2014,46(10):6-11.
LI Bing,ZHANG Zhaogui,WANG Jiajia,LI Sishen. Cloning and functional marker of GDH1 gene in wheat[J]. Shandong Agricultural Sciences,2014,46(10):6-11.
[54] LIANG C G,CHEN L P,WANG Y,LIU J,XU G L,LI T. High temperature at grain-filling stage affects nitrogen metabolism enzyme activities in grains and grain nutritional quality in rice[J]. Rice Science,2011,18(3):210-216.
[55] 李友军,熊瑛,陈明灿,骆炳山. 氮、磷、钾对豫麦50旗叶蔗糖和籽粒淀粉积累的影响[J]. 应用生态学报,2006,17(7):1196-1200.
LI Youjun,XIONG Ying,CHEN Mingcan,LUO Bingshan. Effects of nitrogen,phosphorus and potassium fertilization on sucrose accumulation in flag leaf and starch accumulation in kernel of weak gluten wheat[J]. Chinese Journal of Applied Ecology,2006,17(7):1196-1200.
[56] ALLAN W L,SHELP B J. Fluctuations of γ-aminobutyrate, γ-hydroxybutyrate, and related amino acids in Arabidopsis leaves as a function of the light-dark cycle, leaf age, and N stress[J]. Canadian Journal of Botany,2006,84(8):1339-1346.

