Changes in embryonic development, juvenile growth and physiological adaptation of the cuttlef ish Sepia pharaonis in response to photoperiod manipulation*
Maowang JIANG, Huan CHEN, Shuangnan ZHOU, Qingxi HAN, Ruibing PENG,Xiamin JIANG
Key Laboratory of Applied Marine Biotechnology, School of Marine Sciences, Ningbo University, Ningbo 315211, China
Keyword: photoperiod; embryonic development; growth; physiological changes; cuttlef ish
1 INTRODUCTION
During the spawning season from February to April, adult cuttlef ish migrate to shore, congregate in off shore waters for mating, and lay eggs in the shallow coastal waters (5-40-m deep) (Boletzky, 1983;Tehranifard and Dastan, 2011), where egg cases are not only attached but also oxygenated by ocean currents to satisfy embryonic development and light can regulate the development gonads of the reproductive stock (Roper et al., 1984). In recent years, numerous studies have been conducted on the eff ects of biotic and abiotic factors that inf luence embryonic development (Peng et al., 2015; Lee et al.,2016; O’Brien et al., 2018) and juvenile growth performance (Minton, 2004; Anil et al., 2005).Photoperiod is one of the key environmental factors aff ecting the growth, development, and survival of aquatic organisms (Gehrke, 1994). During evolution,organisms have developed physiological and behavioral mechanisms that allow them to adapt to the circadian rhythm of light. However, photoperiod may be one of the critical factors for embryonic development and juvenile growth, as well as one of the most controllable variables in the hatchery.Currently, little attention has been given to the inf luence of “unnatural” light conditions (constant light or darkness), although they are known to aff ect the development of physiological disturbances in the cuttlef ish and their circadian rhythms (Koueta and Boucaud-Camou, 2001).
The light-dark cycle is one of the most important environmental challenge for organisms to survive in the wild. As a result, light-sensitive circadian clocks have changed in most animals including vertebrates and invertebrates. The photoperiod, an internal“zeitgeber”, is known to aff ect the endogenous rhythms in teleosteas and has been shown to be controlled by a well-developed photoreceptive structure in the pineal gland (Simensen et al., 2000;Tuckey and Smith, 2001). Likewise, transcriptions of opsin have been found in the skin of cephalopods,similar to the retina, which is an eff ector-epistellar photoreceptive body (Cobb et al., 1995; Mäthger et al., 2010). Photoperiod has a notable eff ect on the intrinsic aspects of body’s ontogenesis, including metamorphosis, physiological activity, and sexual maturation (Forsythe et al., 1994; Kamler, 2002;Dong et al., 2011; Qiu et al., 2015). Moreover, it also has several external eff ects such as on hatching rate(Shi et al., 2012), the incubation period of embryos(Downing and Litvak, 2002), body weight of newly hatched larvae (Villamizar et al., 2009), juvenile swimming activity and behaviour (Trotter et al.,2003), and subsequent on survival and growth(Moustakas et al., 2004).
It is well-known that most cuttlef ish depend on sight to detect predators and prey, and are strongly inf luenced by lighting (Groeger et al., 2005; Serb and Eernisse, 2008). For each species, there is a minimum light intensity that allows juveniles to catch prey.However, the light intensities that permit the normal development of juveniles vary considerably between species, from 1 lx in striped bass (Moronesaxatilis) to 3 000 lx in Leopard coral grouper (Plectropomusleopardus) (Boeuf and Le Bail, 1999; Yoseda et al.,2008). Sykes et al. (2014) showed that low light intensity (100 lx) improved better growth and survival(vs. 350 and 1 200 lx) of juvenile cuttlef ish for the f irst 50 days after hatching (DAH). In addition to the light intensity, the photoperiod aff ects the behaviour of juvenile cuttlef ish, and they show high levels of nocturnal activity at the age of 30 days, while at the age of 6 months old, prolonged light had no impact on growth performance (Richard, 1971). In conditions of constant light,Sepiaoffi cinalis, which has been cultivated for several generations, has induced a longer life span, and has grown into larger individuals by inhibiting gonad development and sexual maturation (Forsythe et al., 1994). To date, studies on the eff ect of photoperiod on embryonic development are still rare, and the growth performance of juveniles remains limited to a single study (Koueta and Boucaud-Camou, 2003). However, the fertilized cuttlef ish eggs are transparent, soft, easy to observe with the naked eye, and sensitive to external stimuli(inking in the egg capsule), it is necessary to understand how the photoperiod aff ects the embryonic development of those fertilized eggs to reveal changes in the photosensitivity of the species in the course of growth and development.
The purpose of this study was to explore the eff ects of light∶dark cycles (L∶D) on the performance of embryonic development and newly hatched juvenile,including physiological factors related to stress on embryonic development, digestive enzyme and metabolic enzyme relative to digestive gland and muscles, tissue glycogen content relative to energy marker. This research provides the theoretical basis for optimizing the light environment of fertilized cuttlef ish eggs and newly hatched juveniles.
2 MATERIAL AND METHOD
2.1 Animal
The experiment was carried out at the Laifa Aquaculture Co. Ltd. (29°59′N, 121°99′E) (Zhejiang Province, China). Cuttlef ish (Sepiapharaonis) from a stock of f irst generation broodstock (Jiang et al.,2019). A total of 389 broodstock individuals with a male∶female ratio close to 1∶2 was placed in square concrete tanks (7.8 m×3.8 m×1.6 m, length×width×depth; area: 30 m2), at a culture density of 5-8 inds./m2during the spawning season from February to April in 2020. The broodstock was reared in a closed system,using natural seawater that was f iltered through a f ilter bed and preheated to 23±0.8 ℃ before being pumped into the tank. These cuttlef ish were fed with frozen f ishes (LarimichthyspolyactisandPampusargenteus) and white shrimp (Penaeusvannamei)twice a day (fed ad libitum), with a feeding rate of 3%-5% body weight (BW)/d (Jiang et al., 2018).Eggs were laid in February-April and the same batch of eggs cases was used for this study. Nylon nets with a diameter of 1-1.5 cm were used to collect fertilized eggs. The broodstock was cultivated under the following environmental conditions: salinity 28.7±1.2, temperature 23.8±0.6 ℃, pH 8.13±0.86,dissolved oxygen 5.77±1.13 mg/L, and a natural daynight photoperiod with intensity below 1 000 lx.
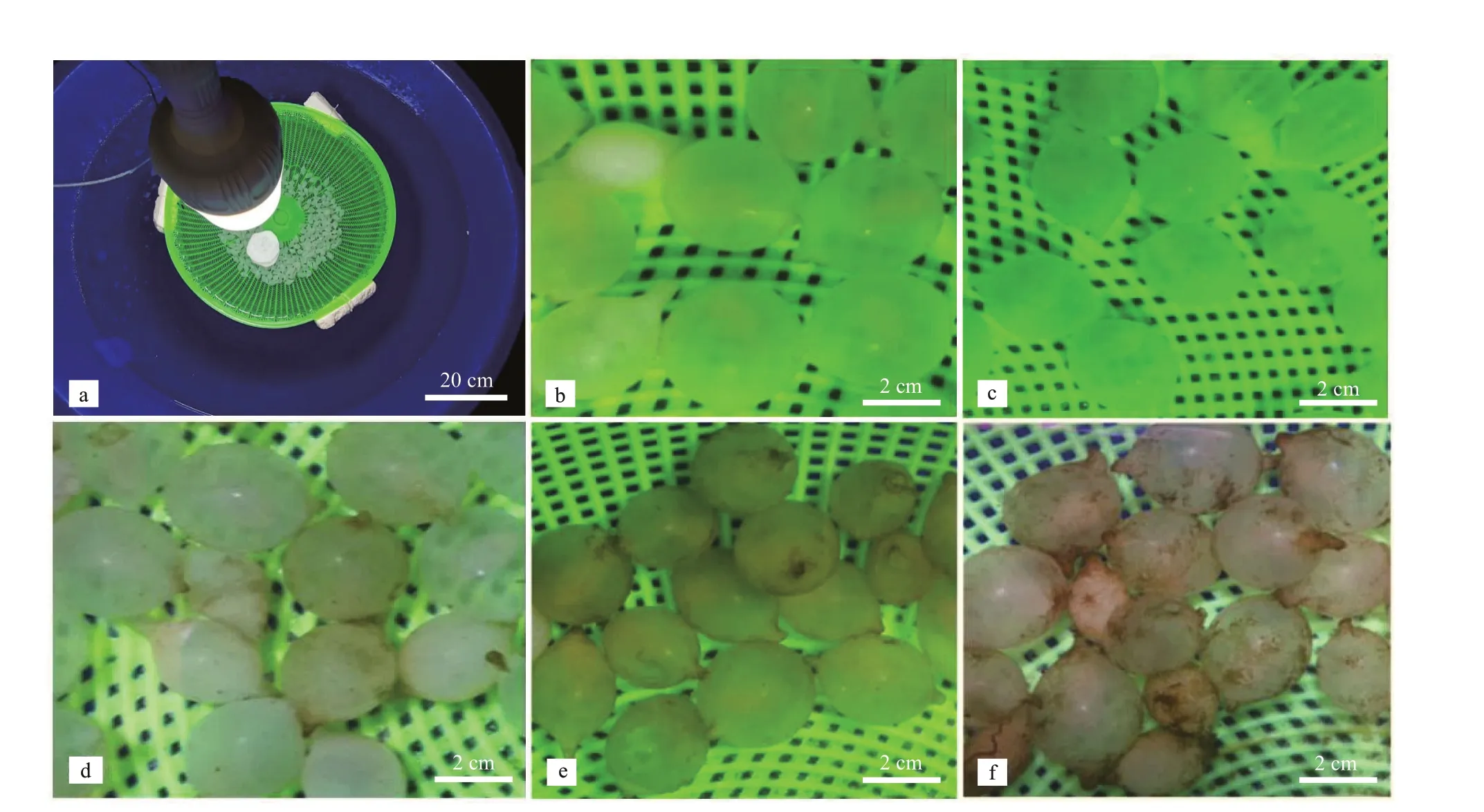
Fig.1 The experimental device and the embryo observation (15 days post spawning) exposure to diff erent photoperiods
2.2 Experimental design
2.2.1 Experiment 1: eff ect of photoperiod on embryonic development
All systems used in this study were composed of f ifteen blue f iberglass cylindrical tanks (diameter: 1 m;water depth: 0.65 m; and volume: 500 L), as described in Jiang et al. (2020). Round plastic baskets (diameter of 0.55 m, height of 0.18 m) with a series of small holes (diameter of 0.3 cm) f loated over the water in each f iberglass cylindrical tank; the eggs were incubated in these suspended baskets, as shown in Fig.1a. Fertilized eggs ofS.pharaoniswere obtained from same batch which hatched on the same day (i.e.,being the same age), and distributed into round plastic basket. Eggs were removed from the cluster individually, with 300 eggs assigned to each of basket and 3 replicates per treatment group (Fig.1a). Natural seawater was f iltered through a f ilter bed and ultraviolet sterilizers prior to being pumped into the tank. The water temperature was controlled at 24.6±0.5 ℃(Samuel and Patterson, 2015; Jiang et al., 2020) using a water bath equipped (1.5 kW) with a digital thermoregulator and immersion heaters (SUNSUN GD-1000, PID). Each tank was supplied with constant gentle ventilation with an air stone and an air lift.During the incubation period, salinity was 29.1±0.4,dissolved oxygen was 6.17±0.26 mg/L, and pH was 8.07±0.25. The salinity, pH, and dissolved oxygen were measured using the YSI Pro Plus instrument(YSI; www.ysi.com), and 30% of the seawater was renewed every third day under the same conditions.
The photoperiod (light∶dark, L∶D) treatments were as follows: 1) constant light (24L∶0D), 2) 18 h light∶6 h dark (18L∶6D), 3) 12 h light∶12 h dark (12L∶12D), 4)6 h light∶18 h dark (6L∶18D), and 5) constant darkness(0L∶24D). The photoperiod was maintained in each treatment with timers. Previous studies have suggested that cuttlef ish acclimatize to artif icial light with no response to stress (Boletzky, 1974; Koueta and Boucaud-Camou, 2003). Recently, we tested the inf luence of light intensity on embryo development by using cool-white f luorescent lamps as a light source and found that they were sensitive to strong light(Zhou et al., 2018). Consequently, an artif icial light(cool-white f luorescent lamps) of low intensity(250-300 lx) was used in this experiment. All tanks were placed in a dark room equipped with one 20W cool-white f luorescent lamp of SEEBEST (QZ130-20W, Xiamen SEEBEST Technology Co. Ltd.,Xiamen, China), mounted 0.50 m above the water surface, and given a range of light intensity between 250 and 300 lx at 0.18 m above the water surface. The light intensity of each treatment was measured at the air-water interface using a ZDS-10 luxmeter(Shanghai, China). Each tank was enclosed in a box of black plastic sheets to prevent the light from leaking to the surrounding tanks. This study was performed in accordance with the recommendations of the Guidelines for the Care and Welfare of Cephalopods in Research (Fiorito et al., 2015). The National Standards for Laboratory Animals of the People’s Republic of China were followed by the authors.
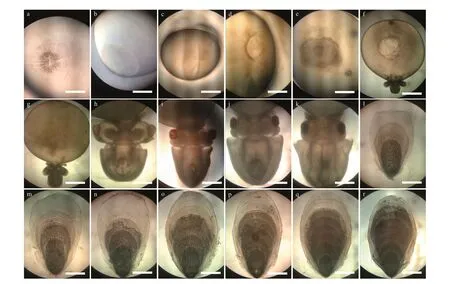
Fig.2 The 18 stages of S. pharaonis embryonic development
Eff ects of photoperiod on embryonic development were assessed according to the following criteria: 1)chronological comparison of the embryo development at diff erent photoperiods, embryonic development ofS.pharaoniswas observed from the f irst day post spawning (hours post spawning, HPS) to the day of hatching under laboratory conditions, and the embryos were photographed with a digital camera (HDRCX450, SONY, Japan) for determining the stages of embryonic development; to observe in a more intuitive and effi cient way the eff ects of the photoperiod on embryonic development, 18 stages(cleavage, blastula, gastrula, blastopore closure,embryonic primordium formation, organogenesis,red-bead, heartbeat, endoskeleton formation, pigment appear, pigment formation, three increments of cuttlebone, four increments of cuttlebone, f ive increments of cuttlebone, six increments of cuttlebone,seven increments of cuttlebone, eight increments of cuttlebone, and nine increments of cuttlebone (i.e.,hatchling cuttlef ish) (Fig.2) were adopted observation points in this experiment; 2) the hatching rate (HR),the incubation period (IP), the hatching period (HP),the yolk shed rate (YSR), the inking rate in the egg capsule (IREC), the mean wet weight and mantle length of the new hatchlings (Gracia-López et al.,2004), were calculated at the end of the experiment.The HR was determined as the percentage of stocked embryos that hatched hatchlings. The IP, which was the time interval between the activation period of the eggs and the period during which all the fertilized eggs hatched. The HP, which was def ined as the total duration from the f irst hatchling to the last one. The YSR was determined as the ratio of the number of yolks shed by diff erent factors to the total number of eggs. The IREC was determined as the ratio of the number of pre-hatchings inked by any mechanical impact to the total number of eggs. The total wet weight of each basket of new hatchlings was quantif ied on one scale (AE AUW120D, maximum 200 g, accuracy 0.000 1 g). Mantle length of hatchling were measured with digital Vernier calipers (0.001 mm) (n=6).
2.2.2 Experiment 2: eff ects of photoperiod on growth and physiological activity of juvenile
The culture system and the photoperiod treatments were identical to the above experiment. A total of 750 juveniles (1DAH, weight 0.221±0.011 g, dorsal mantle length 8.058±0.164 mm,n=50) were randomly divided into f ifteen 500-L f iberglass cylindrical tanks,including 50 juveniles in each basket and 3 replicates per treatment group. Initial stocking density was 150 inds./m2(Correia et al., 2005). New hatchlings were fed with enriched live Artemia (Artemianauplii)and mysids (Hyperacanthomysisbrevirostris) during the f irst 3 days of post-hatching, and then fed with live mysids twice a day ad libitum (Jiang et al., 2020).Feeding rates were set at 20% BW/d, which is considered a satisfactory proportion (Domingues et al., 2004). Feed provided was off ered were adjusted every 7 days to ref lect the new total biomass in each basket, ensure that the prey remained in the basket until the total duration of the test. Tanks and baskets were thoroughly cleaned as required, and the cuttlef ish were placed under dark conditions. The trial period lasted 30 days. Previous researchers have suggested that cuttlef ish are sensitive to intense light (Richard,1971; Sykes et al., 2014). As a result, we used a lowintensity light (250-300 lx) in this test. The water temperature was controlled at 24±0.5 ℃ using a water bath equipped (1.5 kW) with digital thermoregulator and immersion heaters, and 30% of the seawater was refreshed every day under the same conditions. The water conditions were as follows:salinity was 29.4±0.5, dissolved oxygen was 5.69±0.18 mg/L, and pH was 7.97±0.31. During the experiment, all cuttlef ish from each basket were enumerated and measured every 10 days to calculate the survival and growth parameters.
The growth parameters in this experiment were calculated as follows: survival (%)=100×(Nt/N0),weight gain (WG, %)=100×(Wt-W0)×100/W0, and specif ic growth rate (SGR, %/d)=(lnWt-lnW0)×100/T.In these equations,N0is the number of cuttlef ish in the basket at the start of experiment,Ntis the number of cuttlef ish in the basket at the end of experiment,W0is the initial mean wet body weight (g),Wtis the f inal mean wet body weight (g), andTis the experimental period (days).
2.3 Sampling and biochemical analyses
At the end of the experiment, six cuttlef ish per basket were anaesthetized in diluted ethanol (1∶20,Sinopharm Chemical Reagent, Shanghai, China).Samples of digestive glands and muscles were immediately frozen in liquid nitrogen and stored at-80 °C until analysis. The procedures for preparing glycogen and enzyme extract were as follows: 1) the frozen samples were thawed, weighed (~100 mg), and homogenized (3 min) in an ice-cold 50-mmol/L citrate phosphate buff er (pH 7.0) at a ratio of 1∶9 (w/v)using Ultraturrax (T-25) homogenizer; 2) the homogenate was centrifuged at 4 000 r/min (4 °C) for 10 min, and then the supernatant was centrifuged at 12 000 r/min (4 °C) for 15 min; 3) the supernatants were collected and used for further testing within 4 h and kept refrigerated at 4 °C; 4) the total protein content of the crude extracts was measured at 30 °C using bovine serum albumin as a standard at 595 nm using the Bradford (1976). All assay kits were purchased from Nanjing Jiancheng Bioengineering Institute (http://www.njjcbio.com/, Nanjing, China),testing procedures following the manufacturer’s instructions. The assays are brief ly described below.
The glycogen content for digestive gland and muscle samples was measured using the glycogen assay kit. Glycogen concentration was determined using the anthrone reaction method according to Kohyama-Koganeya et al. (2015), free glucose was subtracted from the total glucosyl equivalents for glycogen levels, and sample absorption was read at 620 nm. The following test procedures as our previously described (Jiang et al., 2020). Glycogen level was expressed as per mg of total protein (specif ic level).
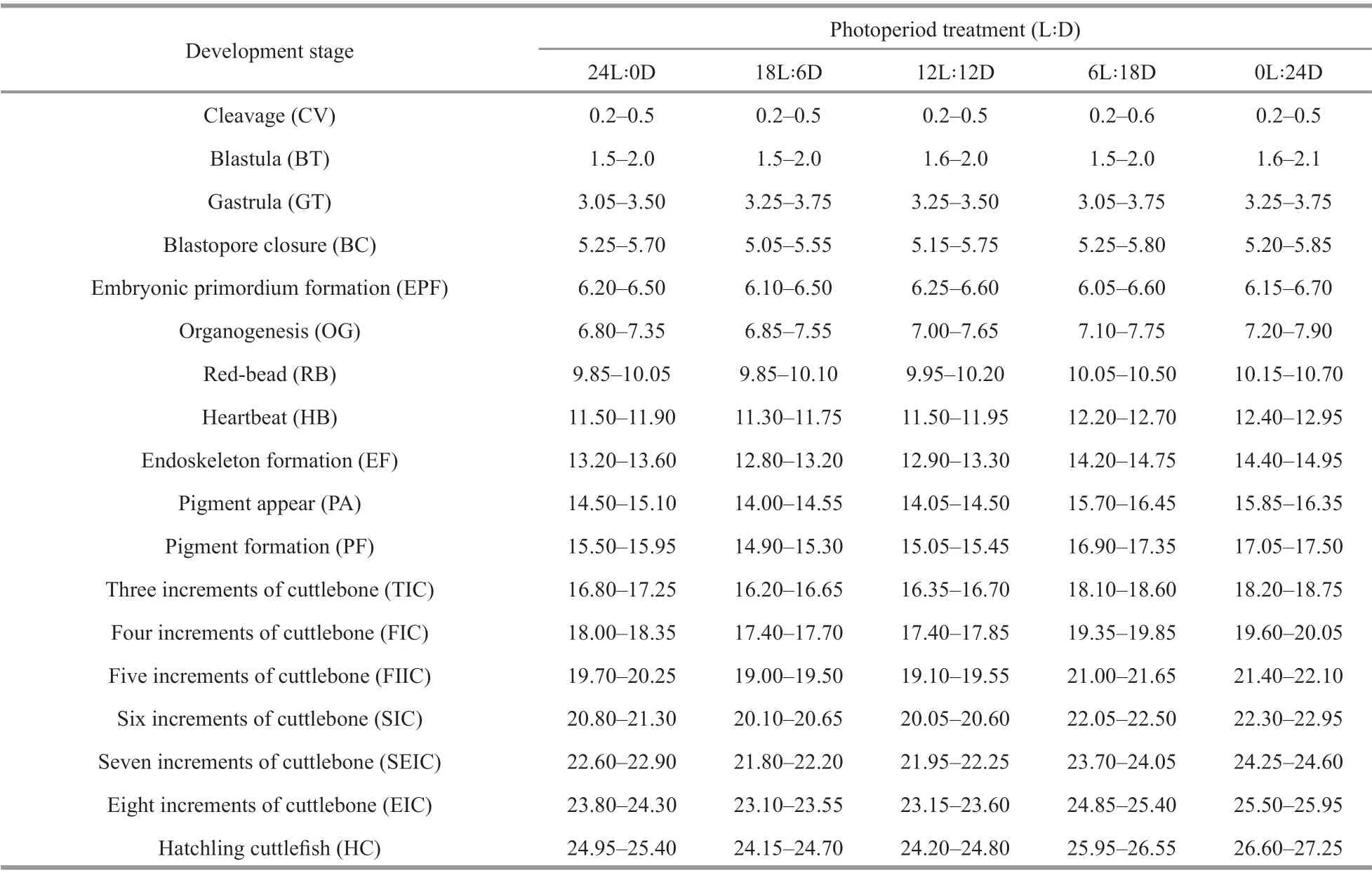
Table 1 Timing of the occurrence of developmental stages exposure to diff erent photoperiods (d)
The activities of digestive gland alkaline phosphatase (ALP), trypsin (TRYP), and lipase (LIP)were determined according to the procedures described by Principato et al. (1982), Tsunematsu et al. (1985), and Pinsirodom and Parkin (2001),respectively. The absorption of ALP (EC 3.1.3.1),TRYP (EC 3.4.4.4), and LP (EC 3.1.1.3) was recorded at 520 nm, 253 nm, and 420 nm, respectively. Enzyme activities were expressed as per mg of total protein(specif ic activity).
The activities of muscle tissue succinate dehydrogenase (SDH), malate dehydrogenase(MDH), hexokinase (HK), and pyruvate kinase(PK) were determined in accordance with procedures described by Palmer et al. (1977),Baldwin and Reed (1976), Tranulis et al. (1996),and Foster and Moon (1986), respectively. The absorption of SDH (EC 1.3.5.1), MDH (EC 1.1.1.37), HK (EC 2.7.1.1), and PK (EC 2.7.1.40)was read at 600 nm, 340 nm, 340 nm, and 340 nm,respectively. Enzyme activities were expressed as per g or/and mg of total protein (specif ic activity).
2.4 Statistical analysis
Data are expressed as mean±standard deviation(means±S.D.). The eff ects of photoperiod on the indices were analyzed using a one-way ANOVA,followed by the Duncan’s test withP<0.05 taken as the statistically signif icant threshold. Analyses were performed using the SPSS program version 20.0(SPSS for Windows). Histograms were drawn in GraphPad Prism 8.0.2 (https://www.graphpad.com/scientif ic-software/prism/). Spearman’s correlation analysis and principal component analysis (PCA)were visualized using the R 4.1.0 packages (https://www.r-project.org/) corrplot and ggord, respectively.
3 RESULT
3.1 Eff ect of photoperiod on the embryonic development time and egg capsule
The embryonic development process and hatching time varied considerably according to the photoperiod during incubation (Table 1). The time from cleavage to red-bead was 9.85-10.70 d at photoperiods treatment, but the diff erence in development time was aggravated after the red-bead stage. The period of embryonic development was similar in the 18L∶6D treatment and the 12L∶12D treatment. Hatching occurred 24.15-24.80 d at 18L∶6D and 12L∶12D treatments, which was 0.7-1.0 d, 1.5-2.0 d, and 2.5-3.0 d shorter than that at 24L∶0D, 6L∶18D, and 0L∶24D treatments, respectively.
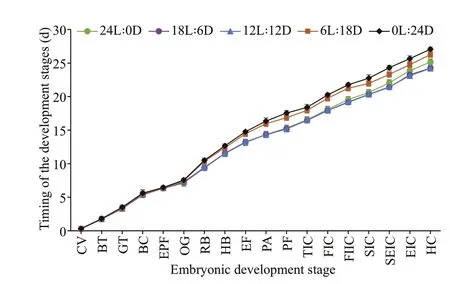
Fig.3 Comparison of embryo development exposure to diff erent photoperiods
Timing of embryo development stages exposed to diff erent photoperiods is shown in Fig.3. The diff erence in embryo development timing did not appear until the red-bead stage, with the red-bead stage appeared earlier in 12L∶12D to 24L∶0D treatments than 6L∶18D to 0L∶24D treatments.Subsequently, the diff erence in the development timing of embryo was more evident, as shown that the seven layers of cuttlebone stage appeared earlier in 12L∶12D and 18L∶6D treatment than 24L∶0D treatment. Until cuttlef ish hatched, the incubation time from 24L∶0D to 0L∶24D treatment were 24.95-25.40, 24.15-24.70, 24.20-24.80, 25.95-26.55, and 26.60-27.25 d, respectively.
The PCA plots for the embryonic development timing (Fig.4), in which each point represents a sample from a diff erent photoperiod treatment, clearly highlights the diff erences between the diff erent lightdark cycles. The f irst and second principal components accounted for 85.4% of the total variation (73.3% and 12.1%, respectively) (Fig.4a). The prof iles of the embryonic development timing were categorized into f ive groups corresponding to each photoperiod treatment.S.pharaonisembryonic development timing was most similar between 12L∶12D and 18L∶6D treatments, whereas 24L∶0D was the furthest away from the other treatments. In Fig.4b, combined with the factor analysis of the principal components of SPSS, showed that the main contributing factors to the f irst principal component were the red-bead stage,heartbeat, endoskeleton formation, pigment appear,six increments of cuttlebone, and their score coeffi cients are all higher than 0.95.
The observation of the egg capsule (15 days post spawning) as shown in Fig.1. During the photoperiods,the egg capsule surface varied greatly with the prolonged incubation time. At 12L∶12D treatment, the algae began to adhere to the surface of the egg capsule,and the amount of algae adhesion increased with the prolonged exposure to light (Fig.1e & f), but it did not appear at 0L∶24D and 6L∶18D treatments (Fig.1b & c).
3.2 Eff ect of photoperiod on hatching rate,incubation period, hatching period, yolk shed ratio, inking rate in the egg capsule, and mean wet weight of hatchling
Photoperiod had a signif icant eff ect on the hatching rate, incubation period, and hatching period, although the hatching period seemed to increase as the length of darkness increased (Table 2). A relatively high hatching rate (>85%), a lower incubation period(<25 d), and an embryos hatching period (<6 d) of the were found in the 12L∶12D to 24L∶0D treatments.These results regarding the incubation period and the hatching period of embryos at diff erent photoperiods,indicated that the photoperiod had no signif icant influence between the 18L∶6D and 12L∶12D treatments (P>0.05). However, the incubation period and hatching period were longer at 0L∶24D treatment than that at other treatments (P<0.05). The highest hatching rate (93.77%±1.33%), the shortest incubation period (23.55±1.25 d), and hatching period(108.63±1.24 h), were observed in the 12L∶12D,24L∶0D, and 24L∶0D treatments, respectively.
Moreover, the photoperiod also inf luenced the yolk shed ratio and inking rate in the egg capsule of the embryos. The yolk shed ratio and the inking rate in the egg capsule signif icantly increased (P<0.05) with constant light and/or darkness, whereas there was no signif icant diff erence (P>0.05) between 18L∶6D,12L∶12D, and 6L∶18D treatments (Table 2). The highest yolk shed ratio (2.30%±0.61%) and inking rate in the egg capsule (1.60%±0.13%) was found in the 24L∶0D and 0L∶24D treatments. Photoperiod had a modest eff ect on the mean mantle length of hatchling,but it was not signif icant (P>0.05). As with the mean wet weight of hatchling, no signif icant diff erence in the mean mantle length between photoperiod treatments (P>0.05).
Spearman was used to analyze correlations between incubation parameters and physiological response values. Figure 5a shows that the hatching rate was strongly positive correlated with the mantle length of hatchling, while a strong negative correlated with the incubation period, and a very strong negative correlation was observed for inking rate in the egg capsule. The mantle length of hatchling showed a moderately negative correlation with the incubation period and the inking rate in the egg capsule. A very strong positive correlation was found between the hatching period and the incubation period.
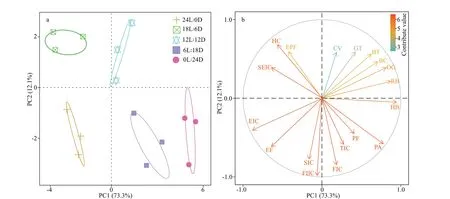
Fig.4 Principal component analysis (PCA) based on the diff erent developmental timing of S. pharaonis embryos exposure to diff erent photoperiods
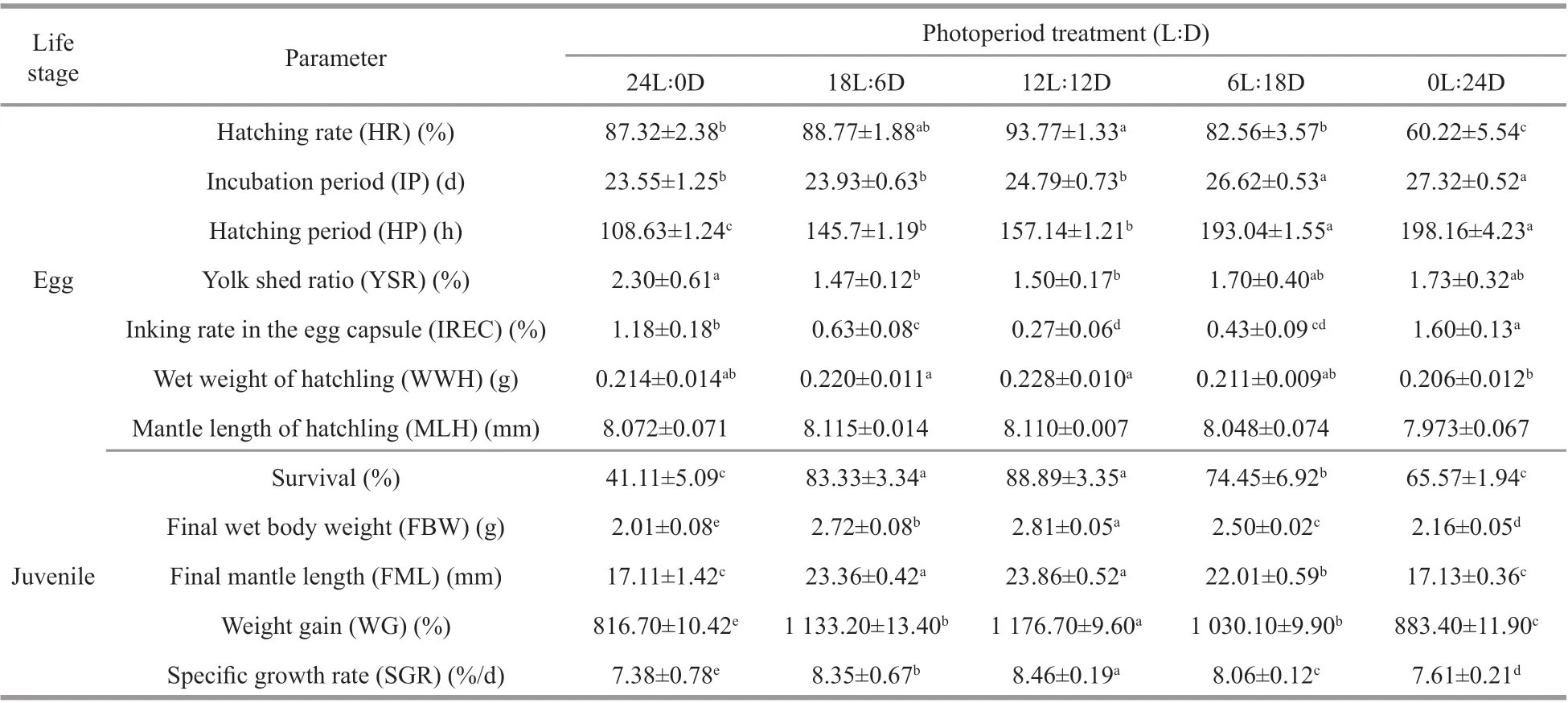
Table 2 Hatching rate, incubation period, hatching period, yolk shed ratio, inking rate in the egg capsule of S. pharaonis eggs, and survival and growth of S. pharaonis juvenile exposure to diff erent photoperiods
3.3 Eff ect of photoperiod on growth performance of S. pharaonis juvenile
Over the f irst 10 d of rearing, the survival of the 12L∶12D and 18L∶6D treatments were not signif icantly diff erent (P>0.05), but were signif icantly higher than those of the 0L∶24D and 24L∶0D treatments (P<0.05),and the minimum level was observed in the 24L∶0D treatment. Subsequently, at the age of 20 d, the survival of the 12L∶12D treatment was signif icantly higher than those of the other groups (P<0.05), and the lowest value was observed in the 24L∶0D and 0L∶24D treatments. After 30 d of rearing, signif icant diff erences in survival were observed among all groups (P<0.05), as follows: 12L∶12D≥6L∶18D≥18L∶6D>0L∶24D≥24L∶0D (Fig.6).
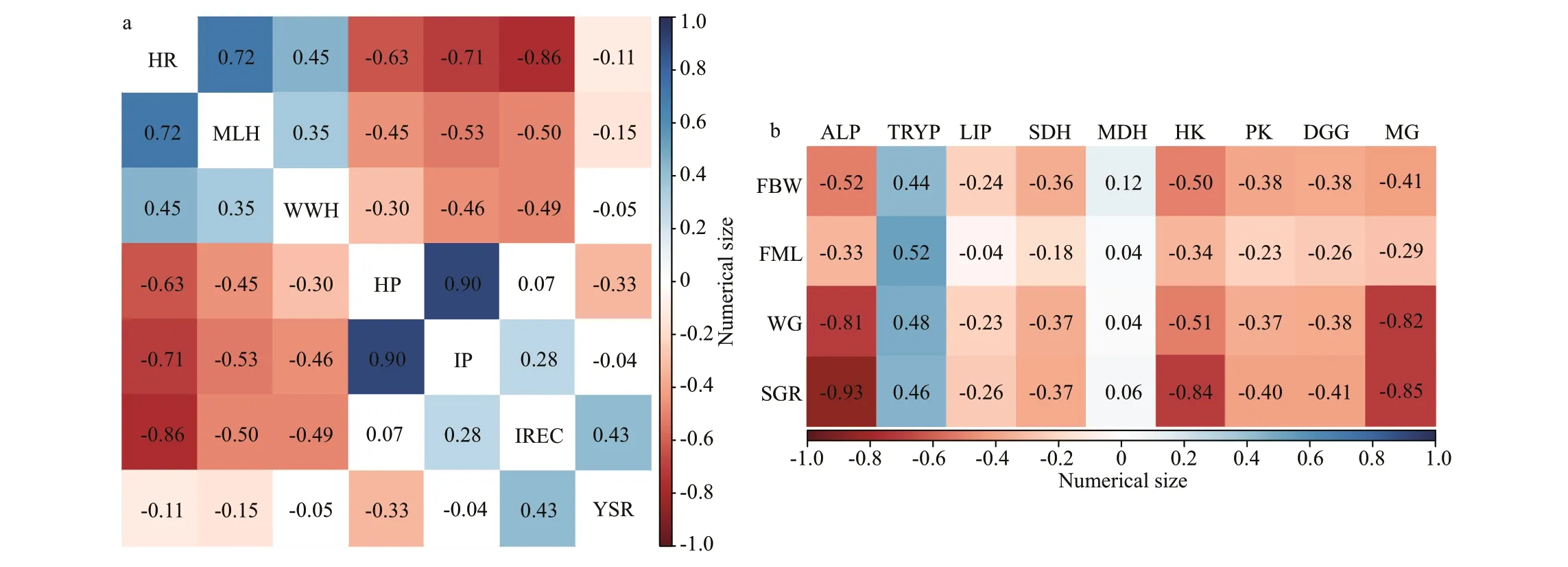
Fig.5 The correlation analysis of incubation parameters, physiological response values during embryonic development stage (a); the correlation analysis of growth performance and the levels of digestive enzymes from the digestive gland and muscle of juveniles (b)
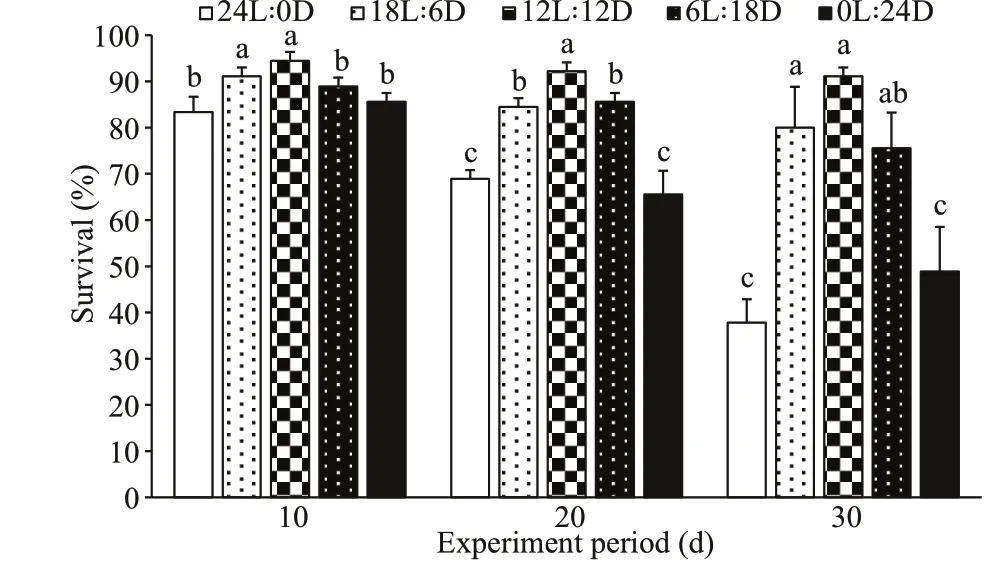
Fig.6 Eff ect of photoperiods on survival of S. pharaonis
Growth of juvenile cuttlef ish (30 DAH) under photoperiod treatments are shown in Table 2. Changes in weight gain and specif ic growth rate corresponded to exposure to diff erent photoperiods. After 30 days of the experiment, signif icant diff erences in the specif ic growth rate were observed among all groups(P<0.05), as follows: 12L∶12D>18L∶6D>6L∶18D>0L∶24D>24L∶0D, were 8.46%±0.19%, 8.35%±0.67%,8.06%±0.12%, 7.61%±0.21%, and 7.38%±0.78% /d,respectively.
3.4 Eff ect of photoperiod on glycogen concentrations and enzyme activities of tissues
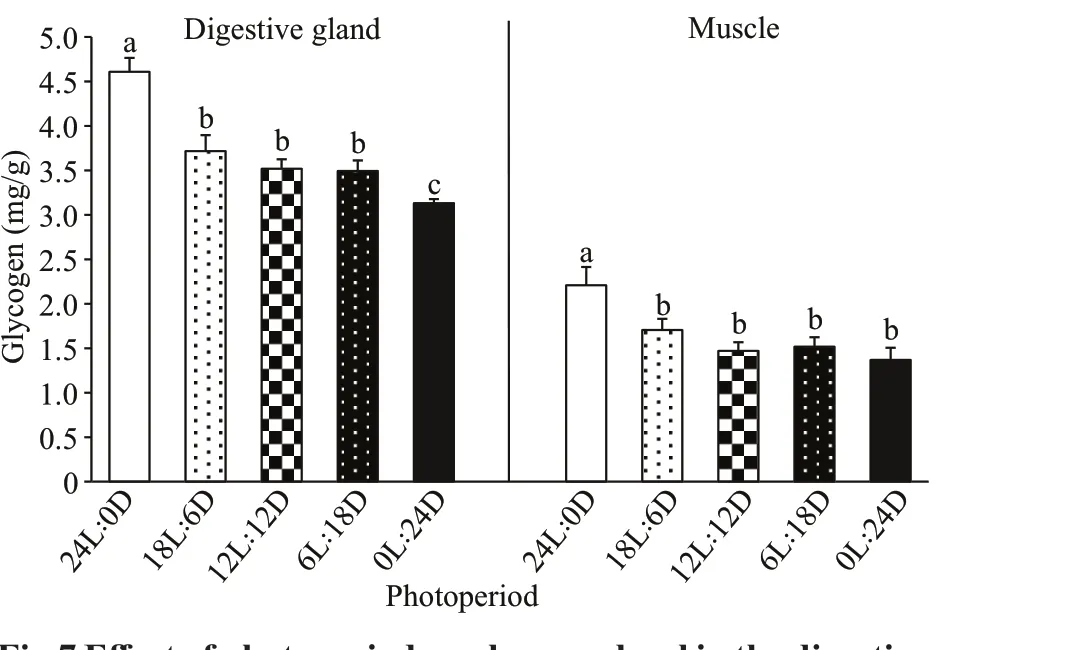
Fig.7 Eff ect of photoperiod on glycogen level in the digestive gland and muscle tissues of S. pharaonis
Glycogen levels in the digestive gland and muscles were signif icantly aff ected by the photoperiod (Fig.7).The glycogen content of the 18L∶6D, 12L∶12D, and 6L∶18D treatments was not signif icantly diff erent(P>0.05), but was signif icantly lower than that of the 24L∶0D treatment (P<0.05) in the digestive gland,and the minimum value was found in the 0L∶24D treatment. Muscle glycogen levels of 24L∶0D treatment was signif icantly higher than in the other groups, and no signif icant diff erences were observed in the other treatments. Moreover, the glycogen levels of the digestive gland (~3.5 mg/g) was twice as high as that of the muscle (~1.5 mg/g).
Table 3 shows the digestive enzyme (alkaline phosphatase, trypsin, and lipase) in the digestive gland of juvenile exposed to photoperiod. Alkaline phosphatase activity did not diff er signif icantly(P>0.05) between 12L∶12D, 6L∶18D, and 0L∶24Dtreatments, whereas the maximum value appeared at 24L∶0D treatment. Photoperiod had a modest eff ect on trypsin, which was signif icantly higher at 24L∶0D treatment than that of other treatments. Changes in lipase and trypsin were consistent with exposure to diff erent light-dark cycles.
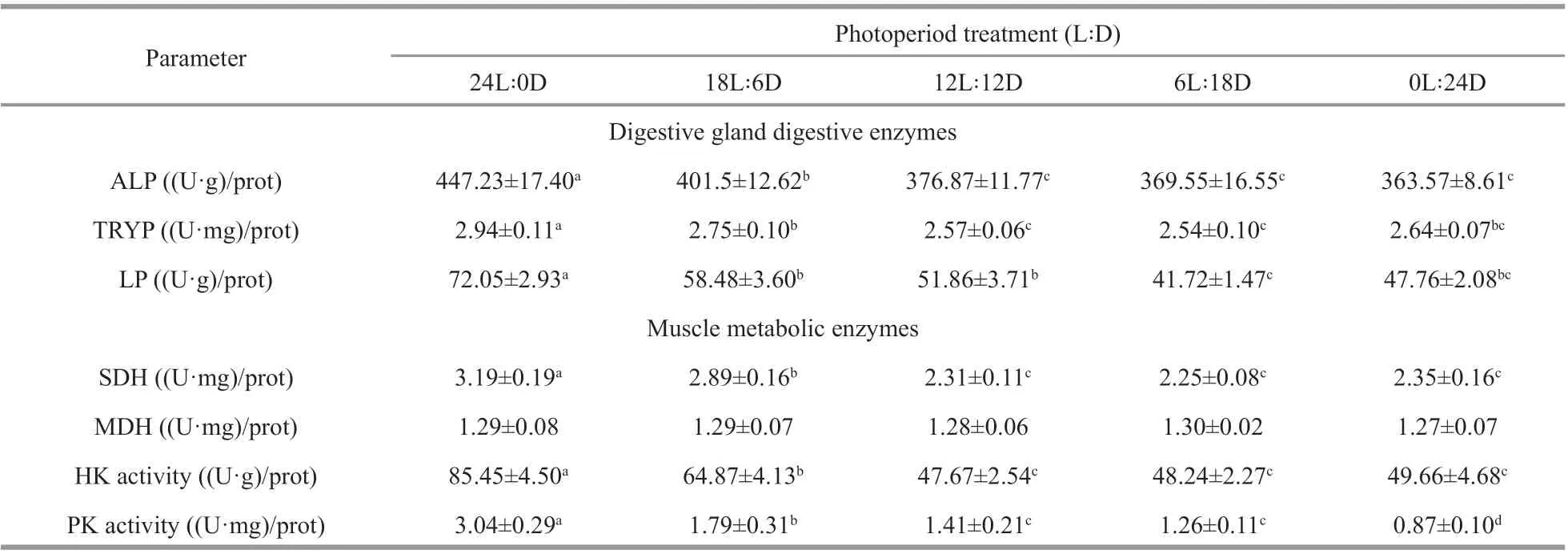
Table 3 Digestive gland digestive enzymes and muscle metabolic enzymes of juveniles exposure to diff erent photoperiods
Muscle metabolic enzyme of juvenile cuttlef ish are presented in Table 3. Muscle malate dehydrogenase did not diff er between treatments (P>0.05), whereas succinate dehydrogenase, hexokinase, and pyruvate kinase activities were signif icantly aff ected by the photoperiod. Succinate dehydrogenase increased signif icantly with the increase in illumination time from 0 to 24 h (P<0.05), the minimum and maximum values appeared at 0L∶24D and 24L∶0D treatments,respectively. Hexokinase activity f irst kept stabilized and then increased, with the maximum value appearing at 24L∶0D treatment, but showed no statistical diff erence (P>0.05) between 0L∶24D,6L∶18D, 12L∶12D, and 18L∶6D treatments. The variation in pyruvate kinase activity was similar to the that of hexokinase activity, the highest level was observed in the 24L∶0D treatment, followed by the 18L∶6D treatment, the minimum value at the 0L∶24D treatment, but no signif icant diff erences between 6L∶18D, and 12L∶12D treatments (P>0.05).
The light cycle has an impact on the physiological activity of the body, Spearman correlation analysis was used to further analyze the correlation analysis between growth and physiological indicators. As shown in Fig.5b, weight gain has a moderate negative correlation with muscle hexokinase, while it has a strong negative correlation with alkaline phosphatase and muscle glycogen content of the digestive glands.The specif ic growth rate was negatively correlated with alkaline phosphatase, hexokinase, and glycogen levels of the digestive glands. A moderate positive correlation of trypsin with growth parameters was observed.
4 DISCUSSION
In cephalopods, encapsulation of egg is essential, as it provides a physical barrier and chemical defence during embryonic development, and allows exchanges with the environment (Benkendorff et al., 2001;Boletzky, 2003). Once a fertilized egg is formed,embryo development is a continuous process. The whole process of embryonic development involves a series of processes of cell diff erentiation, division, and morphogenesis, which are inf luenced internally by genetic expression and externally by environmental factors (Boletzky, 2003; Cinti et al., 2004). Light has an important eff ect on the embryonic development of many marine species, such as increasing the hatching rate, shortening the time to hatch, and improving the viability of the 24 h post hatching larvae (Downing and Litvak, 2002; Shi et al., 2012). In nature,S.pharaonisfertilized eggs in the South China Sea are mostly found in the shallow waters off shore during late spring in February and March, and a natural light cycle was measured approximately 14L∶10D. In the present study, the prolonged photoperiod induced an acceleration of the rate of embryonic development was noted inS.pharaonisembryos, particularly in the red bead stage began to show diff erences. It could also be attested to the short development time, where a diff erence may have appeared but was not found to be signif icant. Prior to retina formation, the pineal gland was shown functional (Andrew et al., 2009). Studies have shown that light stimulation of the pineal gland can aff ect the physiological state of teleost f ish through neuro-modulation or humoralregulation (Falcón et al.,2010). In addition, from the 12L∶12D to 24L∶0D treatments, the individual development speed was obviously accelerated from the red-bead stage to seven layers of cuttlebone stage. It is highly probable that the retinal visual acuity is sensitive to light stimulation after the red-bead stage formation, and the duration of light/day length may accelerate the embryonic development process (Evans et al., 2015). At this moment, the visual function of the cuttlef ish has been fully developed; the retinal photoreceptor is equipped with a complete photosensitive pathway. We inferred from the development rate that the 24-h day length at the late stage of development had an adverse eff ect on the embryos, and the incubation period of 24L∶0D treatment was greater than that of 12L∶12D treatment and 18L∶6D treatment. However, the cornea closure times for diff erent species are not consistent. Lee et al.(2016) showed that the cornea enclosed the eyes ofS.pharaonisat the pigment-formation stage, whereas enclosed corneas occurred in four layers of cuttlebone inS.offi cinalisand 2 DAH inSepioteuthisaustralis(Bozzano et al., 2009). It remains to be determined whether the timing of corneal closure is related to species phototaxis, brain photosensitive vesicles, or retinal visual acuity. The hatching occurred 24-25 d at day lengths of 12-24 h, which was 42-58 h earlier than the day lengths of 0-6 h. PCA analysis showed that the main contributing factors of the f irst principal component were red-bead stage, heartbeat,endoskeleton formation, pigment appear, and six increments of cuttlebone. The development time and duration of these f ive stages varies diff erent with exposure to diff erent photoperiods, which may determine the length of the incubation period.
Incubation and hatching periods are important parameters for evaluating embryonic development.The incubation period refers to the rate of fertilized egg development under certain conditions, and the hatching period provides information on the consistency of the development rate. This study found that the shortest incubation and hatching periods occurred in the 24L∶0D treatment, and that mediumto-long light cycles tended to shorten the incubation and hatching periods. The earliest hatchling was observed at 20-d post spawning in 24L∶0D treatment.However, a decrease in the rate of embryonic development and the timing of hatching were observed with the 0L∶24D and 6L∶24D treatments. The hatching rate is an important indicator in the study of embryonic development for large-scale reproduction. In this study, we found that changes in the hatching rate were not consistent with the photoperiod, and a reduction in the hatching rate was observed with the 0L∶24D and 24L∶0D treatments. This may be related to the extent of environmental adaptation and the adjustment of the endogenous rhythm of the species (Bromage et al.,2001). During embryonic development, the outer membrane becomes more transparent by absorbing water and reduces the thickness of the egg capsule. It is observed that the algae start to adhere to the egg capsule surface 15 d post spawning, and more algae adhere with increased light exposure. However, algal attachment had no signif icant eff ect on hatching, and new hatchlings could break out from the membrane.Egg yolk is greatly reduced as the size of the developing embryo increases. In the present study, we found that egg yolk shedding occurred primarily in the later stages of embryonic development, especially under constant light or darkness. Some hatchlings possessed an external incompletely absorbed yolk sac even after hatching, which they immediately released, if necessary (Anil et al., 2005). We observed that premature hatchlings in the egg capsule are capable of releasing ink when handled vigorously or when changes are detected in the external environment and the ink gland was found to be functional even during the pigmentation stage (Samuel and Patterson, 2015).Twenty-four hours of continuous darkness may not be conducive to embryonic development and, on exposure to light stimulation during the 0L∶24D treatment,premature hatchling inking rate in the egg capsule was found to be three to f ive times higher than that of other treatments. As a result, we conclude that the optimal photoperiods for the optimalS.pharaonisembryonic development are 12-h day lengths.
Our f indings did not only show thatS.pharaonispossesses the characteristic of high growth rates, but also contribute to the optimization of the cuttlefish culture method to ensure more consistent growth and survival based on a welfare approach to the problem.In contrast to the embryonic development stage,during which the cuttlef ish grows based on endogenous nutrition, juvenile cuttlef ish gain energy from hunting at the newly hatched stage, when they are in high demand for food and nutrition.S.pharaonis,similar to most other cephalopods, are highly dependent on vision for prey capture, predator avoidance, and intraspecif ic communication (Hanlon,2007). In this study, the new hatchlings were less sensitive to light and were able to hunt all the time for the f irst few days of their life. In general, the long photoperiod benef its increased feeding, probably because of increased food availability (Boeuf and Le Bail, 1999). This is consistent with the present study,which revealed that increased day length (12-24 h)produced signif icantly higher growth rates with respect to wet weight compared to day lengths of 0-6 h. However, over the f irst 10 d of rearing, the survival rates of juveniles exposed to the 0L∶24D and 18L∶6D treatments did not diff er signif icantly, but were signif icantly higher than those of juveniles in 24L∶0D treatment. Speers-Roesch et al. (2016)showed that cuttlef ish juveniles could tolerate starvation for up to 12 d; however, we found that the specif ic growth rate in 0L∶24D group was much lower than that of other treatments. As a result, constant light and darkness were not benef icial to juvenile growth and survival; constant light had a negative eff ect on juvenile growth, and constant darkness was detrimental to survival (Fig.5). As Richard (1975)indicates, cuttlefish in total darkness had low growth and high mortality as a cumulative eff ect of starvation,which was most common at an early stage. The visual functions of cuttlef ish are limited in the dark and therefore depend more on other organs (such as the developed nervous system and the sense organs for olfactory and light sensitivity) to promote predation,which leads to decrease in food intake due to imprecise location, orientation, and low success rate of capture prey during hunting (Jereb and Roper, 2005). In the present study, the f indings of 0L∶24D and 6L∶18D treatments conformed this interpretation. Specif ic growth rates of juveniles cultivated in 0L∶24D and 6L∶18D treatments were signif icantly lower than those of juveniles grown in 12L∶12D and 18L∶6D treatments. Subsequently, the specif ic growth rate and survival gradually increased as the day length was extended from 6 h to 18 h. Under these photoperiods,cuttlef ish may have greater opportunities to feed,which should result in better growth and survival rates based on access to suffi cient food (Litvak, 1999).Combined with the life habits ofS.pharaonis, which inhabited low latitudes in the Indo-Pacif ic region, it is reasonable to assume that the cuttlef ish preferred a natural photoperiod or a medium-to-high amount of sunlight. Our results indicated that specific growth rate and survival of juvenile cuttlefish had optimal values under a 12L∶12D light cycle, which was consistent with the data obtained fromS.offi cinalis(Koueta and Boucaud-Camou, 2003).
Cephalopods are highly developed marine molluscs with digestive physiological functions that share many similarities to teleosts. The process of maturation of the digestive gland has been described in cephalopods as the progressive development of intracellular and extracellular digestion enzymes during the f irst month of life (Saf i et al., 2018). Several studies have shown that these enzyme activities are related to diet and growth (Perrin et al., 2004; Rosas et al., 2011), or are localized to describe their function in the digestive system (Boucaud-Camou and Roper,1995). The onset of juvenile growth is profoundly aff ected by the transition period from endogenous to exogenous feeding, which is primarily aff ected by digestive enzymes of the digestive glands. Boucaud-Camou et al. (1985) reported that digestive enzymes have shifted from a predominantly acidic intracellular digestion to extracellular alkaline digestion in newly hatched cuttlef ish, and the change/ratio of these enzyme activities could be used as an indicator of the maturation of digestive glands inSepiaduring the early life stages (Saf i et al., 2018). Trypsin is a member of serine protease that specif ically hydrolyzes proteins, which are located in the secretory organs of the digestive tract inS.offi cinalis(Jellouli et al.,2009). Alkaline phosphatase (ALP) catalyzes the hydrolysis of various phosphate-containing compounds and acts as a transphosphorylase at alkaline pH, and is found in the digestive system of cephalopods (Mazorra et al., 2002). In the present study, the photoperiod strongly aff ected the digestive enzyme activity of juveniles from the 1 to 30 DAH.No signif icant diff erence was observed as the day length increased from 6 to 18 h, and a high level of ALP and trypsin activities were observed during 24 h of continuous light exposure. Trypsin and ALP activities were stabilized from 14 DAH in the digestive glands of cuttlef ish, indicating that both two enzymes function as exogenous digestive enzymes(Saf i et al., 2018). It is possible that juveniles need to consider nutrient digestion, absorption, and assimilation of nutritions to meet metabolic needs,rather than the length of the day or prey supply. In other words, continuous light exposure may interfere with digestive physiological controls and physiological rhythms of juvenile (Shi et al., 2012).
Photoperiod manipulation may aff ect food intake,physiological activity, individual behaviour and/or activity. Generally, a long day length can promote better growth because a longer feeding time allows for more food consumption and better absorption(Giri et al., 2002). In this study, however, it was observed that continuous light exposure may have caused the animals to remain in a constant state of activity. High levels of glycogen was found in muscles after 24L∶0D treatment may be due to the high metabolic rate of juveniles. Sykes et al. (2014)reported that in the active portion of the day, the metabolic rate of cuttlef ish was 2.5 to 3 times higher than that during the inactive portion. In the cephalopod muscle, carbohydrates are important fuels, and hexokinase (HK) and pyruvate kinase (PK) enzymes play a major role in the energetic regulation of the glycolysis pathway (Panserat et al., 2001), whereas succinate dehydrogenase (SDH) is involved in both the citric acid cycle and the respiratory electron transfer chain, and roughly ref lects the level of aerobic metabolism (Rutter et al., 2010). In the present study,high levels of HK, PK, and SDH activity were observed in muscles after the 24L∶0D treatment,indicating an increase in the aerobic metabolism of the organism. The constant light on juveniles may have led to suboptimal absorption and assimilation,and thus reduced growth performance compared to that in the 18L∶6D and 12L∶12D treatments where the juveniles were in the “dark” phase. Therefore, the optimal photoperiod is species-specif ic, maximum feeding time is not necessarily the optimal condition,and continuous light may disturb an organism’s endogenous controls. This has been proven in other species, such as the southern f lounderParalichthyslethostigma(Tuckey and Smith, 2001), the catf ishWallagoattu(Giri et al., 2002), the green turtlesCheloniamydas(Southwood et al., 2003), and the tawny puff erTakifuguf lavidus(Shi et al., 2012).Moreover, the continuous darkness did not favour the development of juveniles. In the present study, the growth, survival, digestive gland/system development,and physiological activity of juveniles reared in constant darkness have deteriorated. These results are supported by Villamizar et al. (2009) and Shi et al.(2010), who found that total darkness strongly aff ected the digestive system development of f ish and inhibited animal feeding. Alternatively, juvenileS.pharaonismay display a circadian feeding pattern.Inappropriate light cycles resulted in disturbances of their physiological activity that have compromised the growth and development of cuttlef ish.
5 CONCLUSION
In conclusion, the embryonic development ofS.pharaoniswas strongly aff ected by light-dark cycles.The 12L∶12D cycles provided the best incubation conditions for embryos and produced a large number of high-quality new hatchlings. Furthermore, increases in day length (12L∶12D and 18L∶6D cycles) contributed to improved growth and survival ofS.pharaonisjuveniles from 1 to 30 DAH. Inappropriate light cycles(constant light and darkness) have led to disturbances of physiological activity that have compromised the welfare and survival of cuttlef ish. The results of this study are helpful in increasing the production of this species during embryo incubation and juveniles rearing in aquaculture practice.
6 DATA AVAILABILITY STATEMENT
The data that support the f indings of this study are available from the corresponding author upon reasonable request.
7 ACKNOWLEDGMENT
The authors are grateful to Laifa Aquaculture Co.Ltd. (Zhejiang Province, China) for supplying the experimentalS.pharaoniscuttlef ish hatchlings and providing logistical support throughout the experiment.
 Journal of Oceanology and Limnology2022年5期
Journal of Oceanology and Limnology2022年5期
- Journal of Oceanology and Limnology的其它文章
- Comparison of three f locculants for heavy cyanobacterial bloom mitigation and subsequent environmental impact*
- Eff ect of light intensity on bound EPS characteristics of two Microcystis morphospecies: the role of bEPS in the proliferation of Microcystis*
- Community structure of aerobic anoxygenic phototrophic bacteria in algae- and macrophyte-dominated areas in Taihu Lake, China*
- Tidal water exchanges can shape the phytoplankton community structure and reduce the risk of harmful cyanobacterial blooms in a semi-closed lake*
- Eff ect of random phase error and baseline roll angle error on eddy identif ication by interferometric imaging altimeter*
- Estimating the evolution of sea state non-Gaussianity based on a phase-resolving model*
