火龙果DREB1D基因的克隆及在转基因拟南芥中的功能分析
张璐芳 侯黔东 蔡晓薇 杨鵾 文晓鹏

摘 要:【目的】DREB(dehydration responsive element binding)是一類脱水响应元件结合蛋白,在植物响应高温、干旱、高盐和低温等多种非生物胁迫过程中发挥关键作用。以紫红龙火龙果(Hylocereus monacanthus)为材料,克隆得到DREB转录因子,并命名为HmDREB1D(HU02G01866.1),探究其生物学功能。【方法】构建HmDREB1D基因植物过表达载体,通过亚细胞定位分析HmDREB1D基因在细胞中的位置。异源转化拟南芥,对T3代纯合系转基因拟南芥(OE3、OE4、OE5)进行生物学功能验证。【结果】火龙果HmDREB1D基因的开放阅读框全长723 bp,产生的蛋白定位于细胞核内,属DREB1s亚家族,具有典型的AP2结构域。将HmDREB1D基因转化至拟南芥获得超表达转基因株系,与野生型相比,转基因株系表现出较高的抗逆性。在干旱胁迫下,转基因植株T3代纯合系种子的萌发率高于野生型。转基因植株的叶片在逆境胁迫下表现出更低的电导率及更高的保护性酶活性。实时荧光定量PCR分析显示,RD20、HSP70和COR15A等逆境胁迫响应基因在HmDREB1D基因超表达植株中具有更高的表达量。【结论】过表达HmDREB1D基因通过调控抗逆相关基因表达,加速清除植株内的活性氧,增强植株的抗逆性。
关键词:火龙果;HmDREB1D;功能分析;非生物胁迫;亚细胞定位
中图分类号:S667.9 文献标志码:A 文章编号:1009-9980(2023)07-1330-12
Cloning of DREB1D gene from pitaya and its functional analysis in transgenic Arabidopsis thaliana
ZHANG Lufang, HOU Qiandong, CAI Xiaowei, YANG Kun, WEN Xiaopeng*
(College of Life Sciences/Institute of Agro-bioengineering, Guizhou University/Key Laboratory of Plant Resource Conservation and Germplasm Innovation in Mountainous Region (Ministry of Education), Guiyang 550025, Guizhou, China)
Abstract: 【Objective】 DREB (dehydration responsive element binding) proteins are widely present in plants and are primarily involved in the abiotic stress response of plants. The two primary DREB transcription factors are DREB1 and DREB2, with DREB1 being mostly associated to low temperature and drought stress and DREB2 being primarily related to drought, salt, and high temperature stress. Pitaya (Hylocereus monacanthus) belongs to the cactus plants, because of its high nutritional value and strong resistance stress, it is popular with customers in karst regions like Guizhou and Guangxi. DREBs were found responsive to drought stress in pitaya, leaving the underlying mechanism unrevealed. This study intends to clone HmDREB1D (HU02G01866.1) gene and verify its biological function. 【Methods】 The pCambia35s-HmDREB1D-GFP plant overexpression vector was constructed by seamless cloning technology and transformed into Tobacco. The fluorescence signal of HmDREB1D was observed under a laser confocal microscope to determine the subcellular location. The expression vector of pCambia35s-HmDREB1D was constructed. The HmDREB1D gene was transformed into A. thaliana, a total of 6 transgenic A. thaliana plants were obtained, and 3 overexpressed transgenic Arabidopsis (OE3, OE4 and OE5) plants were chosen for further biological verification. After surface sterilization, HmDREB1D transgenic Arabidopsis (OE3, OE4 and OE5) and the wild type Arabidopsis seeds were sown in 250 mmol·L-1 mannitol 1/2 MS medium for drought stress treatment (16 h/8 h day/night cycle, 24 ℃). The germination rate was counted after 7 days. The seedlings grew in 1/2 MS medium for 7 days, and then were transplanted into 1/2 MS medium containing 250 mmol·L-1 mannitol for drought stress treatment (16 h/8 h day/night cycle, 24 ℃). The root length and fresh weight were measured 7 days after the treatment. Transgenic Arabidopsis (OE3, OE4 and OE5) and the wild type Arabidopsis seeds were surface sterilized, sown in 1/2 MS medium and cultured for 7 days, and then transplanted in pots filled with nutrient soil vermiculite (3∶1) and placed in an artificial climate growth chamber for 4 weeks (16 h/8 h day/night light cycle, 24 ℃). Mock drought 20% PEG6000 (3 d), high temperature 42 ℃ (1 d) and low temperature -20 ℃ (1 h) were subsequently applied. Transgenic Arabidopsis and the wild type Arabidopsis leaves were collected before and after stress treatment for determination of physiological and stress gene expression levels. The relative conductivity was measured by Jenco3020. The activities of peroxidase (POD), superoxide dismutase (SOD) and catalase (CAT) were determined by spectrophotometer. The qRT-PCR technique was used to detect the response gene expression level of transgenic Arabidopsis (OE3, OE4 and OE5) and the wild type Arabidopsis under drought (RD20 and RD22), high temperature (HSP70 and HSFA1D) and low temperature (COR47 and COR15A) stress. 【Results】 A 723 bp open reading frame codes the 243 aa HmDREB1D, which is categorized in DREB1s subfamily because of the AP2 domain. The results of GFP fluorescence signal showed that the pCambia35s-HmDREB1D-GFP was transformed into Tobacco and distributed in the nucleus, while the pCambia35s-GFP protein was distributed on the cell surface, indicating that HmDREB1D protein was located in the nucleus. The HmDREB1D gene was transformed into Arabidopsis. The HmDREB1D gene responded greatly to the drought stress with 250 mmol·L-1 mannitol treatments. The results showed that seed germination rate and seedling growth status of transgenic Arabidopsis were better than the wild type Arabidopsis under drought stress. The germination rate of transgenic plant remained above 87.8%, while the germination rate of wild type Arabidopsis was only 37.3%. The results showed that HmDREB1D gene enhanced the tolerance of transgenic Arabidopsis to drought stress by increasing the germination rate of transgenic Arabidopsis under drought stress, indicating that the germination rate was positively correlated with stress resistance. After drought, high temperature and low temperature stress, transgenic Arabidopsis had not only a better phenotype than the wild type Arabidopsis, but also a significantly higher survival rate than the wild type Arabidopsis (p<0.05). The results showed that transgenic Arabidopsis had strong water retention capacity, which improved the survival rate of transgenic Arabidopsis. The relative conductivity of transgenic Arabidopsis was significantly lower than the wild type Arabidopsis (p<0.05). The damage of transgenic Arabidopsis was less than that of the wild type Arabidopsis under drought, high temperature and low temperature stress, indicating that the stress resistance of transgenic Arabidopsis was higher than that of the wild type Arabidopsis. The antioxidant enzyme activity of the HmDREB1D transgenic Arabidopsis was significantly higher than that of the wild type Arabidopsis (p<0.05). The results showed that transgenic Arabidopsis could improve the antioxidant capacity of transgenic Arabidopsis through the activity of antioxidant enzymes, and then reduce the harm caused by drought, high temperature and low temperature stress. According to the results of qRT-PCR, the expression of stress response genes in transgenic Arabidopsis showed upward trend under drought, high temperature and low temperature stress, and was significantly higher than that of the wild type Arabidopsis (p<0.05). The results showed that HmDREB1D gene enhanced the stress ability of transgenic Arabidopsis under drought, high temperature and low temperature stress by inducing the expression of stress-related genes. 【Conclusion】 Aforementioned results suggest that HmDREB1D might be positively involved in the stress response of pitaya.
Key words: Hylocereus monacanthus; HmDREB1D; Functional analysis; Abiotic stress; Subcellular localization
在自然环境状态下,植物遭受着各种非生物胁迫的威胁。非生物胁迫包括干旱、高温、高盐和低温,是影响植物的正常生长和发育的主要因子[1]。植物通过转录因子(transcription factor,TF)结合启动子特异的元件,调节功能基因的表达以响应外界信号[2]。AP2/ERF是一大类植物特异性转录因子,其特征在于存在稳定的AP2结构域。根据AP2结构域的数量,AP2/ERF被分为4个亚家族AP2、DREB、ERF和RVA[3]。其中,DREB(dehydration responsive element-binding protein)是植物特有的一类转录因子,在逆境应答中发挥重要作用[4]。
近年来,研究人员通过对光皮桦[5]、玉米[6]和沙冬青AP2/ERF[7]转录因子家族鉴定与分析发现,DREB类转录因子参与响应干旱、高温或低温胁迫的应答,这表明DREB可能是植物抗逆相关的关键调节因子。相关研究发现,DREB蛋白能特异性地识别共同的核心序列A/GCCGAC的DRE/C重复(CRT),以调控下游基因的表达进而增强非生物胁迫耐受性[8]。如苔藓类BaDBL1通过诱导应激响应基因的表达,进而增强了转基因植物对干旱和高盐胁迫的耐受性[9]。胡萝卜DcDREB1A基因通过提高转基因植物的活性氧清除能力,使得胁迫反应基因的表达上调,正向调节了转基因植物的抗旱性[3]。海棠MhDREB2A基因通过调控胁迫相关基因的表达,使得转基因植物对干旱胁迫的耐受性增强[10]。百合LIDREB1G基因过表达不仅提高了转基因植物的萌发率和存活率,还促进了胁迫相关基因的表达,使得转基因植物对干旱、高温和低温的耐受性增强[11]。由此推测,不同的植物物种可能是DREB功能存在差异的原因。
火龙果(Hylocereus monacanthus)[12]是贵州、广西等喀斯特地区的特色和优势产业,在农业结构调整和扶贫开发等方面发挥了重要作用。同时,火龙果还具有较高的经济效益和很强的抗逆性。关于火龙果抗旱的机制,前人从功能基因[13]、miRNA[14]和转录因子[15]等方面开展了研究工作。根据笔者团队前期的研究,DREB转录因子参与了火龙果抗旱响应[16]。在此基础上,笔者在本研究中克隆火龙果DREB1D基因开放阅读框(open reading frame,ORF)全长序列,通过异源遗传转化分析其在非生物胁迫应答中的表达特性,并解析其生物学功能,旨在为深刻认识火龙果抗旱机制提供信息,也为抗逆遗传育种提供新资源。
1 材料和方法
1.1 RNA提取及cDNA的合成
以30 d苗龄的紫红龙组培苗(苗高6~8 cm)为试验材料,利用Plant RNA Kit(Omega,上海)提取试剂盒,参照说明书方法提取肉质茎RNA,用1%琼脂糖凝胶电泳检测RNA质量,然后用反转录试剂盒(TaKaRa,日本)合成cDNA第一链。
1.2 HmDREB1D克隆和序列分析
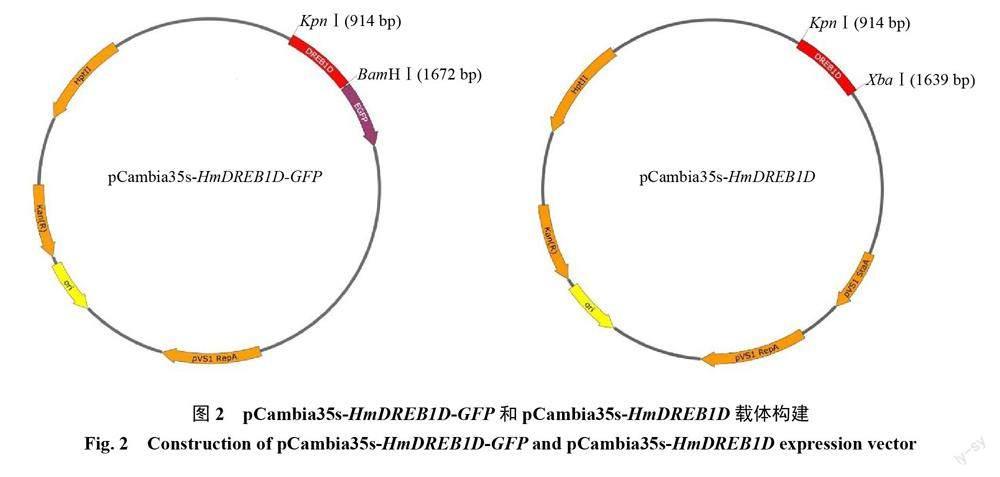
在火龙果基因组数据库(http://www.pitayagenomic.com)中查找DREB片段同源序列[17],并命名为HmDREB1D,利用引物设计网址(https://crm.vazyme.com/cetool/singlefragment.html)中的方法,设计无缝克隆引物(表1)。以cDNA为模板进行PCR扩增,使用胶回收試剂盒(TaKaRa,日本)对目的片段回收纯化。选用即用型无缝克隆试剂盒(生工,上海)对将目的片段与pCambia35s-EGFP载体进行连接,连接条件为50 ℃、20 min,将重组质粒转化于大肠杆菌感受态细胞DH5α(全式金,北京),采用PCR技术鉴定阳性菌落并送上海生物工程有限公司(Sangon)测序,将构建的过表达载体命名为pCambia35s-HmDREB1D。
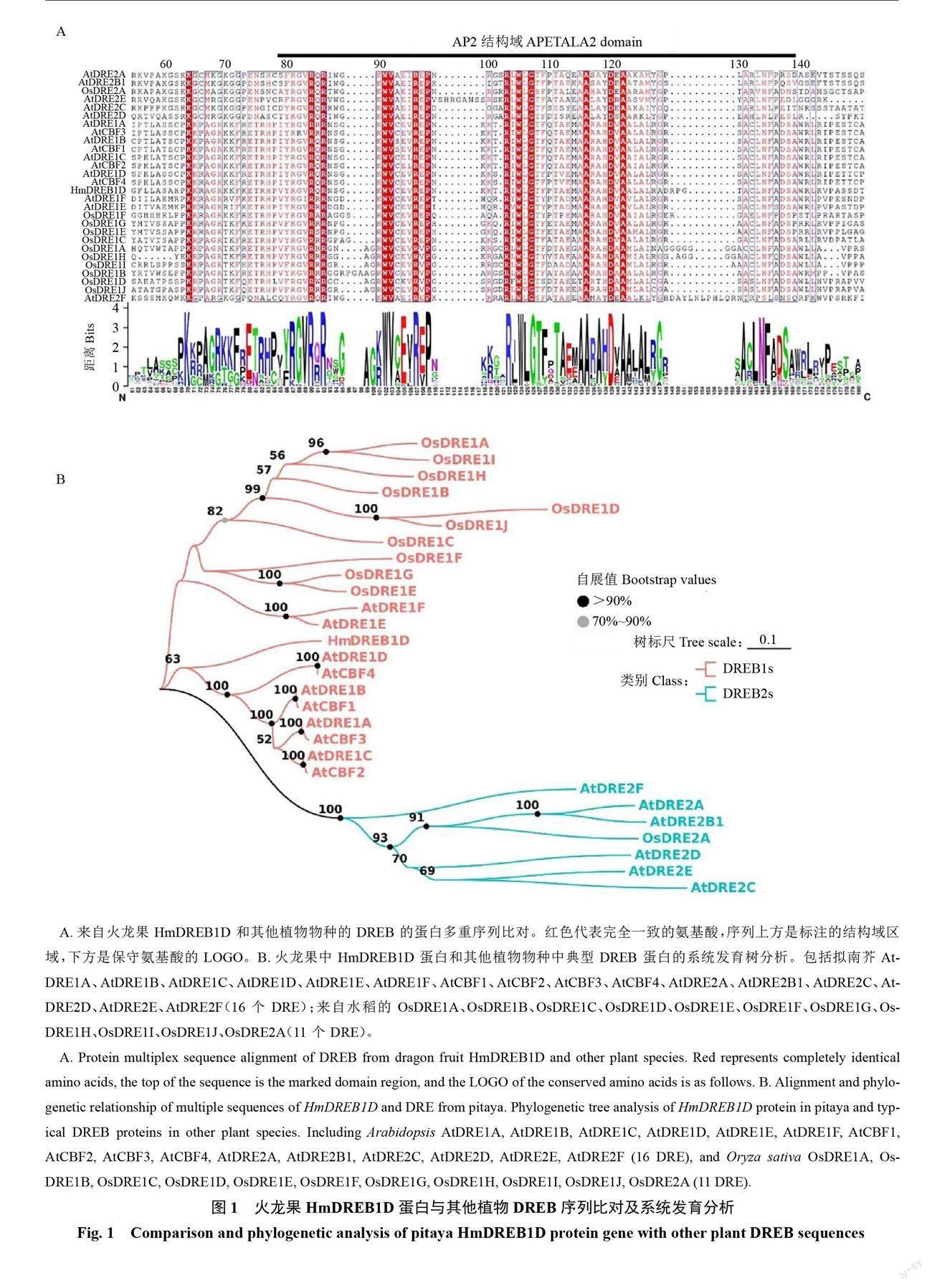
对HmDREB1D蛋白与其他植物中的DREB蛋白序列进行多序列比对和进化分析,多序列比对使用https://espript.ibcp.fr/ESPript/cgi-bin/ESPript.cgi进行,利用MEGA11进行系统进化树构建,设置Bootstrap为1000次,其他参数默认。
1.3 HmDREB1D基因亚细胞定位分析
以pCambia35s-EGFP载体为基础,采用无缝克隆技术克隆HmDREB1D蛋白序列,方法见1.2。设计引物去除终止密码子(表1),构建植物过表达载体pCambia35s-HmDREB1D-GFP。将验证正确的pCambia35s-HmDREB1D-GFP重组质粒和pCambia35s-GFP空载质粒,采用冻融法分别转入农杆菌感受态GV3101中。参照文献[18]中的方法,将重悬菌液注射于1月龄的本氏烟草下表皮(背面)。避光培养72 h后,撕取下表皮制片,在激发波长488 nm和发射波长510 nm条件下,用激光共聚焦显微镜TCS-SP8(Leica 德国)观察并拍照。
1.4 HmDREB1D基因过表达拟南芥的产生
采用浸花法[19]浸染野生型拟南芥,收取种子后。在含50 g·L-1潮霉素的1/2 MS培养基中进行转基因植株的筛选,利用DNA试剂盒(天根,北京)对阳性植株进行DNA提取,使用特异性引物(表1)对转基因进行PCR验证。
利用Plant RNA Kit提取试剂盒(Omega,上海),提取转基因和野生型拟南芥的RNA,并合成cDNA第一条链。使用Primer Premier 5.0软件设计HmDREB1D特异性荧光定量引物(表1),参照Biomarker Fast2×SYBR Green qPCR试剂盒(Thermo Fisher Scientific,北京)进行实时荧光定量PCR(quantitative real-time PCR,qRT-PCR),表达水平由梯度荧光定量PCR系统检测(Bio-Rad,美国)。设置3个生物学重复和3次技术重复,以Actin基因为拟南芥内参基因(表1)。根据最终的Ct值,使用2-ΔΔCt的分析方法计算HmDREB1D基因的表达水平[20],并挑选高表达的3个株系用于后续功能研究。
1.5 转基因拟南芥非生物胁迫的耐受性评价
选用T3代超表达株系和野生型种子进行表面灭菌,对于萌发干旱胁迫试验,将种子播种于含250 mmol·L-1甘露醇的1/2 MS培养基中,在植物生长室中培养,培养条件为16 h(24 ℃),黑暗8 h(20 ℃)。每处理播种30粒,3个生物学重复,7 d后统计萌发率,萌发率(%)=种子萌发数/供试种子总数×100。对于生长特性试验,将种子播种于普通1/2 MS培养基中培养7 d,然后转移至含250 mmol·L-1甘露醇的1/2 MS培养基中,每处理20株幼苗,3个生物学重复,7 d后测定其根长度和鲜质量。
为了进一步分析HmDREB1D转基因植株对各逆境胁迫的耐受性,选择5周龄的T3代超表达和野生型株系,进行不同的逆境胁迫处理。对于干旱胁迫处理,将20% PEG6000溶液从植物根部浇灌3 d,每天50 mL,然后再浇水5 d。对于高温胁迫处理,将植物放置42 ℃下处理1 d,正常条件下(24 ℃)恢复5 d。对于低温胁迫处理,植物在-20 ℃下处理1 h,正常条件下(24 ℃)恢复5 d,统计存活率。同时,分别在干旱胁迫(3 d)、高温胁迫(1 d)和低温胁迫(1 h),采集处理前后植物的葉片用于物理指标测定和应激基因表达分析的模板。
参考电导仪法[21]测定相对电导率,采用电导仪Jenco 3020(JENCO,美国)进行试验操作,每个试验3次重复。计算公式为:相对电导率(%)=C1/C2?100(C1:煮沸前的电导率;C2:煮沸后的电导率)。而过氧化物酶(peroxidase,POD)、超氧化物歧化酶(superoxide dismutase,SOD)和过氧化氢酶(catalase,CAT)活性,选择POD、SOD和CAT检测试剂盒(索莱宝,北京),按说明书进行测定。
1.6 应激相关基因表达分析
选取已经被证实的与应激相关的基因,包括干旱应激反应基因RD20、RD22[22],热休克基因HSP70、HSFA1D[23-24]和低温应激基因COR47、COR15A[25],引物序列见表1。收集胁迫处理前后的叶片,提取RNA并合成cDNA,以拟南芥Actin为内参基因,采用qRT-PCR技术检测HmDREB1D基因逆境胁迫处理下的表达水平,检测及表达量分析参照1.4进行。
1.7 数据统计学分析
所有数据分析使用Excel 2010软件统计并绘制柱形图,数据显示为3个独立试验的平均值±SE(n=3;生物学重复)。使用单项方差分析*p<0.05和**p<0.01进行统计分析。
2 结果与分析
2.1 HmDREB1D基因克隆与序列分析
克隆得到DREB1D基因,并命名为HmDREB1D。通过对其他植物中的DREB蛋白序列进行多序列比对,发现HmDREB1D基因属于AP2超家族,且具有典型的AP2保守结构域(图1-A)。系统进化分析表明,DREB家族转录因子可分为2个亚家族(DREB1s和DREB2s)。HmDREB1D蛋白属于DREB1s亚组,且HmDRE1D蛋白序列与拟南芥AtDRE1D蛋白序列具有高度相似性(图1-B)。
2.2 HmDREB1D基因过表达载体构建
选取测序正确的菌株进行过夜摇菌,提取质粒进行PCR验证,得到预期723 bp的基因片段,表明成功构建过表达载体pCambia35s-HmDREB1D-GFP(图2-A)和pCambia35s-HmDREB1D(图2-B)。
2.3 HmDREB1D基因亚细胞定位
为了验证HmDREB1D蛋白在体内的亚细胞位置,将构建的pCambia35s-HmDREB1D-GFP载体,通过瞬时转化注射于烟草叶片中,激光共聚焦显微镜跟踪观察HmDREB1D蛋白的亚细胞定位。结果显示(图3),pCambia35s-HmDREB1D-GFP的GFP荧光主要定位于细胞核,而GFP蛋白均分布于整个细胞表面,说明HmDREB1D蛋白定位在细胞核中,作为转录因子发挥作用。
2.4 HmDREB1D拟南芥遗传转化与生长特性
为了分析HmDREB1D基因的功能,将HmDREB1D基因在拟南芥中过表达,共获得6株超表达HmDREB1D转基因拟南芥植物。筛选3株高表达转基因纯合系(OE3、OE4、OE5)(图4-A)进一步分析。
对T3代超表达株系幼苗期干旱胁迫耐受性进行统计分析。野生型拟南芥的萌发率仅为37.3%,而转基因株系的萌发率保持在87.8%以上(图4-B~C)。转基因株系的根长度、鲜质量均显著高于野生型(图4-D~F)。结果表明,过表达HmDREB1D基因显著提高了对干旱胁迫的耐受性。
2.5 逆境胁迫下超表达株系的表型分析
选择5周龄的野生型和转基因拟南芥进行干旱和高、低温胁迫处理(图5)。结果表明,在干旱胁迫3 d后,野生型植株发生明显皱缩且叶片泛黄,而过表达HmDREB1D转基因株系叶片没有发生明显的皱缩,且叶片仍是大片绿色;正常浇水5 d后野生型存活率仅为21.8%,而转基因的存活率OE3为44.3%,OE4为82.6%,OE5为64.6%(图5-A)。在高温胁迫24 h后,野生型叶片明显干燥,而大多数过表达转基因植株仍然保持新鲜叶片,正常温度(24 ℃)下恢复5 d后野生型存活率为20%,而转基因的存活率OE3为43.3%、OE4为82.6%、OE5为61.3%(图5-B)。在低温胁迫下1 h后,野生型大部分被冻伤,叶片大多数变黄或萎蔫,而转基因拟南芥仍保持叶片鲜绿,正常温度(24 ℃)下恢复5 d后,野生型存活率为21.7%,而转基因的存活率OE3为52.3%,OE4为84.0%,OE5为66.7%(图5-C)。过表达HmDREB1D基因显著提高了转基因拟南芥在干旱、高温和低温胁迫下的存活率。
2.6 逆境下超表达株系的生理生化反应
除了存活率外,还包括生理特征,如相对电导率和抗氧化活性酶(POD、SOD、CAT)活性的测定。在正常条件下,野生型与转基因株系之间的生理参数无显著差异,而在干旱和高、低温胁迫条件下,转基因拟南芥中相对电导率显著低于野生型(图6-A),且POD、SOD、CAT的活性显著高于野生型(图6-B~D)。这些数据表明,过表达HmDREB1D基因通过提高抗氧化活性,降低细胞膜损伤程度,进而增强了转基因拟南芥抗干旱、高温和低温胁迫的能力。
2.7 逆境下超表达株系应激相关基因的表达分析
为了了解HmDREB1D基因如何增强对逆境胁迫响应的能力,采用qRT-PCR技术检测转基因株系和野生型中相关应激基因表达水平(图7)。结果显示,在正常条件下转基因植物中应激响应基因的表达水平出现上调,但是在干旱、高温和低温胁迫处理下,与野生型相比,转基因植物体内干旱(RD20、RD22)、高温(HSFA1D、HSP70)和低温(COR47、COR15A)应激基因的表达水平显著上调。这表明,HmDREB1D基因通过上调应激相关基因的表达来增强转基因植物对不同非生物胁迫的应激能力,从而提高转基因植物抗干旱、高温和低温的能力。
3 討 论
本研究从火龙果肉质茎中克隆得到HmDREB1D基因,多序列比对和进化分析表明,该基因具有典型的AP2结构域,属于A1(DREB1)亚组。有研究表明,A1亚组主要参与干旱诱导,而不参与低温诱导[26-27]。但也有研究发现,A1亚组不仅能参与低温诱导,还参与高温诱导[28-29]。逆境胁迫下,植物的生长状态和存活率在一定程度上可以体现出植物对胁迫环境的响应情况。Ren等[30]研究发现,过表达AmDREB3转基因植物在干旱、高盐和高温胁迫下的生长状态和存活率明显优于野生型。本研究中发现,与野生型相比,转基因拟南芥在干旱、高温和低温胁迫下表现出更好的生长状态和更高的存活率。逆境胁迫下,植物体内的相对电导率与植物抗逆性呈负相关[21],本研究中测得逆境胁迫下转基因拟南芥的相对电导率显著低于野生型。正常环境下,植物体内的活性氧清除系统使细胞内活性氧(reactive oxygen species,ROS)保持在较低的水平,以保证植物正常生长[31]。当植物受到逆境胁迫时,抗氧化酶活性的增强能有效地帮助植物避免细胞膜氧化损伤[3],本研究中测得过表达HmDREB1D转基因拟南芥在逆境胁迫下体内抗氧化酶POD、SOD和CAT的活性均显著高于野生型。以上研究结果表明,过表达HmDREB1D转基因拟南芥通过降低逆境胁迫所产生的伤害,提高了植物在逆境胁迫下的存活率,有效地缓解了逆境胁迫对膜系统造成的损伤,进而提高了植物对逆境胁迫的抗逆能力。
除了测定生理指标,本研究还通过qRT-PCR技术对转基因植物进行逆境胁迫响应基因表达水平分析,在逆境胁迫下转基因植物与野生型相比表现出更高的表达水平。相关研究发现,DREB转录因子通过与许多逆境胁迫相关基因的DRE/CRT相互作用,使得相关基因的表达上调,从而提高它们在逆境胁迫下的表达能力[32]。苔藓ScDREB8基因通过上调干旱、高盐和热休克相关响应基因的表达,增强了植物对逆境胁迫的耐受性[33]。因此,过表达HmDREB1D基因可能通过提高应激响应基因的表达水平,产生各种能抵御植物氧化胁迫的物质,进而增强了植物对各种逆境胁迫的耐受性。综上所述,DREB转录因子对火龙果逆境胁迫的响应具有关键调控作用,但其分子调控机制还需要进一步的研究。
4 结 论
从火龙果中克隆得到的HmDREB1D基因属于核定位基因。通过异源转化拟南芥,验证了HmDREB1D基因激活了一系列逆境响应基因的表达,增强了火龙果对干旱、高温和低温胁迫的耐受性。本研究为火龙果抗性研究的分子机制和新种质的创制奠定了基础。
参考文献 References:
[1] 易家宁,王康才,张琪绮,董雨青,毛晓敏,邓艳婷. 干旱胁迫对紫苏生长及品质的影响[J]. 核农学报,2020,34(6):1320-1326.
YI Jianing,WANG Kangcai,ZHANG Qiqi,DONG Yuqing,MAO Xiaomin,DENG Yanting. Effects of drought stress on growth and quality of Perilla frutescens[J]. Journal of Nuclear Agricultural Sciences,2020,34(6):1320-1326.
[2] ZHU J K. Abiotic stress signaling and responses in plants[J]. Cell,2016,167(2):313-324.
[3] LI T,HUANG Y,KHADR A,WANG Y H,XU Z S,XIONG A S. DcDREB1A,a DREB-binding transcription factor from Daucus carota,enhances drought tolerance in transgenic Arabidopsis thaliana and modulates lignin levels by regulating lignin-biosynthesis-related genes[J]. Environmental and Experimental Botany,2020,169:103896.
[4] ZHOU Y X,ZHOU W,LIU H,LIU P,LI Z G. Genome-wide analysis of the soybean DREB gene family:Identification,genomic organization and expression profiles in response to drought stress[J]. Plant Breeding,2020,139(6):1158-1167.
[5] 黃奕孜,钱旺,邱姗,王文新,黄华宏,林二培. 光皮桦AP2/ERF基因家族鉴定与表达分析[J]. 浙江农林大学学报,2022,39(6):1183-1193.
HUANG Yizi,QIAN Wang,QIU Shan,WANG Wenxin,HUANG Huahong,LIN Erpei. Identification and expression analysis of AP2/ERF gene family in Betula luminifera[J]. Journal of Zhejiang A & F University,2022,39(6):1183-1193.
[6] 王雷立,董柯清,张严玲,刘青青,王翠玲. 玉米DREB转录因子家族的全基因组鉴定与分析[J]. 湖南农业大学学报(自然科学版),2022,48(3):270-281.
WANG Leili,DONG Keqing,ZHANG Yanling,LIU Qingqing,WANG Cuiling. Genome-wide identification and analysis of DREB transcription factor family in maize[J]. Journal of Hunan Agricultural University (Natural Sciences),2022,48(3):270-281.
[7] CAO S L,WANG Y,LI X T,GAO F,FENG J C,ZHOU Y J. Characterization of the AP2/ERF transcription factor family and expression profiling of DREB subfamily under cold and osmotic stresses in Ammopiptanthus nanus[J]. Plants,2020,9(4):455.
[8] SHARMA V,GOEL P,KUMAR S,SINGH A K. An apple transcription factor,MdDREB76,confers salt and drought tolerance in transgenic tobacco by activating the expression of stress-responsive genes[J]. Plant Cell Reports,2019,38(2):221-241.
[9] LIANG Y Q,LI X S,YANG R R,GAO B,YAO J X,OLIVER M J,ZHANG D Y. BaDBL1,a unique DREB gene from desiccation tolerant moss Bryum argenteum,confers osmotic and salt stress tolerances in transgenic Arabidopsis[J]. Plant Science,2021,313:111047.
[10] 党明青,王京平,冉昆,刘加芬,李慧峰. 泰山海棠抗旱基因MhDREB2A的克隆与功能鉴定[J]. 沈阳农业大学学报,2022,53(4):462-468.
DANG Mingqing,WANG Jingping,RAN Kun,LIU Jiafen,LI Huifeng. Cloning and functional idenfication of drought-resistant related gene in Malus hupehensis[J]. Journal of Shenyang Agricultural University,2022,53(4):462-468.
[11] LIU B J,ZHOU Y,LAN W,ZHOU Q,LI F,CHEN F,BAO M Z,LIU G F. LlDREB1G,a novel DREB subfamily gene from Lilium longiflorum,can enhance transgenic Arabidopsis tolerance to multiple abiotic stresses[J]. Plant Cell,Tissue and Organ Culture (PCTOC),2019,138(3):489-506.
[12] FERNANDES A C F,DE SOUZA A C,RAMOS C L,PEREIRA A A,SCHWAN R F,DIAS D R. Sensorial,antioxidant and antimicrobial evaluation of vinegars from surpluses of Physalis (Physalis pubescens L.) and red pitahaya (Hylocereus monacanthus)[J]. Journal of the Science of Food and Agriculture,2019,99(5):2267-2274.
[13] NIE Q,GAO G L,FAN Q J,QIAO G,WEN X P,LIU T,PENG Z J,CAI Y Q. Isolation and characterization of a catalase gene “HuCAT3” from pitaya (Hylocereus undatus) and its expression under abiotic stress[J]. Gene,2015,563(1):63-71.
[14] LI A L,WEN Z,YANG K,WEN X P. Conserved miR396b-GRF regulation is involved in abiotic stress responses in pitaya (Hylocereus polyrhizus)[J]. International Journal of Molecular Sciences,2019,20(10):2501.
[15] 葛菲,聶琼,乔光,张婷,吴艳,文晓鹏. 转火龙果过氧化氢酶基因烟草植株的获得及其抗旱性分析[J]. 西南大学学报(自然科学版),2016,38(11):57-63.
GE Fei,NIE Qiong,QIAO Guang,ZHANG Ting,WU Yan,WEN Xiaopeng. Gain of the HuCAT genetic tobacco and analysis on drought stress resistance of transgenic tobacco[J]. Journal of Southwest University (Natural Science Edition),2016,38(11):57-63.
[16] 樊庆杰. 火龙果应答干旱胁迫的分子基础[D]. 贵阳:贵州大学,2013.
FAN Qingjie. Molecular basis of Pitaya response to drought stress[D]. Guiyang:Guizhou university,2013.
[17] CHEN J Y,XIE F F,CUI Y Z,CHEN C B,LU W J,HU X D,HUA Q Z,ZHAO J,WU Z J,GAO D,ZHANG Z K,JIANG W K,SUN Q M,HU G B,QIN Y H. A chromosome-scale genome sequence of pitaya (Hylocereus undatus) provides novel insights into the genome evolution and regulation of betalain biosynthesis[J]. Horticulture Research,2021,8(1):164.
[18] ZHENG L P,YAO J N,GAO F L,CHEN L,ZHANG C,LIAN L L,XIE L Y,WU Z J,XIE L H. The subcellular localization and functional analysis of Fibrillarin2,a nucleolar protein in Nicotiana benthamiana[J]. BioMed Research International,2016,2016:2831287
[19] CLOUGH S J,BENT A F. Floral dip:A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana[J]. The Plant Journal,1998,16(6):735-743.
[20] SCHMITTGEN T D,LIVAK K J. Analyzing real-time PCR data by the comparative CT method[J]. Nature Protocols,2008,3(6):1101-1108.
[21] 葛菲. 火龙果CAT基因在烟草中的遗传转化及功能分析[D]. 贵阳:贵州大学,2016.
GE F. Genetic transformation and functional characterization of pitaya CAT gene in tobacco[D]. Guiyang:Guizhou University,2016.
[22] KANG G J,YAN D,CHEN X L,LI Y,YANG L F,ZENG R Z. Molecular characterization and functional analysis of a novel WRKY transcription factor HbWRKY83 possibly involved in rubber production of Hevea brasiliensis[J]. Plant Physiology and Biochemistry,2020,155:483-493.
[23] NIU X,LUO T L,ZHAO H Y,SU Y L,JI W Q,LI H F. Identification of wheat DREB genes and functional characterization of TaDREB3 in response to abiotic stresses[J]. Gene,2020,740:144514.
[24] LU X,YANG L,YU M Y,LAI J B,WANG C,MCNEIL D,ZHOU M X,YANG C W. A novel Zea mays ssp. mexicana L. MYC-type ICE-like transcription factor gene ZmmICE1,enhances freezing tolerance in transgenic Arabidopsis thaliana[J]. Plant Physiology and Biochemistry,2017,113:78-88.
[25] YANG X Y,WANG R,HU Q L,LI S L,MAO X D,JING H H,ZHAO J T,HU G B,FU J X,LIU C M. DlICE1,a stress-responsive gene from Dimocarpus longan,enhances cold tolerance in transgenic Arabidopsis[J]. Plant Physiology and Biochemistry,2019,142:490-499.
[26] GUTTIKONDA S K,VALLIYODAN B,NEELAKANDAN A K,TRAN L S P,KUMAR R,QUACH T N,VOOTHULURU P,GUTIERREZ-GONZALEZ J J,ALDRICH D L,PALLARDY S G,SHARP R E,HO T H D,NGUYEN H T. Overexpression of AtDREB1D transcription factor improves drought tolerance in soybean[J]. Molecular Biology Reports,2014,41(12):7995-8008.
[27] HAAKE V,COOK D,RIECHMANN J,PINEDA O,THOMASHOW M F,ZHANG J Z. Transcription factor CBF4 is a regulator of drought adaptation in Arabidopsis[J]. Plant Physiology,2002,130(2):639-648.
[28] FENG W Q,LI J,LONG S X,WEI S J. A DREB1 gene from zoysiagrass enhances Arabidopsis tolerance to temperature stresses without growth inhibition[J]. Plant Science,2019,278:20-31.
[29] YANG W,LIU X D,CHI X J,WU C A,LI Y Z,SONG L L,LIU X M,WANG Y F,WANG F W,ZHANG C,LIU Y,ZONG J M,LI H Y. Dwarf apple MbDREB1 enhances plant tolerance to low temperature,drought,and salt stress via both ABA-dependent and ABA-independent pathways[J]. Planta,2011,233(2):219-229.
[30] REN M Y,WANG Z L,XUE M,WANG X F,ZHANG F,ZHANG Y,ZHANG W J,WANG M Y. Correction:Constitutive expression of an A-5 subgroup member in the DREB transcription factor subfamily from Ammopiptanthus mongolicus enhanced abiotic stress tolerance and anthocyanin accumulation in transgenic Arabidopsis[J]. PLoS One,2019,14(12):e0227290.
[31] 徐小艷,姚新转,吕立堂,赵德刚. 烟草NtNAC1基因的克隆及其在烟草中的抗旱功能分析[J]. 植物生理学报,2018,54(6):1085-1094.
XU Xiaoyan,YAO Xinzhuan,L? Litang,ZHAO Degang. Cloning of NtNAC1 gene from Nicotiana tabacum and its analysis of drought-resistant function[J]. Plant Physiology Journal,2018,54(6):1085-1094.
[32] AGARWAL P K,AGARWAL P,REDDY M K,SOPORY S K. Role of DREB transcription factors in abiotic and biotic stress tolerance in plants[J]. Plant Cell Reports,2006,25(12):1263-1274.
[33] LIANG Y Q,LI X S,ZHANG D Y,GAO B,YANG H L,WANG Y C,GUAN K Y,WOOD A J. ScDREB8,a novel A-5 type of DREB gene in the desert moss Syntrichia caninervis,confers salt tolerance to Arabidopsis[J]. Plant Physiology and Biochemistry,2017,120:242-251.

