棉织物过度氧化引起破洞的检测方法
张劲峰 姚春婵 张翊翔 洪剑寒 余国建 孙月平

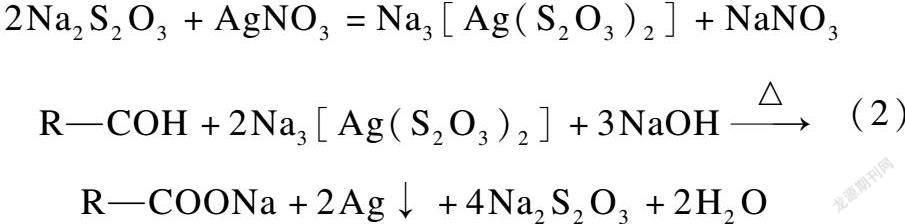
摘要: 棉织物在氧漂工序中容易出现破洞现象,为了查清破洞产生的原因,本文将不同破洞的棉织物放入20 mL硫代硫酸银络合物测试溶液中煮沸2 min,用去离子水冲洗后观察破洞周围的颜色变化。结果表明,由机械等外力造成的破洞,破洞口与布面颜色一致;因局部过度氧化引起的破洞,破洞口颜色比布面颜色深。通过X射线衍射仪、扫描电镜和X射线光电子能谱仪对其进行表征,得出棉织物经测试溶液检测后,表面有银析出,颜色深的洞口含银量比其他部位多,表明这类棉织物破洞是因为局部过度氧化造成的。
关键词: 棉织物;氧漂;破洞;金属离子;醛基纤维素;银原子
中图分类号: TS111.913
文献标志码: A
文章编号: 1001-7003(2023)05-0074-06
引用页码: 051110
DOI: 10.3969/j.issn.1001-7003.2023.05.010
作者简介:
张劲峰(1975),男,工程师,主要从事面料研发及设备改造的研究。通信作者:洪剑寒,教授,jhhong@usx.edu.cn。
棉纤维加工成服装后由于其柔软舒适的触感、丝光处理后的光泽度、吸湿透气性和抗静电性优良而深受消费者的青睐[1-3]。棉花生长环境复杂形成众多天然杂质,而在纺纱和织造等工序中加入抗静电剂、润滑剂、浆料和油剂等,坯布需在高温、强碱、高浓度的氧化剂条件下进行前处理及漂白,去除棉纤维中的杂质、发色基团和共生物,以增加面料的白度、鲜艳度和毛效,提高面料的染色性能[4-6]。棉織物漂白大多采用无污染、性价比高、漂白效果好及去杂能力强的双氧水(H2O2)。H2O2在pH值为碱性且高温时快速释放HOO-,与色素共轭结构中的羰基和醌基等发生氧化反应生成醛基,起到漂白和脱色作用[7-8]。棉纤维在氧化剂存在的条件下羟基很不稳定,容易被氧化生成醛基纤维素,过度氧化使棉纤维分子链在苷键处断裂,造成纤维强力下降引起破洞。在国家要求高能耗印染企业节能减排情况下,大多数染厂对棉布漂白运用冷轧堆一浴一步的高效短流程工艺。这种工艺所加的H2O2比传统工艺多3倍以上,粘有金属离子的棉织物更容易过度氧化而产生破洞[9-11],如何减少棉织物氧漂破洞成为染厂从业者的目标。崔浩然[12]和李兴娜等[13]总结了铁离子的主要来源:生产过程中黏附的油污和铁锈;生产水管中的铁锈水;生产机械上的铁锈和铁屑;设备维修保养粘上油污、铁屑和锈渍。提出预防的措施有:加强坯布检验,铁锈渍用酸清洗;氧漂用过滤水,超过12 h停机,要放掉管道里滞留的水;烧碱高位槽注满碱液沉淀24 h使用澄清部分;氧漂浸轧槽要定期清洗并用磁铁吸除槽内铁杂质;烧毛汽油也要沉淀后才可使用,以防铁锈粉和铁屑吸到汽化器中;氧漂时不能使用丝光回收NaOH液,使用饱和蒸汽且不能使用过热蒸汽;氧漂时加入螯合剂;设备维修保养做好卫生工作,以防金属离子带入槽中。
李兴娜等[13]提出了两种方法检验破洞原因是铁离子造成的,一种是亚铁氰化钾和盐酸溶液,如果布面颜色变成普鲁士蓝则证明含有铁离子,反应式如下:
另一种是菲啰啉铁试剂,如果溶液颜色变红色,则说明棉织物中含有铁离子。但这两种方法只是证明布中或氧漂处理液中含有铁离子,如果是其他金属离子,则不能检测出来,更不能实际反映出布面上黏附情况,如果氧漂处理时溶液中恰好有微量的金属离子,且分布均匀,不会过度氧化引起破洞。而烧毛、勾挂、纱线强力差、受潮发霉、粘上其他化学助剂等产生的这类破洞会被误认为过度氧化引起的,很难追查产生的原因。
本试验主要探讨棉织物实际黏附铁离子的表现情况,棉织物经双氧水漂白处理后生成的醛基纤维素,容易被氧化成含有相同碳原子数的羧酸。如Fehling(斐林)和Tollens(多伦)等弱氧化试剂可以使醛基氧化,利用这个特性,让金属银原子直接在布面上析出,根据布面和破洞口析出量的多少形成颜色深浅,判断双氧水分解激烈程度,从侧面证明棉织物上的破洞是否因过度氧化引起的?如果证明破洞是过度氧化引起的,对于尚未进入生产流程的坯布则可以根据文献[12-13]提出的措施去预防,甚至加多一道酸洗工序提前去除棉布上的金属离子。
1 试 验
1.1 材料与设备仪器
试验材料:9.8 tex×9.8 tex、433根/10 cm×433根/10 cm、平方米质量105 g/m2的全棉府绸(绍兴孚亨纺织科技有限公司),8%硝酸银(实验室自制),螯合分散剂ST121、多功能精炼剂ST233(四川益欣科技有限责任公司),双氧水(omf 30%)、冰醋酸、高锰酸钾、硫酸、硫代硫酸钠(大苏打)、烧碱、FeCl3(上海国药集团化学试剂有限公司)。
设备仪器:SU8010场发射扫描电子显微镜(日本日立公司),Gemini S Ultra X射线衍射仪(英国牛津衍射有限公司),K-Alpha型X射线光电子能谱仪、Nicolet IS20傅里叶变换红外光谱仪(美国赛默飞公司),DHG-9123A电热恒温鼓风干燥箱(上海仪电科学仪器股份有限公司),AS-24恒温水浴振荡器(温州方圆仪器有限公司),标准光源灯箱(深圳市天友利标准光源有限公司)。
1.2 工艺流程
1.2.1 冷堆工艺
全棉府绸坯布→二浸二轧漂白(轧余率125%)→打卷→堆置→热水清洗→醋酸中和水洗→冷水清洗→烘干。工艺配方:烧碱35 g/L,双氧水30 g/L,多功能精炼剂ST233 25 g/L,螯合分散剂ST121 3 g/L,堆置温度40 ℃,堆置时间22 h。
1.2.2 破洞检测工艺
将含量为8%的硝酸银溶液1 mL,20%硫代硫酸钠(大苏打)和20%烧碱混合溶液2 mL,加入17 mL去离子水中,迅速摇匀后,放入待测的破洞布片5 g,在100 ℃煮2 min后用清水冲洗,观察面料破洞口周围变色情况。化学反应方程如下:
1.3 测试与表征
1.3.1 测H2O2的分解率
配制测试液A:H2O2 30 g/L,50 mL;测试液B:H2O2 30 g/L,NaOH 2 g/L,50 mL。
将测试液A、B分别放入两个锥形瓶内,在水浴振荡锅中煮沸30 min,然后从中取出5 mL的氧漂处理液移入100 mL锥形瓶,再加入6 mol/L的H2SO4溶液10 mL并摇匀,然后用0.1 mol/L的KMnO4溶液进行滴定试验,当滴定测试液颜色放置30 s后仍呈现红色即为终点。记录此时KMnO4溶液的体积,滴定5次并算出平均值。由下式计算出H2O2的分解率:
式中:V0为处理前消耗的KMnO4体积,mL;V为氧漂试样30 min消耗的KMnO4体积,mL。
1.3.2 红外光谱分析
采用傅里叶红外光谱仪对面料上析出的Ag进行衰减全反射检测,扫描频率32 s-1,光谱范围为4 000~400 cm-1,分辨率4 cm-1。
1.3.3 X射线衍射(XRD)测试
采用X射线衍射(XRD)对棉织物上所粘的材料在管电压40 kV、管电流100 mA、扫描速率10 °/min、2θ为5°~80°条件下进行表征及分析。
1.3.4 X射线(XPS)分析
采用X射线光电子能谱仪(配备有Al-Kα X射线源1 486.6 eV)对棉织物上所粘的材料通过压片法进行表征,分辨率0.5 eV,灵敏度80 KCPS。
1.3.5 扫描电镜观察
采用扫描电镜(SEM)对棉織物上所粘的材料进行形貌观察及分析,放大倍数1 500倍,工作电压1.0 kV。
2 结果与分析
2.1 铁离子和烧碱对双氧水分解率的影响
根据1.3.1测试方法,分别在去离子水、自来水及含铁离子(FeCl3)10 mg/L的溶液中加入测试液A与B,在100 ℃水浴锅中处理30 min后测试双氧水的分解率,同时在测试液B中加入5 g坯布进行氧漂测试,得到如表1所示的数据。
由表1测试液A试验结果可知,在去离子水中H2O2的分解速度很慢,只有3.3%;当水溶液中含有铁等金属离子时,尽管含量只有10 mg/L,H2O2的分解速度都在加快。这与梁海波等[14-15]水中含Cu2+的试验结果大致相同,同时又提出生物酶、机器壁、极小或有棱角的物体和胶体等表面粗糙的物体都有加速催化H2O2氧化的作用。测试液B由于加了烧碱,在煮沸条件下能大幅加快H2O2的分解率。但在相同条件下与坯布同浴,H2O2的分解率下降了近一半,原因可能是坯布中含有共生物、油剂、润滑剂、浆料及抗静电剂等,对H2O2分解有抑制作用。
2.2 不同破洞检测后银原子在棉布上析出情况
先用滴管在棉织物上滴上2滴含量为10 mg/L的FeCl3溶液放置24 h后,跟正常的棉织物一起进行氧漂处理,取铁离子氧漂后破洞棉织物,再分别取烧毛、勾挂、纱线强力差、受潮发霉引起的破洞经正常工艺的双氧水漂白后的5 g棉织物,一起由1.2.2破洞检测工艺得到如图1所示的对比照片。图1各小图中,左边是各种破洞氧漂后的布面情况,右边是经破洞检测后的布面情况。
由图1可知,由烧毛、勾挂、纱线强力差、受潮发霉引起的破洞经破洞检测工艺测试后,破洞洞口四周与整体棉布颜色一致,说明破洞受到氧漂的程度与整体棉布一样;反观由铁离子氧漂后引起的破洞棉布经破洞检测工艺测试后,破洞洞口四周明显比与整体棉布颜色要深很多,说明在洞口周围氧化纤维素与Ag+反应剧烈,从而析出更多的银原子,进一步说明破洞是由于过度氧化引起的。
2.3 棉布的红外图谱分析
分别对退浆后的棉坯布、双氧水漂白和金属引起氧漂破洞检测后的棉织物,在4 000~400 cm-1进行红外光谱测试,如图2所示。
通过分析图2中退浆后的棉坯布与双氧水漂白棉织物的红外特征吸收峰可知,经过双氧水漂白后的棉织物在1 743 cm-1出现了新的特征吸收峰,即CO典型伸缩振动峰,说明棉织物经过双氧水氧化后有醛基生成;氧漂布与破洞检测后的棉织物对比红外光谱可知,氧漂布在1 743 cm-1出现了新的特征吸收峰经破洞检测后消失,说明醛基与银离子发生了氧化还原反应后,醛基消失,1 743 cm-1吸收峰也随之消失。对比退浆后的棉坯布与双氧水漂白棉织物红外光谱,在1 107 cm-1处消失红外吸收峰值,棉纤维分子伯醇基、仲醇基内—OH键吸收峰,表明分子内—OH在氧化过程中发生了反应。这是棉纤维C6原子上的伯醇基(—OH)氧化为醛基和羧基;C2、C3原子上的两个仲醇基氧化成二醛基纤维素。对比退浆后的棉坯布与双氧水漂白棉织物红外光谱,2 850 cm-1处为—CH2非对称伸缩吸收峰,此处的峰强度稍有增强,这可能是在双氧水在漂白过程中棉纤维的C6原子处使—CH2OH先氧化生成醛基,如果氧化剂足够多,那么再氧化生成了羧基。氧漂布与破洞检测后的棉织物对比红外光谱可知,在2 850 cm-1生成峰消失,说明生成和醛基与银离子发生氧化还原反应,2 850 cm-1处的峰也随之消失。对比退浆后的棉坯布与双氧水漂白棉织物红外光谱,棉坯布红外光谱图中983 cm-1和1 459 cm-1处消失的红外吸收峰是吡喃环上C2、C3原子上的—OH不对称伸缩振动峰,说明棉纤维素在氧化过程中C2、C3原子处的两个仲醇基氧化成二醛基纤维素,在碱性环境下再生成二羧基纤维素,使葡萄糖剩基环进行了开环反应从而发生破裂。
2.4 棉布的表征
2.4.1 棉布的X射线衍射分析(XRD)
对经过氧漂和破洞测试的样品进行XRD分析,结果如图3所示。与氧漂棉布相比,破洞检测的样品在2θ为38.2°、44.5°、64.8°和77.9°处都出现了特征衍射峰,分别对应着在棉布上析出的黑色银原子(111)、(200)、(220)和(311)晶面[16-18]。由于检测液中的银离子含量远少于棉布中的氧化纤维素含量,全部以银原子形式析出,故没有氧化银或其他银的化合物衍射峰出现。
2.4.2 棉布的X射线光电子分析(XPS)
由图4(a)可知,氧漂棉布在285.7 eV处的峰对应的是C1s,在531.3 eV处的峰对应的是O1s;经破洞检测后的棉布除了上述的C1s峰和O1s峰外,还在370 eV左右处出现新的峰,对应的是Ag3d峰,表明Ag原子成功在棉布表面析出。利用高分辨XPS谱图(4(b)),分析棉布中Ag3d的化学态,破洞检测后的棉布367.7 eV处的峰是Ag3d5/2,373.6 eV处的峰是Ag3d3/2,两个分峰结果表明银离子被还原成单质银附着在棉布表面[17-18]。
2.4.3 棉布的掃描电镜分析(SEM)
由图5可知,氧漂后的棉纤维表面非常光洁干净,无凸起颗粒,但经破洞检测后的棉纤维表面出现了细微的黑色颗粒物,这是析出的银原子在纤维表面发生了团聚。
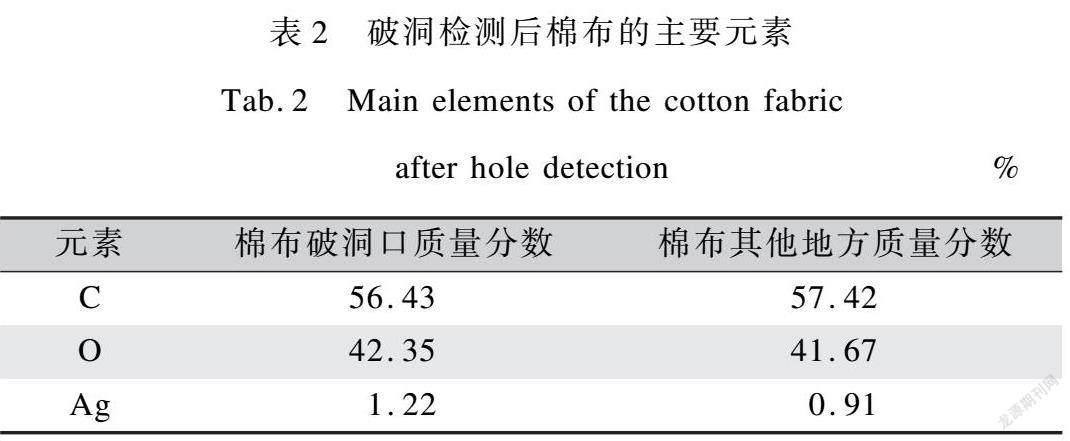
对滴FeCl3的棉布进行氧漂破洞检测,分别对破洞口(颜色深)和其他部位(颜色较浅)进行SEM,得到棉布的主要元素为C、O和Ag,如表2所示。
由表2可知,棉布颜色较深的破洞口处金属银含量较多,说明棉布在局部含较多Fe3+时进行氧漂,纤维受氧化程度较其他地方激烈,易产生破洞。
3 结 论
对烧毛破洞、勾挂破洞、纱线强力差破洞、受潮发霉破洞和过度氧化破洞用自制试剂分别进行破洞检测,并对各自破洞口的颜色与整体棉布颜色进行对比,根据颜色深浅得出氧化程度的差别,可得出如下结论:
1) 棉织物表面金属离子的存在,导致双氧水加速分解,碱性时分解更快,坯布中含有共生物、油剂、润滑剂、浆料及抗静电剂等对H2O2分解有抑制作用。
2) 棉织物金属离子团聚导致过度氧化,破洞口有更多的黑色银原子析出,出现破洞口颜色较布面深;而烧毛破洞、勾挂破洞、纱线强力差破洞、受潮发霉破洞与布面颜色一样,说明氧化程度一致。
参考文献:
[1]程佩, 傅佳佳, 王蕾, 等. 预处理对棉织物免烫整理效果的影响[J]. 纺织学报, 2021, 42(9): 126-130.
CHENG Pei, FU Jiajia, WANG Lei, et al. Influence of pretreatment on anti-wrinkle finishing of cotton fabrics[J]. Journal of Textile Research, 2021, 42 (9): 126-130.
[2]周伟, 林更旺, 贾言星, 等. 丝光棉针织物的染色工艺及性能探究[J]. 纺织导报, 2021(4): 68-72.
ZHOU Wei, LIN Gengwang, JIA Yanxing, et al. Research on dyeing and performance of mercerized cotton knitted fabrics[J]. China Textile Leader, 2021(4): 68-72.
[3]岳任芳, 於琴. 原棉散纤维双面覆网连续轧蒸练漂工艺[J]. 印染, 2021, 47(10): 21-24.
YUE Renfang, YU Qin. Pad-steaming continuous scouring-bleaching process of raw cotton bulk fiber with double-sided covering net[J]. China Dyeing & Finishing, 2021, 47(10): 21-24.
[4]李勇. 棉混纺机织物的清洁短流程前处理工艺[J]. 染整技术, 2021, 43(11): 31-33.
LI Yong. Clean short process pretreatment technology for cotton blended woven fabric[J]. Textile Dyeing and Finishing Journal, 2021, 43(11): 31-33.
[5]李红彪. 棉针织布节能氧漂新工艺[J]. 印染, 2019, 45(10): 21-23.
LI Hongbiao. Energy-saving peroxide bleaching of cotton knitted fabric[J]. China Dyeing & Finishing, 2019, 45(10): 21-23.
[6]石桂刚, 杨文芳. 棉织物前处理方法的研究现状与展望[J]. 染整技术, 2020, 42(7): 6-11.
SHI Guigang, YANG Wenfang. Research status and prospects of cotton fabric pretreatment methods[J]. Textile Dyeing and Finishing Journal, 2020, 42(7): 6-11.
[7]王壮, 王雪燕, 李钰颖, 等. 双氧水低温漂白促进剂研究进展[J]. 染整技术, 2020, 42(4): 9-16.
WANG Zhuang, WANG Xueyan, LI Yuying, et al. Overview of hydrogen peroxide low temperature bleaching accelerator[J]. Textile Dyeing and Finishing Journal, 2020, 42(4): 9-16.
[8]崔双双, 张艳, 高加勇, 等. 棉织物低温氧漂活化剂的制备[J]. 纺织学报, 2016, 37(7): 88-92.
CUI Shuangshuang, ZHANG Yan, GAO Jiayong, et al. Synthesis of peroxide activator for low-temperature bleaching of cotton fabric[J]. Journal of Textile Research, 2016, 37(7): 88-92.
[9]赵文杰, 张晓云, 钟毅, 等. 棉针织物的冷轧堆前处理与染色[J]. 纺织学报, 2016, 37(6): 76-82.
ZHAO Wenjie, ZHANG Xiaoyun, ZHONG Yi, et al. Cold pad-batch pretreatment and dyeing of cotton knits[J]. Journal of Textile Research, 2016, 37(6): 76-82.
[10]施勤, 刘峥嵘. 印染加工过程中常见的质量问题及分析[J]. 纺织科技进展, 2012(1): 47-48.
SHI Qin, LIU Zhengrong. Analysis on common quality problems in dyeing process[J]. Progress in Textile Science & Technology, 2012(1): 47-48.
[11]徐杨, 陈泽敏, 高艳娥, 等. DTPA的合成及在氧漂中的應用[J]. 印染, 2021, 47(10); 16-20.
XU Yang, CHEN Zemin, GAO Yane, et al. Synthesis of DTPA and its application to oxygen bleaching[J]. China Dyeing & Finishing, 2021, 47(10): 16-20.
[12]崔浩然. 如何预防稀薄棉织物的氧漂破洞[J]. 印染, 2014, 40(20): 60.
CUI Haoran. How to prevent oxygen bleaching holes in thin cotton fabrics[J]. China Dyeing & Finishing, 2014, 40(20): 60.
[13]李兴娜, 王立强, 韩祖彬, 等. 氧漂破洞产生的原因分析及防止方法[J]. 染整技术, 2019, 41(12): 43-45.
LI Xingna, WANG Liqiang, HAN Zubin, et al. Analysis of the cause of oxygen bleaching hole and prevention method[J]. Textile Dyeing and Finishing Journal, 2019, 41(12): 43-45.
[14]梁海波, 薛琴芳. 氧漂破洞的产生及防止[J]. 针织工业, 2005(1): 37-39.
LIANG Haibo, XUE Qinfang. A research on the reasons of holes and tackling methods for oxygen bleaching treatment[J]. Knitting Industries, 2005(1): 37-39.
[15]梁海波, 薛琴芳. 针织物氧漂破洞的产生及防止[J]. 印染, 2005(2): 11-12.
LIANG Haibo, XUE Qinfang. Prevention of hole of knitted fabric during oxygen bleaching[J]. China Dyeing & Finishing, 2005(2): 11-12.
[16]KHALILABAD M S, YAZANSHENAS M E, NATEGHI M R, et al. Effect of cationization on adsorption of silver nanoparticles on cotton surfaces and its antibacterial activity[J]. Cellulose, 2009, 16(6): 1147-1157.
[17]KWAK W G, OH M H, GONG M S, et al. Preparation of silver-coated cotton fabrics using silver carbamate viathermal reduction and their properties[J]. Carbohydrate Polymers: Scientific and Technological Aspects of Industrially Important Polysaccharides, 2015, 115: 317-324.
[18]闫利峰. 纳米银整理棉织物的制备及其抗菌性能[J]. 印染助剂, 2020, 37(1): 29-32.
YAN Lifeng. Preparation and antibacterial properties of cotton fabric treated by nanosilver[J]. Textile Auxiliaries, 2020, 37(1): 29-32.
Abstract: In recent years, cotton fabrics, especially summer clothing, have been increasingly favored by young people in domestic market for their mercerization, moisture absorption, breathability and soft touch, and the market potential demanding for cotton cloth has been huge. Under the requirement of energy saving and emission reduction for high energy-consumption printing and dyeing enterprises by the Chinese government, most dyeing plants have adopted the more efficient short process of one-bath one-step cold pad-batch for cotton cloth bleaching. The hydrogen peroxide added in this process is over three times more than that of the traditional process. After oxygen bleaching mercerization of the cotton cloth, local excessive oxidation occurs and oxygen bleaching holes are more likely to occur. In the production process of cotton cloth, the selection process of different cotton cloth specifications is different, resulting in complex reasons for holes. Therefore, it is necessary to find out the causes of holes in the production process and formulate corresponding preventive measures and technological processes. In this experiment, aldehyde cellulose produced by bleaching cotton cloth with hydrogen peroxide was oxidized by a self-made weak oxidation reagent, so that metal silver atoms could be directly precipitated on the cloth surface. According to the number of silver atoms precipitated from the cloth surface and the hole opening, the color depth was formed, the intensity of hydrogen peroxide decomposition was judged, and whether the hole on cotton cloth was caused by excessive oxidation was proved from the side. Then, 1 mL of 8% silver nitrate solution, and 2 mL of mixed solution of 20% sodium bicarbonate and 20% caustic soda were added into 17 mL of deionized water. Then, the mixed solution was shaken up quickly. Subsequently, 5g of cloth with holes was boiled in the solution for 2 min under 100 ℃, and then it was washed with deionized water. The color difference around the hole of that fabric was observed. The cotton cloth with holes were characterized by Fourier transform infrared spectroscopy, X-ray diffraction, scanning electron microscope (SEM) and X-ray photoelectron spectroscopy. The test results of the self-made solution show that the holes caused by mechanical and other external forces have the same color as the holes on the cloth surface, indicating that the silver content precipitated is basically the same. As for holes caused by local overoxidation, the color of the opening is darker than the cloth surface, and the silver content of the hole area is obviously higher than that of other parts. During the test, it is found that the decomposition rate of hydrogen peroxide is affected by temperatures, pH values and metal ions, and the symbionts and textile auxiliaries in gray cloth can inhibit the decomposition of hydrogen peroxide. Fourier transform infrared spectroscopy shows that the structure of most cotton fiber polysaccharides changes into dialdehyde cellulose after oxygen bleaching. After treatment with the self-made hole breaking detection reagent, the aldehyde group and silver ions have oxidation-reduction reaction, and the aldehyde group disappears. Through characterizing the cotton cloth tested by the self-made hole detection reagent, it is concluded that the black particulate matters on the cotton cloth surface are silver atoms. The hole areas such as burnt holes, hook holes, weak yarn strength holes and damp mildew holes are of the same shade as the cloth surface. Nevertheless, compared with the main elements of the cloth surface, there are more black silver atoms in the hole than in other places, which makes the color darker than in other places.
As the demand for oxygen bleached mercerized cotton cloth is gradually increasing in domestic market, it is significant to determine the causes of holes for production prevention, which will be beneficial to cost saving and energy conservation and emission reduction. For the cotton cloth with holes during the production process, the semi-finished cotton cloth can be treated through lower tension process on the machines, increase the friction between yarns by adding adhesives, or even be changed to the overflow or air cylinder with low tension for dyeing, so as to reduce the probability of holes as much as possible and minimize the loss. In the future, the weaving mill will equip the magnetic metal cloth inspection machine in the gray cloth inspection. As long as the fabric has more than a certain amount of metal ions, the metal alarm device on the cloth inspection machine will raise the alarm to prevent the problematic gray cloth flowing into the next process, which guarantees the good quality.
Key words: cotton fabric; oxygen bleaching; hole; metal ion; aldocellulose; silver atom

