宁夏不同子产区霞多丽葡萄内源激素及果实品质差异研究
王宁 张艳霞 李栋梅 王振平


摘 要:【目的】揭示贺兰山东麓不同子产区霞多丽葡萄果实发育过程中内源激素含量差异,了解不同产区果实品质形成的变化规律。【方法】以镇北堡产区(ZBB,砾石土,志辉源石酒庄)、玉泉营产区(YQY,风沙土,玉泉国际酒庄)、鸽子山产区(GZS,淡灰钙土,西鸽酒庄)3个子产区霞多丽葡萄为试材,使用超液相色谱-质谱联用仪(ultra performance liquid chromatography tandem mass spectrometry,UPLC-MS)测定葡萄果实发育过程中果皮、果肉和种子中脱落酸(abscisic acid,ABA)、生长素(indole acetic acid,IAA)、赤霉素(gibberellic acid,GA3)、异戊烯腺嘌呤(isopentene adenine,2-IP)和反式玉米素核苷(trans zeatin riboside,tZR)含量,使用高效液相色譜法(high performance liquid chromatography,HPLC)测定葡萄果实中葡萄糖、果糖、苹果酸、酒石酸含量。采用实时荧光定量PCR法(quantitative real-time PCR,qRT-PCR)检测脱落酸代谢途径相关基因表达量。【结果】在霞多丽葡萄果实生长发育过程中,各组织ABA含量在E-L 35时期达到峰值,GZS最高;GA3总体呈“M”形变化趋势;IAA含量总体呈下降趋势,E-L 35之后种子中IAA在较低水平浮动;果皮中2-IP含量逐渐上升后在采收期下降。3个子产区霞多丽葡萄果实各组织内源激素含量存在差异,在转色期差异显著。在不同风土特征条件下,果实百粒质量和可滴定酸含量均为ZBB最高;果实单糖(果糖、葡萄糖)含量和固酸比表现为YQY>GZS>ZBB;苹果酸含量为ZBB>GZS>YQY;酒石酸含量则YQY最高,GZS最低。E-L 35时期VvNCED在GZS果实中表达量显著高于其他两个产区,ZBB霞多丽葡萄果实中VvHYD2表达量显著高于GZS和YQY。【结论】YQY种植的霞多丽葡萄含糖量和固酸比高于其他产区,表明霞多丽葡萄对YQY的风土有更好的适应性。ZBB砾石土含氮量高,有机质丰富,霞多丽葡萄果实中IAA、tZR含量高于其他产区,ABA含量低于其他产区,果实含糖量低,酸度高,成熟度较低。
关键词:霞多丽葡萄;产区;内源激素;果实品质
中图分类号:S663.1 文献标志码:A 文章编号:1009-9980(2023)07-1374-12
Effect of different terroir conditions on endogenous hormones and berry quality of Chardonnay at the East Helan Mountains
WANG Ning1, ZHANG Yanxia2, LI Dongmei1*, WANG Zhenping1*
(1School of Agricultural, Ningxia University, Yinchuan 750021, Ningxia, China; 2School of Life Science, Ningxia University, Yinchuan 750021, Ningxia, China)
Abstract: 【Objective】 This study aimed to investigate the changes of endogenous hormone content and fruit quality of Chardonnay grape cultivated in different terroir conditions, and to clarify the relationship between the endogenous hormones and fruit quality in order to provide a theoretical basis for Chardonnay flavor formation in each sub-production region of the Eastern foothills of the Helan Mountain in Ningxia. 【Methods】 We used Chardonnay (Vitis vinifera L.) in three sub-production areas of ZhenBeiBu (Gravelly soil, ZBB, Yuanshi vineyard), YuQuanYing (Aeolian soils, YQY, Chateau yuquan) and GeZiShan (Sierozem, GZS, Xige estate) as experimental materials. The berries were sampled at five periods, E-L 33 (Berries still hard and green), E-L 35 (Berries begin to colour and enlarge), E-L 37 (Berries not quite ripe) and E-L 38 (Berries harvest-ripe), and were frozen with liquid nitrogen. The berry skin, pulp and seed were ground to powder in liquid nitrogen and 10 mL of 80% methanol was added to 1 g of the powder. The solution was extracted overnight in the dark at 4 ℃ in a shaker. The mixture was then centrifuged at 12 000 × g and 4 ℃ for 5 min and the supernatant was collected. The extraction process was repeated three times and the collected supernatant was pooled and the methanol was evaporated off by nitrogen blowing apparatus. The remaining material was extracted by adding 1.5 times of petroleum ether, and the ether phase was discarded. The same volume of methyl for mate was added for extraction and the ester phase was collected. The ester phase obtained was blown dry by nitrogen blowing apparatus, dissolved with chromatographic methanol, passed through a 0.22 μm filter membrane and stored at -20 ℃. The endogenous hormone content of abscisic acid (ABA), indole acetic acid (IAA), gibberellin A3 (GA3), isopentene adenine (2-IP), and trans zeatin riboside (tZR) in fruit skin, flesh and seed during fruit development were determined by UPLC-MS (Ultra Performance Liquid Chromatography Tandem Mass Spectrometry). The contents of glucose, fructose, malic acid and tartaric acid in the fruits were determined by HPLC. The contents of individual sugar, organic acid were determined by HPLC according to a previously described method with minor modifications. The juice was contrifuged at 12 000 ×g for 10 min, The supernatant was extracted and filtered through a 0.22 ?m filter and used for subsequent analysis. The separating column were Hypersil GOLDTM Amino (250 mm×4.6 mm, 5 ?m) and C18 (250 mm×4.6 mm, 5 ?m) column. The acetonitrile-water mixture (75∶25) and methanol-KH2PO4 mixture (3∶97) were the mobile phase at flow rate of 0.5 mL·min-1 and 0.8 mL·min-1. The column temperature of 25 ℃ and injection volume of 10 μL were adopted. The analysis was carried out with three technical replicates and the corresponding sugar and acid content were calculated using a standard curve. The 100-grain weight, titratable acid and total soluble solids content of grape berries were determined. Quantitative real-time PCR (qRT-PCR) was used to detect the expression levels genes related to abscisic acid metabolism, total RNA was used as the template for reverse transcription according to the Prime ScriptTM RT Reagent Kit with gDNA Eraser Reverse Transcription Kit instruction. The VvActin was selected as the internal reference, and the primers of the VvNCED2, VvNCED4, VvAAO3 and VvHYD2 genes were designed with Primer 5. 【Results】The fruit quality indexes of the same variety cultivated in the three areas were significantly different. The glucose content of Chardonnay fruit at harvest was 84 mg·g-1 in YQY, 76 mg·g-1 in GZS, and 75 mg·g-1 in ZBB, and the fructose content was 105 mg·g-1 in YQY and 84 mg·g-1 in ZBB. The malic acid continued to decline in Chardonnay fruit development, with 9.6 mg·g-1 in the fruit of ZBB at maturity, 39% higher than that of GZS. The content of the tartaric acid did not change significantly at the later stage of fruit development, with the highest content in YQY and the lowest in GZS at maturity. During the growth of Chardonnay fruit, the ABA content in the fruit tissues of the three areas was highest during the E-L 35 period and gradually decreased as the fruit matured, and the ABA content in the fruit of the GZS was higher than that of the other areas; the GA3 content in the peel had a peak during the E-L 36 period, the GA3 content in the flesh showed an “M” change trend. The IAA content in the peel and flesh showed a decreasing trend, the IAA content in the peel in ZBB appellation was the highest and it was lower in the seeds after E-L 35, with insignificant differences among areas; the 2-IP content in the peel gradually increased in GZS and YQY and decreased at harvest; the tZR content in the flesh of Chardonnay showed an increasing trend, with the lowest tZR content in YQY. The endogenous hormone content of Chardonnay fruits in the three sub-appellations differed from each other, and the differences were significant at the E-L 35. The VvNCED expression during E-L 35 was significantly higher in the fruit of GZS than those of the other two production areas, and the VvHYD2 expression in the fruit of ZBB was significantly higher than those of GZS and YQY. 【Conclusion】 The sugar content and the solidity-acid ratio of Chardonnay grown in YQY aeolian soils were higher than that in other regions, indicating that Chardonnay was better adapted to YQYs terroir. The ZBB gravel soil has the highest nitrogen content and rich organic matter, and the fruit of Chardonnay grown there had higher IAA and tZR content, lower ABA content than the fruits in other producing areas, and had lower sugar content, higher acidity content and lower ripeness than the fruits in the other two areas.
Key words: Chardonnay grape; Production regions; Endogenous hormones; Fruit quality
葡萄(Vitis vinifera L.)是世界上种植广泛的水果作物之一,种植区域为除南极洲以外的所有大陆[1]。宁夏贺兰山东麓酿酒葡萄种植条件得天独厚,是生产优质葡萄酒的绝佳产区。但由于贺兰山东麓冲积扇,不同地块土质形成差异较大,不同子产区之间存在风土差异。风土条件的差异对葡萄品质的形成以及特色葡萄酒的生产发挥着重要作用[2-3],风土主要包括了气候[4]、土壤、地形、海拔[5-6]和生物多样性等因素[7-8]。
气候作为风土系统的组成部分之一,为葡萄生长发育提供必不可少的光、热、水、气条件。气候在空间上(区域气候)和时间上(年效应)均有变化,对酿酒葡萄品质形成至关重要,尤其与葡萄果实酸度密切相关[9]。良好的气候条件可以让葡萄树体生长健壮,果实完全成熟,使得葡萄有较高的含糖量和协调的糖酸比,为生产优质葡萄酒提供保障。土壤为葡萄生命活动提供了所必需的水和营养元素,从而保证了葡萄生长发育的正常进行。不同的土壤理化特性直接影响着葡萄根系的生长和养分吸收。虽然酿酒葡萄在世界各地广泛种植,有着强的适应性,但葡萄和葡萄酒的成分仍会因土壤类型的不同而影响最终的口感。前人研究表明土壤相对贫瘠,可以为葡萄根系深扎提供有利的条件,能够汲取多种类的营养成分满足自身生长需要,葡萄在胁迫环境下生长,风土特色会有更好的表达[10]。研究表明,土壤中微量元素含量与葡萄可滴定酸含量呈正相关,土壤氮素供应水平可影响葡萄的代谢物[11],含氮过高会对葡萄的库源关系和冠层微气候产生负面影响,降低葡萄产量和品质[12]。此外,土壤中水分有效性还会影响葡萄激素的平衡,进而影响果实品质。
长期以来,植物内源激素被认为是植物适应外界环境的中枢调节因子,复杂的植物激素信号通路使植物能够激活有效的生理反应[13]。当暴露于不利环境中时气孔关闭是植物最早作出的反应,ABA是气孔关闭的正向调节因子,而IAA和CTK是气孔关闭的负向调节因子[14-15]。此外,ABA和CTK等内源激素通过整合环境刺激和内源信号,进而调控植物对非生物胁迫的防御反应[16-17]。与此同时,内源激素作为一种必不可少的生长调节剂,引导植物适应外界环境,增强植物对环境的耐受性[18]。目前国内外有关葡萄的风土研究众多,然而,这些研究大多侧重于特定因素对果实某单一品质的影响,而一般不考虑风土综合的影响,通过内源激素说明不同风土条件影响葡萄果实品质还鲜有报道。
笔者在本研究中以霞多丽葡萄为试验材料,探究霞多丽葡萄对宁夏贺兰山东麓3个重要葡萄酒产区不同风土的适应性,分别从果实品质和内源激素层面进行分析比较,以期为风土影响霞多丽葡萄不同品质形成提供理论基础和实践依据。
1 材料和方法
1.1 试验地概况
试验地分别位于镇北堡产区(ZBB,志辉源石酒庄),玉泉营产区(YQY,玉泉国际酒庄)和鸽子山产区(GZS,西鸽酒庄)。葡萄园均为南北行向,常规管理。各试验地气候数据(从2021年5月25日至9月1日收集),均由试验地小气象站获得,如表1所示。
1.2 试验材料
霞多丽葡萄果实按照E-L系统[19]采自5个物候期,分别是E-L 33(绿果期)、E-L 35(果实开始转色)、E-L 36(果实转色完成)、E-L 37(果实未完全成熟)和E-L 38(果实成熟期)。采样兼顾阴阳面,果穗上中下部位随机剪取霞多丽葡萄果实300粒。采后立即用液氮速冻,装入泡沫冰盒运回实验室,保存在-80 ℃冰箱。
1.3 实验方法
1.3.1 土壤基本指标的测定 在转色期对各葡萄园每个采样区,以树干为中心,采集距离主干30 cm的行内土壤,土层分别为0~30、>30~60、>60~90 cm。将土运回实验室除去石块和植物残根等杂物,风干后过2 mm筛子,用于土壤理化性质、肥力指标的测定。各土壤类型及其3个土层深度各有3个重复。采用雷磁pH计测定pH;采用重铬酸钾法测定土壤有机质含量;采用自动凯氏定氮法测定土壤全氮含量;采用HCLO4-H2SO4消煮钼锑抗比色法测定土壤全磷含量,采用醋酸铵浸提-火焰光度法测定土壤速效钾含量[20]。
1.3.2 果实基本理化指標的测定 使用电子天平测定葡萄果实的百粒质量;将葡萄榨汁后取上清液,通过酸碱中和滴定法测定可滴定酸(TA)含量;可溶性固形物(TSS)含量采用手持折光仪测定。
1.3.3 果实不同组织内源激素的测定 采用超高效液相色谱串联质谱法[21]。提取:用镊子将霞多丽葡萄浆果的果皮、果肉和种子分离,各组织在液氮中研磨。取1 g左右样品粉末用5 mL预冷的80%甲醇4 ℃提取12 h。4 ℃,8000 r·min-1离心15 min,分离上清液,4 mL 80%甲醇复提2次,每次2 h。合并上清液,氮吹至2 mL,用1.5倍体积石油醚萃取3次,弃醚相。水相用1倍体积甲酸甲酯萃取3次,收集酯相,氮吹至干,用1 mL色谱甲醇进行溶解,-20 ℃条件下保存备用,样品进样前用0.22 μm滤膜过滤。测定:采用高效液相色谱-质谱联用仪(ACQUITY UPLC I class+QDa,美国Waters)对葡萄果肉、果皮、种子中的ABA、IAA、GA3、2-IP、tZR进行测定。采用岛津C18色谱柱(150×4.6 mm,5 μm)。液质条件参照龚明霞等[22]的报道,色谱条件:以100%甲醇(A)和含0.1%甲酸水溶液(B)为流动相,柱温:40 ℃。梯度洗脱程序:0~2 min,35% A;2~6 min,35%~45% A;6~9 min,45%~50% A;9~15 min,50%~60% A;15~18 min,60%~100% A;18~20 min,100%~35% A;20~22 min,35% A,流速:0.3 mL·min-1,上样量:5 μL。质谱条件:采用电喷雾离子源负离子模式(ESI-)检测ABA和GA3含量,采用正离子模式(ESI+)检测IAA、tZR和2-IP含量。离子化电压(+5500/-4500 V);温度550 ℃;气帘气压力30 psi(约200 kPa)。
1.3.4 果实单糖和有机酸含量的测定 采用高效液相色谱法[23]。在液氮条件下将霞多丽葡萄果实磨成粉末,称取0.2 g放入离心管中,然后加入1.8 mL ddH2O稀释。将稀释后的果汁过0.22 μm的针头式过滤器,加入进样瓶,利用UltiMate 3000型高效液相色谱仪测定。糖测定色谱条件为:示差检测器,Hypersil GOLDTM Amino色谱柱(250 mm×4.6 mm,5 ?m),流动相为乙腈∶水(体积比75∶25),流速:0.5 mL·min-1,柱温:25 ℃,进样量:10 μL。有机酸测定色谱条件为:紫外检测器,C18色谱柱(250 mm×4.6 mm,5 ?m),流动相:甲醇、0.01 mol·L-1 KH2PO4体积比3∶97,pH=2.5,流速:0.8 mL·min-1,柱温:35 ℃,检测波长为210 nm,进样量:10 μL。
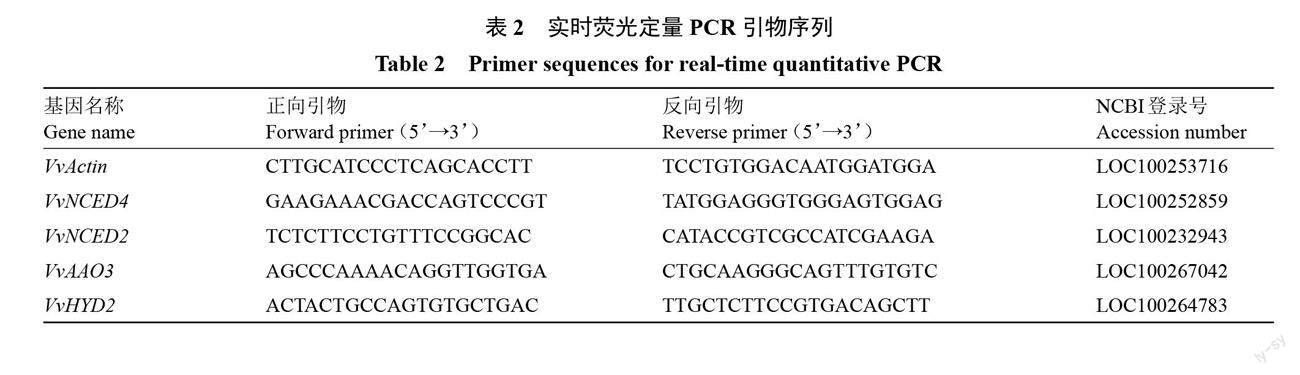
1.3.5 总RNA的提取和实时荧光定量PCR 总RNA提取采用RNAprep Pure多糖多酚植物总RNA提取试剂盒;以总RNA为模板,利用参照PrimeScriptTM RT reagent Kit with gDNA Eraser反转录试剂盒说明书进行反转录;选取VvActin为内参基因,VvNCED4、VvAAO3、VvNCED2、VvHYD2基因的引物用Primer 5.0设计,引物由生工生物工程(上海)公司合成(表2)。qRT-PCR反应体系为20 μL:cDNA(200 ng·μL-1)1 μL,上游引物和下游引物各0.4 μL,2×Perfecstar 10 μL,ddH2O 8.2 μL;qRT-PCR扩增程序为94 ℃ 2 min、94 ℃ 10 s、60 ℃ 30 s,第2步至第3步循环40次。每个模板设3次生物学重复,取其平均值,目的基因的相对表达量用2-ΔΔCt计算[24]。
1.4 数据处理
本试验所得数据使用SPSS 26.0进行方差显著性分析,显著水平p<0.05,采用Excel 2010和RStudio对数据进行整理和作图,所有试验数据均为3次重复。
2 结果与分析
2.1 不同产区土壤理化指标分析
如表3所示,不同产区因土壤类型不同,土壤指标差异较大。各产区土壤pH值均在8.2以上,属于弱碱性土壤,3种土壤的pH值均随着土层深度加深逐渐升高,不同土壤间并没有显著性差异。不同土壤类型中全氮的变化为:随着土层的深度增加,>60~90 cm处GZS淡灰钙土的全氮含量比0~30 cm土层增加了64.6%,而ZBB砾石土、YQY风沙土全氮含量随着土层深度的增加逐渐降低。土壤中各土层的全磷含量表现为:ZBB砾石土>GZS淡灰钙土>YQY风沙土;土壤速效钾含量:GZS淡灰钙土>ZBB砾石土>YQY风沙土。对于土壤中的有机质变化,各土层呈现相同趋势:ZBB砾石土>GZS淡灰钙土>YQY风沙土。
2.2 不同产区霞多丽葡萄果实不同组织内源激素的比较
如图1所示,在霞多丽葡萄果实发育过程中,不同组织中ABA含量呈现先升高后降低的趋势,且均在转色期达到峰值。E-L 35时期各产区霞多丽葡萄ABA含量差异最大:GZS>YQY>ZBB。GZS果皮中ABA含量是YQY的2.3倍和ZBB的15.0倍;GZS果肉中ABA含量分别是YQY和ZBB的4.4倍和39.9倍;GZS种子中ABA含量是YQY的3.7倍和ZBB的18.8倍。由图1得出果皮中GA3含量在E-L 36时期有一个明显峰值,ZBB霞多丽葡萄果皮GA3含量最高为88 ng·g-1,较GZS和YQY分别高出13%和37.5%。果肉中GA3的含量变化有2个峰值,第1个峰值出现在E-L 35时期(GZS>YQY>ZBB),在E-L 37时期,霞多丽葡萄在所有产区中都出现第2个峰值,其含量为YQY>GZS>ZBB。种子中GA3含量整体呈下降趋势,并且其含量水平高于果皮和果肉。霞多丽葡萄果皮中IAA含量在整个果实发育期呈逐渐下降趋势,各产区之间IAA含量存在差异:ZBB>YQY>GZS。果肉中各产区IAA含量均在E-L 35时期开始下降;种子中IAA含量从E-L 33时期开始急剧下降;E-L 35之后没有较大浮动;在整个发育期中,种子中IAA在E-L 33时期含量最高。
如图2所示,霞多丽葡萄果皮中2-IP含量在E-L 37时期均达到峰值(ZBB除外)。种子中YQY在E-L 37时期达到峰值(16 ng·g-1),ZBB和GZS在E-L 38时期达到峰值,分别为10 ng·g-1和18 ng·g-1。在霞多丽葡萄果实发育过程中,不同组织tZR积累模式存在差异,E-L 35时期,果皮中tZR含量在ZBB最高为110 ng·g-1。tZR含量在果肉中随果实发育期逐渐上升,YQY产区在各时期均低于其他2个产区。种子中各产区tZR含量呈下降趋势,在成熟期趋于平稳。
2.3 不同产区霞多丽葡萄果实品质比较
由表4可知,采收期(E-L 38)ZBB的霞多丽葡萄果实百粒质量为158.2 g,分别高于GZS 13.5%和YQY 32.3%;可溶性固形物含量無显著差异;可滴定酸含量:ZBB>GZS>YQY;采收期3个产区霞多丽葡萄果实固酸比之间有显著差异,YQY果实成熟度最高,GZS次之,ZBB最低。
由图3可知,在霞多丽葡萄果实发育过程中,单糖(葡萄糖、果糖)含量变化趋势相同,总体呈上升趋势。采收期,YQY霞多丽葡萄果实葡萄糖含量分别高出GZS 10.5%和ZBB 12%。E-L 38时期果糖含量差异显著,YQY果糖含量(ρ)最高,为105.7 g·L-1,分别高出GZS 14%、ZBB 26%。霞多丽葡萄果实苹果酸呈下降趋势,采收期苹果酸含量:ZBB>GZS>YQY。酒石酸与苹果酸表现不同,逐步下降后在成熟后期基本不变,采收期酒石酸含量在YQY果实中最高为8.4 g·L-1,在GZS霞多丽葡萄果实中最低,为6.8 g·L-1。
2.4 霞多丽葡萄果实品质和内源激素的相关性分析
为了更进一步说明果实品质和内源激素的关系,进行了相关性分析,由图4可知,可溶性固形物和葡萄糖、果糖含量呈极显著正相关(p<0.01),可溶性固形物含量与可滴定酸、苹果酸、酒石酸含量呈显著负相关,百粒质量与单糖含量呈显著正相关(p<0.05),果皮中2-IP含量与葡萄糖、果糖含量呈显著正相关(p<0.05),IAA、tZR含量与单糖含量呈显著负相关(p<0.05)。果肉中2-IP含量与ABA含量呈显著正相关(p<0.05)。
2.5 不同产区对脱落酸代谢相关基因表达的影响
如图5所示,通过对3个产区霞多丽葡萄果实不同组织的内源激素含量进行分析,发现在果皮、果肉和种子中,E-L 35时期3个产区霞多丽葡萄果实中ABA含量较其他激素差异最大。于是对各产区霞多丽葡萄ABA生物合成和分解代谢相关基因进行定量,9-顺式环氧类胡萝卜素双加氧酶(NCED)是ABA生物合成的关键限速酶,本试验中研究了2个编码NCED的基因(VvNCED2和VvNCED4),VvNCED在霞多丽葡萄果实中表达量先上升后下降,在E-L 35时期高表达,GZS果实中表达量显著高于其他2个产区,VvNCED2表达量分别是YQY的3.73倍和ZBB的8.16倍,VvNCED4是YQY的36.19倍、ZBB的8.95倍。ABA生物合成的最后一步是AAO3将脱落醛氧化生成ABA,VvAAO3表达量在果实发育过程中逐渐下降。E-L 33时期,YQY果实中VvAAO3表达量最高,是GZS的2.69倍、ZBB的5.87倍。然而其他时期,3个产区之间差异不大。VvHYD2是负责ABA羟基化分解代谢的基因,随着果实发育呈先上升后下降的变化趋势。E-L 36时期之前VvHYD2表达量在ZBB霞多丽葡萄果实中的表达量显著高于GZS和YQY。
3 讨 论
酿酒葡萄栽培历史悠久,具有不同性状(大小、形状、颜色、味道等)的葡萄品种被选择性地种植,而风土与这些性状息息相关[25]。葡萄浆果的酸度不仅影响葡萄酒的风味,而且影响葡萄酒的口感。本研究中,果实可滴定酸含量ZBB最高,可能与成熟季节不同产区的环境因子有关:首先ZBB植被覆盖率高,气候凉爽,不同微气候会使葡萄果实产生不同的含酸量[7];其次ZBB砾石土的特点是矿质离子丰富,但是土质黏重、通透性较差,造成葡萄成熟度低,酸度较之其他产区要高。固酸比是体现产区特色的重要评价指标,常用于评价果实的成熟程度和风味特色,在一定范围内,固酸比越高,越能酿造出具有特色的葡萄酒[26]。ZBB与其他产区相比固酸比最低,且差异显著,这与陈仁伟等[26]的研究结果一致,他们认为砾石土葡萄园可以通过适当延后采收来获得更有特色的高质量葡萄酒。与此同时,本研究中风沙土种植的葡萄成熟度较高,果实糖分含量高;灰钙土种植的葡萄成熟度适中,品质形成较为适宜,与王锐[27]的研究结果一致。这两种土壤虽表层贫瘠,但透气性较好,利于葡萄扎根,种植的葡萄高糖适酸,为酿造优质葡萄酒提供了保障。
本研究结果表明,ZBB葡萄果皮中IAA含量最高,这可能与其土壤有机质含量丰富、氮含量高有关。前人研究发现土壤施氮量的增加对植物各器官中IAA含量有显著影响,并能促进松木幼苗的生长[28]。细胞分裂素主要在果实坐果期起着重要作用,一般以活性较高的tZR、2-IP为主。ZBB因土壤高含氮量和丰富的有机质导致霞多丽葡萄果实中tZR含量高于其他产区,这与前人[29]研究一致,施用氮肥通过上调细胞分裂素合成关键酶基因的表达,会促进反式玉米素型细胞分裂素的合成。本试验霞多丽葡萄果皮和果肉中,2-IP含量在发育过程中增加,与B?ttcher等[30]的研究结果一致,这表明2-IP可能参与了葡萄成熟过程。此外,还有研究报道细胞分裂素可诱导与细胞扩张、细胞壁相关的基因表达,引起细胞壁特性变化[31]。因此,在一定程度上,转色结束后2-IP含量的升高影响浆果细胞的扩张。GZS霞多丽葡萄ABA含量高于其他产区,2-IP含量也高于其他产区,并且在果实果肉中2-IP含量与ABA含量呈显著正相关(p<0.05),这两种激素之间似乎存在某种联系,有待进一步研究。
脱落酸(ABA)在调节植物发育成熟和对环境刺激(如水分亏缺、盐碱胁迫等)的适应中起着至关重要的作用[32]。本试验中,不同产区霞多丽葡萄果实内源激素ABA差异最大,表现为GZS>YQY>ZBB。而GZS与ZBB果实ABA含量差异的根本原因可能是葡萄园土壤含水量的不同。有研究发现,适度水分胁迫诱导了内源ABA含量的增加,有利于葡萄品质的提升[33]。本研究中,GZS霞多丽葡萄单糖含量高,酒石酸含量低,说明GZS葡萄处于适度水分胁迫状态。此外,前人研究认为气候和土壤通过根系水分吸收和叶片水分流失相互作用控制浆果品质[34],优良的果实品质是对葡萄植株适度水分条件的反映[35-36]。9-顺式环氧类胡萝卜素双加氧酶(NCED)催化9-顺式新黄质合成黄氧素,被认为是ABA生物合成的關键,可被干旱、盐渍等胁迫迅速诱导,并在内源ABA积累中发挥主要作用[37]。本研究表明,GZS霞多丽葡萄果实合成NCED酶的基因表达量显著高于ZBB,同时GZS霞多丽葡萄果实各组织内源ABA含量也显著高于ZBB,二者结果相互印证。Luchi等[38]研究发现VvNCED的表达控制内源ABA水平,且受干旱胁迫的影响。对于ZBB霞多丽葡萄果实ABA含量显著低于其他两个产区还有一种解释,即果实中ABA含量由生物合成和分解代谢共同决定。ZBB霞多丽葡萄果实中分解ABA的基因VvHYD2的表达量显著高于GZS和YQY,也有可能是其ABA含量过低的原因。然而ZBB霞多丽葡萄果实中合成ABA基因的表达量低于其他产区,同时,分解ABA的基因表达量显著高于其他产区,具体原因有待进一步探究。
4 结 论
本研究以霞多丽葡萄为试验材料,通过对宁夏不同子产区果实内源激素和果实品质的测定,发现YQY葡萄含糖量、固酸比高于其他两个产区,表明霞多丽葡萄对于YQY的风土有更好的适应性;ZBB土壤含氮量高、有机质丰富,果实生长类激素,如IAA、tZR含量高于其他产区,果实成熟时的ABA含量低于其他产区,果实成熟度较低,可适当延后采收。
参考文献 References:
[1] MART?NEZ-BRACERO M,ALC?ZAR P,VELASCO-JIM?NEZ M J,CALDER?N-EZQUERRO C,GAL?N C. Phenological and aerobiological study of vineyards in the Montilla-Moriles PDO area,Cordoba,southern Spain[J]. The Journal of Agricultural Science,2018,156(6):821-831.
[2] 李玉梅,韦霞霞,李彦彪,贺雅娟,马维峰,马宗桓,毛娟,陈佰鸿. ‘美乐和‘蛇龙珠葡萄在甘肃不同产区的品质评价[J]. 经济林研究,2020,38(4):152-160.
LI Yumei,WEI Xiaxia,LI Yanbiao,HE Yajuan,MA Weifeng,MA Zonghuan,MAO Juan,CHEN Baihong. Quality analysis of ‘Merlot and ‘Cabernet Gernischt grapes in different producing areas of Gansu Province[J]. Non-Wood Forest Research,2020,38(4):152-160.
[3] 周鹏辉,李泽福,李进. 3个白色酿酒葡萄品种在蓬莱不同产区的栽培特性及果实品质对比研究[J]. 山西果树,2016(2):3-6.
ZHOU Penghui,LI Zefu,LI Jin. Comparative study on cultivation characteristics and fruit quality of three white wine grape varieties in different producing areas of Penglai[J]. Shanxi Fruit,2016(2):3-6.
[4] 乔振羽. 不同坡向微气候对酿酒葡萄霞多丽生长发育及果实品质的影响[D]. 银川:宁夏大学,2022.
QIAO Zhenyu. Effects of microclimates of different slope aspects on the growth,development and fruit quaility of wine grape Chardonnay[D]. Yinchuan:Ningxia University,2022.
[5] 毛如志,張国涛,杜飞,邓维萍,邵建辉,赵新节,何霞红,朱书生. 大香格里拉河谷区海拔梯度变化与玫瑰蜜葡萄品质形成的关系[J]. 果树学报,2016,33(3):283-297.
MAO Ruzhi,ZHANG Guotao,DU Fei,DENG Weiping,SHAO Jianhui,ZHAO Xinjie,HE Xiahong,ZHU Shusheng. Study on the relationship between the berry quality composition and altitude gradient for Rose Honey in the Widely Shangri-La Valley[J]. Journal of Fruit Science,2016,33(3):283-297.
[6] 赵亚蒙,尹春晓,梁攀,乐小凤,张振文. 不同海拔对刺葡萄果实风味物质的影响[J]. 果树学报,2018,35(10):1197-1207.
ZHAO Yameng,YIN Chunxiao,LIANG Pan,YUE Xiaofeng,ZHANG Zhenwen. Effects of altitude on berry flavor compounds in spine grapes[J]. Journal of Fruit Science,2018,35(10):1197-1207.
[7] DOBREI A,DOBREI A G,NISTOR E,PO?TA G,M?L?ESCU M,BALINT M. Characterization of grape and wine quality influenced by terroir in different ecosystems from romania cultivated with feteasc? neagr?[J]. Scientific Papers-Series B-Horticulture,2018,62:247-253.
[8] VAN LEEUWEN C,ROBY J P,PERNET D,BOIS B. Methodology of soil-based zoning for viticultural terroirs[J]. Bulletin de l'OIV,2010,83(947):13.
[9] VAN LEEUWEN C,FRIANT P,CHON? X,TREGOAT O,KOUNDOURAS S,DUBOURDIEU D. Influence of climate,soil,and cultivar on terroir[J]. American Journal of Enology and Viticulture,2004,55(3):207-217.
[10] MACKENZIE D E,CHRISTY A G. The role of soil chemistry in wine grape quality and sustainable soil management in vineyards[J]. Water Science and Technology,2005,51(1):27-37.
[11] CHENG X H,LIANG Y Y,ZHANG A,WANG P P,HE S,ZHANG K K,WANG J X,FANG Y L,SUN X Y. Using foliar nitrogen application during veraison to improve the flavor components of grape and wine[J]. Journal of the Science of Food and Agriculture,2021,101(4):1288-1300.
[12] WHITE R E,BALACHANDRA L,EDIS R,CHEN D L. The soil component of terroir[J]. Journal International Des Sciences De La Vigne et DuVin,2007:41(1):9-18.
[13] BERENS M L,BERRY H M,MINE A,ARGUESO C T,TSUDA K. Evolution of hormone signaling networks in plant defense[J]. Annual Review of Phytopathology,2017,55:401-425.
[14] CRIZEL R L,PERIN E C,SIEBENEICHLER T J,BOROWSKI J M,MESSIAS R S,ROMBALDI C V,GALLI V. Abscisic acid and stress induced by salt:Effect on the phenylpropanoid,L-ascorbic acid and abscisic acid metabolism of strawberry fruits[J]. Plant Physiology and Biochemistry,2020,152:211-220.
[15] KARIMI R,GHABOOLI M,RAHIMI J,AMERIAN M. Effects of foliar selenium application on some physiological and phytochemical parameters of Vitis vinifera L. cv. Sultana under salt stress[J]. Journal of Plant Nutrition,2020,43(14):2226-2242.
[16] LI N,EURING D,CHA J Y,LIN Z,LU M Z,HUANG L J,KIM W Y. Plant hormone-mediated regulation of heat tolerance in response to global climate change[J]. Frontiers in Plant Science,2021,11:627969.
[17] ILYAS M,NISAR M,KHAN N,HAZRAT A,KHAN A H,HAYAT K,FAHAD S,KHAN A,ULLAH A. Drought tolerance strategies in plants:A mechanistic approach[J]. Journal of Plant Growth Regulation,2021,40(3):926-944.
[18] ARNAO M B,HERN?NDEZ-RUIZ J,CANO A,REITER R J. Melatonin and carbohydrate metabolism in plant cells[J]. Plants,2021,10(9):1917.
[19] COOMBE B G. Growth stages of the grapevine:Adoption of a system for identifying grapevine growth stages[J]. Australian Journal of Grape and Wine Research,1995,1(2):104-110.
[20] 魯如坤. 土壤农业化学分析方法[M]. 北京:中国农业科技出版社,2000:12.
LU Rukun. Methods of soil agrochemical analysis[M]. Beijing:China Agriculture Scientech Press,2000:12.
[21] 高江曼,孟莹,刘庆,王童孟,刘美迎,李汶冰,惠竹梅,张振文. 赤霞珠葡萄生长发育过程中内源激素的变化及其与果实成熟的关系[J]. 食品科学,2017,38(7):167-175.
GAO Jiangman,MENG Ying,LIU Qing,WANG Tongmeng,LIU Meiying,LI Wenbing,XI Zhumei,ZHANG Zhenwen. Changes in endogenous hormones during the development of Vitis vinifera L. cv. Cabernet Sauvignon and their relationship with berry ripening[J]. Food Science,2017,38(7):167-175.
[22] 龔明霞,王日升,何龙飞,王萌,赵虎,吴星,何志. 超高效液相色谱-三重四级杆串联质谱法同时测定植物组织中多种激素[J]. 分析科学学报,2016,32(6):789-794.
GONG Mingxia,WANG Risheng,HE Longfei,WANG Meng,ZHAO Hu,WU Xing,HE Zhi. Simultaneous determination of multiple phytohormones in plant tissues by ultra-high performance liquid chromatography-triple quadrupole tandem mass spectrometry[J]. Journal of Analytical Science,2016,32(6):789-794.
[23] 李栋梅,王振平,李相怡,孙思捷,刘博洋,李嘉佳,王磊,王世平. 根域限制对玫瑰香葡萄果实糖酸及酚类物质和内源激素的影响[J]. 果树学报,2022,39(3):376-387.
LI Dongmei,WANG Zhenping,LI Xiangyi,SUN Sijie,LIU Boyang,LI Jiajia,WANG Lei,WANG Shiping. Effect of root restriction on the quality and endogenic hormone of grape berry(Vitis vinifera L. ‘Muscat Hamburg)[J]. Journal of Fruit Science,2022,39(3):376-387.
[24] LI D M,PANG Y J,LI H,GUO D H,WANG R Q,MA C,XU W P,WANG L,WANG S P. Comparative analysis of the gene expression profile under two cultivation methods reveals the critical role of ABA in grape quality promotion[J]. Scientia Horticulturae,2021,281:109924.
[25] BARTHA I,JURJ B,BUTA M,NEGRU?IER C,PAULETTE L. Analysis of terroir components in the vineyard of Silvania[J]. ProEnvironment,2021,14(45):7-19.
[26] 陈仁伟,张晓煜,杨豫,王静,张亚红,胡宏远,丁永平. 贺兰山东麓砾石葡萄园赤霞珠最佳采收期的确定[J]. 中国农业气象,2020,41(9):564-574.
CHEN Renwei,ZHANG Xiaoyu,YANG Yu,WANG Jing,ZHANG Yahong,HU Hongyuan,DING Yongping. Determination of the optimal harvest period for the grape variety Cabernet Sauvignon in gravel vineyard at the eastern foothills of Helan Mountain[J]. Chinese Journal of Agrometeorology,2020,41(9):564-574.
[27] 王锐. 贺兰山东麓土壤特征及其与酿酒葡萄生长品质关系研究[D]. 杨凌:西北农林科技大学,2016.
WANG Rui. Relationship between soil quality with grape growth and composition at the eastern foot of Helan Mountain wine production regions[D]. Yangling:Northwest A&F University,2016.
[28] PENG Y H,CHEN K L,WANG G L,WEI F R,MA Y P. Nitrogen addition regulates allometric growth by changing the distribution patterns of endogenous hormones in different organs of Pinus tabuliformis[J]. Research Square,2021. DOI:10.21203/rs.3.rs-339221/v1.
[29] KAMADA-NOBUSADA T,MAKITA N,KOJIMA M,SAKAKIBARA H. Nitrogen-dependent regulation of de novo cytokinin biosynthesis in rice:The role of glutamine metabolism as an additional signal[J]. Plant and Cell Physiology,2013,54(11):1881-1893.
[30] B?TTCHER C,BURBIDGE C A,BOSS P K,DAVIES C. Changes in transcription of cytokinin metabolism and signalling genes in grape (Vitis vinifera L.) berries are associated with the ripening-related increase in isopentenyladenine[J]. BMC Plant Biology,2015,15:223.
[31] BRENNER W G,RAMIREDDY E,HEYL A,SCHM?LLING T. Gene regulation by cytokinin in Arabidopsis[J]. Frontiers in Plant Science,2012,3:8.
[32] SUN L,ZHANG M,REN J,QI J X,ZHANG G J,LENG P. Reciprocity between abscisic acid and ethylene at the onset of berry ripening and after harvest[J]. BMC Plant Biology,2010,10:257.
[33] 陳祖民. 水分胁迫对‘美乐葡萄不同组织内源激素和多胺的影响[D]. 银川:宁夏大学,2021.
CHEN Zumin. Effects of water stress on endogenous hormones and polyamines in different tissues of ‘Merlot grape[D]. Yinchuan:Ningxia University,2021.
[34] TRAMONTINI S,VAN LEEUWEN C,DOMEC J C,DESTRAC-IRVINE A,BASTEAU C,VITALI M,MOSBACH-SCHULZ O,LOVISOLO C. Impact of soil texture and water availability on the hydraulic control of plant and grape-berry development[J]. Plant and Soil,2013,368(1):215-230.
[35] JU Y L,YANG B H,HE S,TU T Y,MIN Z,FANG Y L,SUN X Y. Anthocyanin accumulation and biosynthesis are modulated by regulated deficit irrigation in Cabernet Sauvignon (Vitis vinifera L.) grapes and wines[J]. Plant Physiology and Biochemistry,2019,135:469-479.
[36] VAN LEEUWEN C,TR?GOAT O,CHON? X,BOIS B,PERNET D,GAUDILL?RE J P. Vine water status is a key factor in grape ripening and vintage quality for red Bordeaux wine. How can it be assessed for vineyard management purposes[J]. Journal International Des Sciences De La Vigne et DuVin,2009,43(3):121-134.
[37] PASHKOVSKIY P P,VANKOVA R,ZLOBIN I E,DOBREV P,IVANOV Y V,KARTASHOV A V,KUZNETSOV V V. Comparative analysis of abscisic acid levels and expression of abscisic acid-related genes in Scots pine and Norway spruce seedlings under water deficit[J]. Plant Physiology and Biochemistry,2019,140:105-112.
[38] LUCHI S,KOBAYASHI M,TAJI T,NARAMOTO M,SEKI M,KATO T,TABATA S,KAKUBARI Y,YAMAGUCHI-SHINOZAKI K,SHINOZAKI K. Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase,a key enzyme in abscisic acid biosynthesis in Arabidopsis[J]. The Plant Journal,2001,27(4):325-333.

