Community structure of aerobic anoxygenic phototrophic bacteria in algae- and macrophyte-dominated areas in Taihu Lake, China*
Limei SHI , Yuanfeng CAI , , Xiaoli SHI , Min ZHANG , , Qingfei ZENG ,Fanxiang KONG , Ping XU
1 State Key Laboratory of Lake Science and Environment, Nanjing Institute of Geography and Limnology, Chinese Academy of Sciences, Nanjing 210008, China
2 State Key Laboratory of Soil and Sustainable Agriculture, Institute of Soil Science, Chinese Academy of Sciences, Nanjing 210008, China
3 The Philips Institute of Oral and Craniofacial Molecular Biology, Virginia Commonwealth University, Richmond, VA 23298-0566, United States of America
Abstract Aerobic anoxygenic phototrophic bacteria (AAPB) represent a major group of bacterioplankton assemblages in many water systems and some are assumed to be closely associated with phytoplankton.However, studies on relationships between AAPB and cyanobacterial blooms are in scarcity. The dynamics of the abundance and diversity of AAPB was compared based on pufM gene in Meiliang Bay (featured by cyanobacterial blooms) and East Bay (featured by macrophyte) of Taihu Lake, a shallow subtropical lake in the East China plain. AAPB abundance was not signif icantly diff erent between the two sites, and they were positively correlated with dissolved organic carbon (DOC) concentration. The ratios of AAPB to total bacteria varied from 3.4% to 11.5% and peaked in winter in both sites. No signif icant diff erences of AAPB community compositions were detected between the two sites, but there was a separation between warm seasons (June, August, and October) and cold seasons (December, February, and April). Rhizobiales and Limnohabitans-like pufM sequences were signif icantly contributors for the diff erence between two seasons,and specially enriched in cold seasons. Chlorophyll a (Chl a) and DOC were the most signif icant variables inf luencing the AAPB community structure. Furthermore, Porphyrobacter and Rhodospirillales-like pufM sequences were positively correlated with Chl a, indicating potential inf luence of cyanobacterial blooms on these AAPB taxa. These results suggested that diverse AAPB ecotypes coexisted in Taihu Lake, and their ecological role in carbon cycling in the lake may not be ignored.
Keyword: aerobic anoxygenic phototrophic bacteria; cyanobacterial blooms; dissolved organic carbon;Alphaproteobacteria; chlorophyll a
1 INTRODUCTION
Aerobic anoxygenic phototrophic bacteria (AAPB)are heterotrophs that can obtain additional energy from light, and exhibit phototrophy and heterotrophy without producing oxygen (Yurkov and Beatty, 1998;Kolber et al., 2001). They are widely distributed in oceans, estuaries, lakes, and rivers (Koblížek et al.,2006; Mašín et al., 2008), and may be remarkable contributors to the productivity and carbon cycling of aquatic ecosystems (Jiao et al., 2010). The growth rate and effi ciency in organic carbon utilization of these bacteria are higher than those of other strict heterotrophs, and can survive diverse environmental conditions and outgrow competitors (Koblížek et al.,2007; Cepáková et al., 2016).
In freshwater systems, AAPB may constitute various fractions ranging from 1% to 80% of the total bacterioplanktonic community in diff erent trophic lakes (Mašín et al., 2008, 2012; Čuperová et al., 2013;Fauteux et al., 2015). AAPB are phylogenetically diverse and mainly composed of Alpha-, Beta-, and Gammaproteobacteria. The taxonomic compositional structure of AAPB varies in diff erent aquatic systems.Alpha- and Gammaproteobacteria-like AAPB are generally dominant in saline water systems (Allgaier et al., 2003; Jiao et al., 2007; Jiang et al., 2009; Lehours et al., 2010; Jeanthon et al., 2011; Boeuf et al., 2013),while Betaproteobacteria-like AAPB are dominant in freshwater systems (Waidner and Kirchman, 2005,2008; Ferrera et al., 2017b). Betaproteobacteria-like AAPB are dominant in Lake Stechlin, an oligotrophic freshwater lake, whereas Alphaproteobacteria-like AAPB dominate the humic matter-rich southwest basin of Lake Grosse Fuchskule, another freshwater body(Salka et al., 2011). Sphingomonadales-like AAPB affi liated with Alphaproteobacteria are dominant in an alpine oligotrophic lake (Čuperová et al., 2013). The variability in the AAPB community structure is likely attributed to environmental factors, such as trophic status, light attenuation, salinity gradients, inorganic nitrogen levels, total phosphorus contents, dissolved organic carbon (DOC), and temperature of aquatic environment where they inhabit (Jiang et al., 2010;Lehours et al., 2010; Caliz and Casamayor, 2014).
Additional to the above-mentioned abiotic factors,close associations between AAPB and phytoplankton has been demonstrated in cyanobacterial mats,microalgae, and dinof lagellate cultures in some pioneer works (Allgaier et al., 2003; Green et al., 2004;Goecke et al., 2013). Specif ic response of AAPB to diff erent algal species has also been observed (Chen et al., 2011). Due to the increased relative abundance of light-dependent AAPB, bacterial community composition in phycosphere was linked closely to light intensity (Piwosz et al., 2020). Distribution of AAPB is aff ected by the availability of organic carbon derived from phytoplankton found in global oceans(Kolber et al., 2001; Jiao et al., 2007; Zhang and Jiao, 2007). Rhodobacteracea-like AAPB affi liated with Alphaproteobacteria become dominant when chlorophylla(Chla) and nutrient concentrations in an oligotrophic area in the Northwestern Mediterranean increase (Ferrera et al., 2014). These studies indicated close relationships between AAPB and phytoplankton.Moreover, in our study on bacteria in phycosphere of cyanobacteria, we also observed that AAPB are associated not only with cyanobacterial cultures but also with cyanobacterial colonies (Shi et al., 2010).So we proposed that there may be some association between AAPB and cyanobacterial blooms which occur in freshwaters and spread worldwide quickly under climate warming (Paerl and Paul, 2012).
Taihu Lake is well known for annual cyanobacterial blooms, which have greatly changed bacterial communities. However, whether the compositions of AAPB are inf luenced by cyanobacterial blooms is still unknown. Given special feature of AAPB and their importance in carbon and nutrient cycling,it is urgent to reveal their distribution pattern in Taihu Lake. In this study, we compared AAPB populations in Meiliang Bay characterized by dense cyanobacterial blooms and East Bay characterized by macrophyte in Taihu Lake in China. In Meiliang Bay,with annual average Chl-aconcentrations throughout the water column increased from 23 μg/L in 1992 to peaks around 50 μg/L in 1998, cyanobacterial bloom biomass steadily remained at high levels up to 2010(Xu et al., 2017). Coverage of cyanobacterial blooms remained moderate and stable until 2015, and then reached another peak around 2017 (Jia et al., 2019).In East Bay, remote sensing mapping revealed a decreased aquatic vegetation presence frequency during 2003-2014 (Zhang et al., 2016). Macrophyte cover declined linearly from 36% in 2002 to 17% in 2015, which was largely due to the loss of submerged macrophytes, which decreased signif icantly from 88.5% in 2000 to 45.8% in 2013 (Zhang et al., 2019).Here, we performed distance-based redundancy analysis (dbRDA) and Pearson correlation analysis to determine the key environmental factors driving AAPB distribution.
2 MATERIAL AND METHOD
2.1 Study area
Taihu Lake is located between 30°56′N-31°33′N and 119°53′E-120°36′E in Jiangsu Province, East China, and is the third-largest freshwater lake in China. With a surface area of 2 338 km2, a mean depth of 1.9 m, and a water residence time of approximately 284 days (Qin et al., 2007), the lake is used as the drinking water source of several cities, including Shanghai, Suzhou, Wuxi, and Huzhou. In addition, it is an important water source for irrigation for farming,industry, and recreational activities (Qin et al., 2007).However, with rapid economic development, the lake water has been seriously polluted. The lake as a whole is eutrophic and aff ected by cyanobacterial blooms dominated byMicrocystisspp. (Chen et al.,2003; Ma et al., 2016). Most of the lakes, especially Meiliang Bay, suff ered from cyanobacterial blooms.The eastern region (the East Bay) is mesotrophic and mainly covered by submerged macrophytes. In our study, water samples were collected in the Meiliang Bay (Site N, 31°25′53.8″N, 120°12′42.5″E) and the East Bay (Site E, 31°3′5″N, 120°27′59.8″E) of Taihu Lake (Fig.1).
2.2 Sample collection and handling
Water samples (0-0.5-m depth) were collected using water sampler bimonthly during August 2011 to June 2012. Water temperature, pH, and dissolved oxygen(DO) were determined in situ using an YSI 6600 multiparameter underwater sensor (Yellow Springs Instrument, Model, USA). Nutrient concentration including dissolved inorganic nitrogen, phosphorus,total nitrogen (TN), and phosphorus (TP) were measured using a Skalar auto analyzer (Skalar, San Plus System). For determination of Chlaand phycocyanin(PC), 100 mL of water samples were f iltered using GF/F f ilters (Whatman, 47 mm), and the f ilters were extracting with 90% acetone for Chlaand 0.1-mol/L phosphate buff er (pH 6.8) for PC respectively, and absorbance of the extracts was measured accordingly using a spectrof luorophotometer (ModelRF-5301PC;Shimadzu) (Asai et al., 2001). The dissolved organic carbon (DOC) concentration in f iltrate was analyzed with a total organic carbon analyzer (TOC-5000,Shimadzu, Tokyo, Japan). For determination of AAPB, 100 mL of water samples was f iltered through polycarbonate f ilters (Millipore, 0.2-μm pore size, 47-mm diameter) using moderate vacuum, and the f ilters were stored at -70 °C until DNA extraction.
2.3 DNA extraction
Community DNA was extracted from the 0.2-μm f ilters using xanthogenate-sodium dodecyl sulfate(SDS) DNA extraction protocols according to Tillett and Neilan (2000). Brief ly, f ilters were cut into small pieces, put into a 2-mL sterile Eppendorf tube with 50-μL TER (10-mmol/L Tris-HCl, pH 7.4; 1-mmol/L EDTA, pH 8; 100-μg m/L RNase A), 750-μL freshly made XS buff er (1% potassium ethylxanthogenate;100-mmol/L Tris-HCl, pH 7.4; 20-mmol/L EDTA,pH 8; 1% sodium dodecyl sulfate; 800-mmol/L ammonium acetate) were added, then the tubes were incubated at 70 °C for 120 min, after that the tubes were vortexed for 10 s before placed on ice for 30 min. The supernant DNA was precipitated with isopropanol, washed with 70% ethanol, and dissolved in 50-μL sterile distilled water.
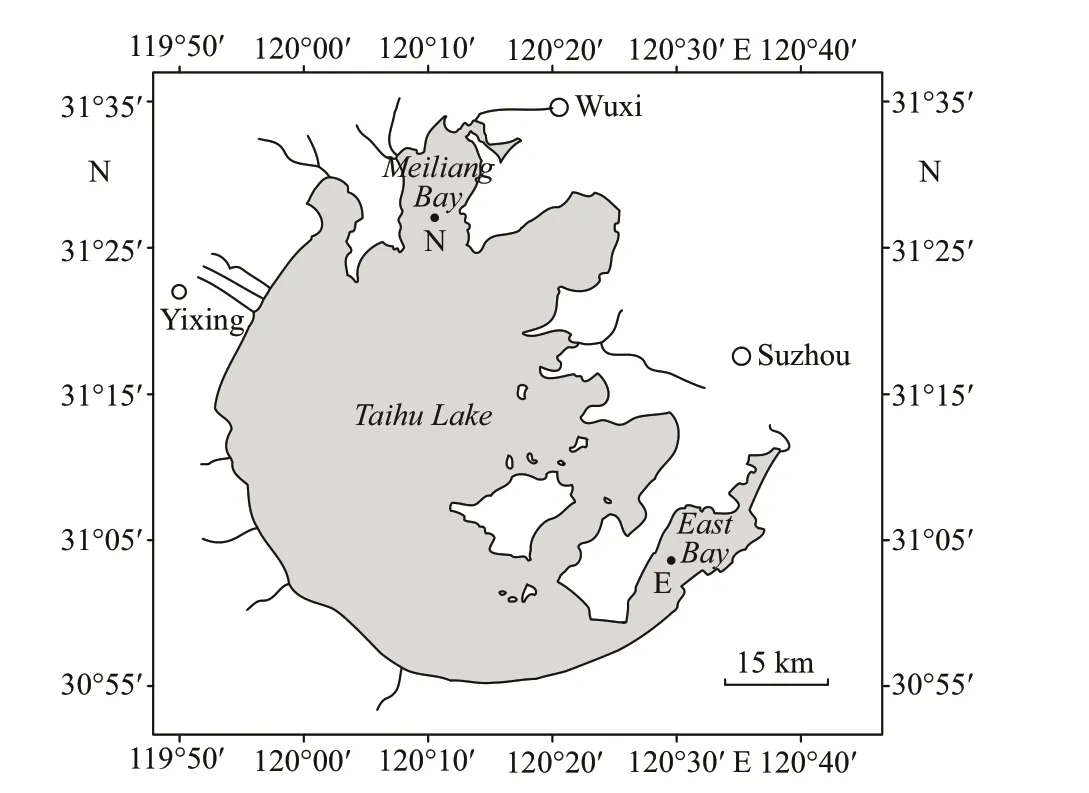
Fig.1 Map of Taihu Lake and the sampling sites (N and E)
2.4 Quantitative real-time PCR (qPCR)
The abundances of AAPB, total bacteria were determined by qPCR based on their specif ic primers using Master cycler ep Realplex (Eppendorf,Germany). For AAPB, PCR amplif ications of the partial sequences of thepufMgene were conducted using the forward primerpufM557F(5′-TACGGSAACCTGTWCTAC-3′) and reverse primerpufM750R (5′-CCATSGTCCAGCGCCAGAA-3′) (Achenbach et al., 2001; Béjà et al., 2002; Du et al., 2006; Hu et al., 2006; Jiao et al., 2007). The PCR products were 193-bp fragments. The reaction mixtures (25 μL) contained 12.5-μL SYBR Premix Ex Taq (TaKaRa, Japan) and 2.5 pmol of each primer. The amplif ication conditions consisted of 94 °C for 4 min,followed by 30 cycles at 94 °C for 1 min, 52 °C for 1 min, and 72 °C for 1 min, and an extension at 72 °C for 5 min (Du et al., 2006). For total bacteria, qPCR amplif ications was conducted by using Bac331F(5′-TCCTACGGGAGGCAGCAGT-3′)/Bac797R(5′-GGACTACCAGGGTCTAATCCTGTT-3′) (Nadkarni et al., 2002; Jiang et al., 2009). The PCR products were gel-purif ied, and cloned intoEscherichiacoliDH5α cells following the manufacturer’s instructions.Inserts in the clones were conf irmed through PCR using the above specif ic primers and subsequent electrophoresis. Dilution series (103to 108copies/μL)of the purif ied plasmids containing the target genes were used as standard DNA templates to make the standard curves. QPCR for AAPB and total bacteria was conducted in triplicate as described above except the amplif ication cycles were changed from 30 to 45.The standard curve was determined by plotting the threshold cycle number (Ct) values versus the standard DNA concentrations, thus DNA concentrations in samples can be calculated based on theirCtvalues.
2.5 PCR and clone library construction
The partial sequences ofpufMgene of AAPB were amplif ied using the primer setpufM557F andpufM750R, as described above. PCR reaction mixtures(25 μL) contained 0.2 mmol/L of each primer, 20-ng template DNA, 1.5-mmol/L MgCl2, 0.2 mmol/L of each dNTP, and 5-U Taq DNA polymerase (TaKaRa,Japan). A total of 12pufMgene clone libraries were constructed from the 12 samples. For each sample,the PCR products ofpufMgene amplif ied in triplicate were pooled, ligated to a pGEM-T easy vector(Promega Corp., USA), and cloned intoE.coliDH5α cells. Fifty positive clones were randomly selected from each clone library, examined through PCR by using the vector primers T7 and SP6, and sequenced with an ABI DNA sequencer. All of the clone sequences retrieved from this study were subjected to a chimera test with the CHIMERA_CHECK program available in the Ribosomal Database Project (RDP)website (Cole et al., 2003). A total of 482 nonchimericpufMsequences were grouped into operational taxonomic units (OTUs) with 6% divergence of nucleic acid sequence (Zeng et al., 2007) by using Mothur (Schloss et al., 2009). Rarefaction analysis computed in Mothur was conducted to estimate the total diversity in each clone library. The coverage of each clone library was determined with the formula[1-(OTUN/sequenceN)]×100, where OTUNis the number of OTUs, and sequenceNis the total number of sequences (Jiang et al., 2010).
2.6 Phylogenetic analysis
Almost the completepufMsequences longer than 900 bp of the cultured species and the environmental clones were retrieved from GenBank database (http://www.ncbi.nlm.nih.gov/Genbank/). Sequences were automatically aligned with Clustal W in Mega 4.0.ApufMdatabase containing 190 aligned sequences was then imported into the ARB database (http://www.arb-home.de/). A backbone tree was calculated from these sequences with a maximum-parsimony algorithm and saved as a positional tree server in the ARB software package. The partialpufMsequences obtained in this study were then inserted into the preestablished core tree by using the ARB parsimony tool and maintaining the overall tree topology without changes. The trees were then exported as Newick f iles and edited online via the Interactive Tree of Life (iTOL) (http://itol.embl.de) (Letunic and Bork, 2007). The phylogenetic affi liations ofpufMsequences were based on the phylogenetic tree and compared with the sequences in the National Center for Biotechnology Information (NCBI) database via the Basic Local Alignment Search Tool (BLAST)(http://blast.ncbi.nlm.nih.gov/Blast.cgi). The sequences obtained in this study were deposited in the European Molecular Biology Laboratory (EMBL) database under accession numbers HF947097 to HF947271.
2.7 Statistical analysis
Nonmetric multidimensional scaling (NMDS)analysis based on Bray-Curtis algorithm distance matrix was performed for all samples on the OTU level. Distance-based redundancy analysis (dbRDA)was used to examine the inf luence of detected environmental factors including temperature, pH,DO, TN, NH4+, NOxˉ (NO2ˉ+NO3ˉ), TP, PO43ˉ, DOC,and Chlaon the dynamics of AAPB composition.Signif icance of variables was assessed with Monte-Carlo permutation tests (999 unrestricted permutations). All these analyses were performed with the “vegan” package (Oksanen et al., 2013) of R software (R Development Core Team 2012). Taxa that were signif icantly diff erent between the two sites and two major diff erent seasons were detected using the Bioconductor-edgeR package (version 3.2.4) (Robinson et al., 2010). In addition, Pearson correlations were run to investigate the relationship between environmental factors and dominantpufMgenotypes using SPSS version 17.0 software (SPSS Inc., Chicago, IL, USA).
3 RESULT
3.1 Environmental parameter
Seasonal variation of environmental variables and nutrient concentrations of surface water are shown in Table 1. Water temperature varied from 4.4 °C to 30.9 °C throughout the year. The oxygen concentration varied from 5.7 to 13.6 mg/L, and was signif icantly negatively correlated with temperature(R=-0.88,P<0.01,n=12). In Site N, cyanobacterial blooms dominated byMicrocystisoccurred from Juneto October. The Chl-aand PC concentration varied from 1.96 to 65.15 μg/L and 3.62 to 157.80 μg/L respectively, with a maximum value in June. In Site E, submersed macrophytes were dominant with relatively low cyanobacterial biomass. The concentrations of Chlaand PC varied from 3.30 to 5.59 μg/L and 0.85 to 2.99 μg/L respectively. There were signif icantly correlated relationships between PC and Chla(Pearson correlation,R=0.993,P<0.001). TN in Site N was signif icantly higher than that in Site E (P<0.01, ANOVA). Temperature was signif icantly higher while DO was signif icantly lower in warm seasons (June, August, and October)than that in cold seasons (December, February, and April) (P<0.05, ANOVA). There were no signif icant diff erences of other detected environmental variables between the two sites, or between warm and cold seasons.
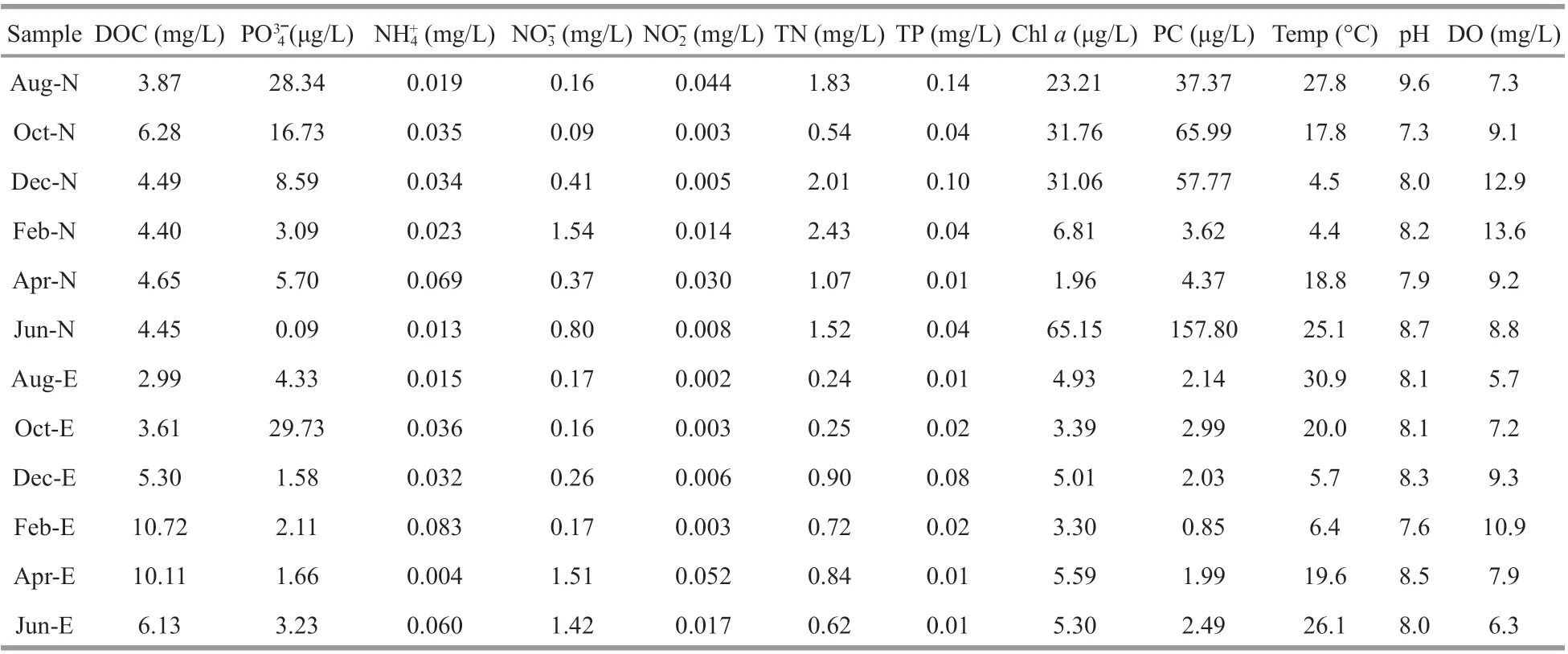
Table 1 Basic physico-chemical characteristics of water samples from Taihu Lake
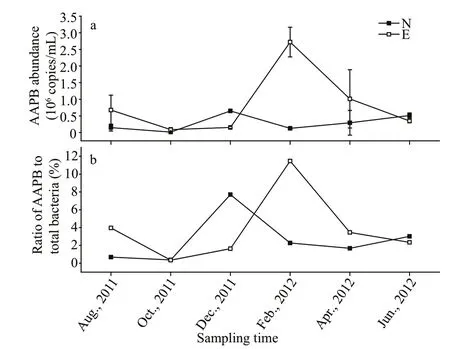
Fig.2 Abundance of AAPB based on pufM gene (a) and the ratio of AAPB to total bacteria (b) in the two sampling sites in Taihu Lake
3.2 Abundance of AAPB
QPCR based on thepufMgene showed that AAPB in Site N varied from 0.01×106(±10 260) to 0.65×106(±10 360) copies/mL, whereas that in Site E varied from 0.08×106(±58 900) to 2.72×106(±446 000) copies/mL(Fig.2a). Amplif ication effi ciencies of 1.11-1.32 were obtained withR2values of 0.985 to 0.990. The ratio of AAPB to total bacteria in Sites N and E ranged from 0.68% to 7.70%, and from 0.34% to 11.47%,respectively, with peak values in December or February when temperature was around 4 °C in Site N and E, respectively (Fig.2b). Pearson correlation analysis revealed that abundance of AAPB was positively correlated with DOC concentrations(R=0.741,P<0.01,n=12).
3.3 Phylogenetic analyse of pufM gene
A total of 482 clones (243 clones from Site N and 239 clones from Site E) were obtained from the 12pufMgene clone libraries, and a total of 136 distinct OTUs were identif ied after the sequences were grouped at 94% nucleic acid sequence similarity.Among the 136 OTUs, 57 and 43 were unique in Sites N and E, respectively, and 36 were shared by the two sites (Fig.3a). The genetic diversity ofpufMin Sites N and E varied from 2.26 to 3.19 and from 1.73 to 2.88, respectively. The coverages ofpufMin Sites N and E varied from 33% to 57% and from 41% to 70%, respectively (Table 2). The rarefaction curves appeared to be far from the saturation level (Fig.3b).
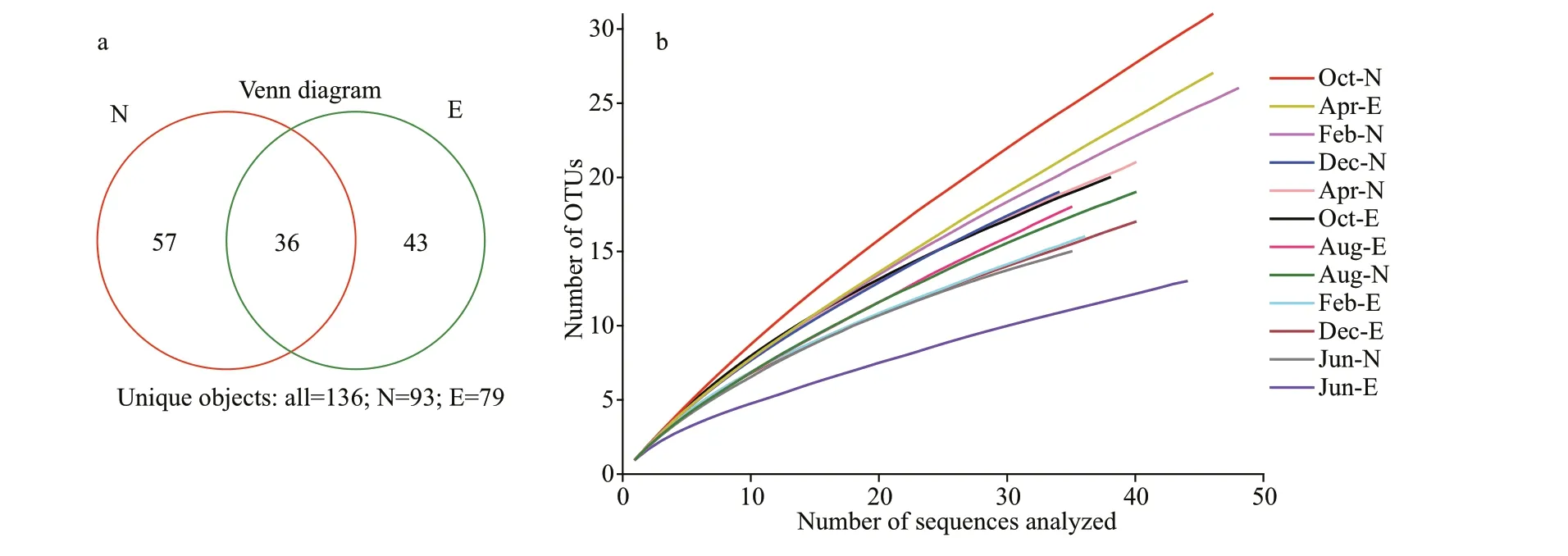
Fig.3 Diversity of AAPB genotypes
Most OTUs exhibited 80% to 97% similarity withpufMof the cultured anoxygenic phototrophs.Phylogenetic analysis demonstrated that mostpufMgene sequences were affi liated with the Alphaproteobacteria clade (Fig.4).Alphaproteobacteria-likepufMsequences were dominated by three orders/genera, namely,Rhizobiales-,Rhodobacter-, andPorphyrobacterlike organisms (Fig.5).Limnohabitans-likepufMsequences affi liated with Betaproteobacteria were also a dominant group from December to April in both sites (Fig.5).
No signif icantly diff erent genotypes were observed between the two sites. MostpufMsequences were present in both sites, butSandarakinorhabdusandMethylobacterium-likepufMsequences were only detected in Site N, andBlastomonas-likepufMsequences were only detected in Site E (Fig.5).NMDS analysis revealed that no signif iciant diff erence occurred between the two sampling sites,but a separation between warm seasons (June, August,and October) and cold seasons (December, February,and April) existed (Fig.6). EdgeR analysis revealed that 1 OTU affi liated with Rhizobiales and 5 OTUs affi liated withLimnohabitans-likepufMsequences signif icantly contributed to the diff erence between the two seasons, and they were specially enriched in cold seasons (P<0.05, false discovery rate (FDR)-corrected) (Table 3).
3.4 Distribution of pufM diveristy in relation to environmental factors
DbRDA results illustrated that Chlaand DOC were the most signif icant variables (Monte Carlo test,P<0.05) in the community composition (Fig.7).Besides, temperature and PO43ˉ were also major contributors to the variance. The f irst two axes accounted for 52.7% (Axis 1, eigenvalue=0.13) and 22.4% (Axis 2, eigenvalue=0.06) of the variations of AAPB community composition.Porphyrobacter,Rhodospirillales,Sandarakinorhabdus-likepufMsequences were closely associated with Chla,whereas Gammaproteobacteria,Sphingomonas,Novosphingobium-likepufMsequences were closely related with temperature and DOC (Fig.7). Pearson correlation analyses revealed thatLimnohabitanslikepufMsequences were negatively correlated with temperature (R=-0.69,P<0.05) and positively correlated with DOC (R=0.61,P<0.05).RhodobacterlikepufMsequences were positively correlated with temperature (R=0.58,P<0.05). RhizobialeslikepufMsequences were negatively correlated with Chla(Pearson correlation,R=-0.6,P<0.05),whereasPorphyrobacterand Rhodospirillales-likepufMsequences were positively correlated with Chla(Pearson correlation,R=0.85 andR=0.72,respectively,P<0.01).Sandarakinorhabdus-likepufMsequences were also positively correlated with Chla(Pearson correlation,R=0.61,P<0.05).
4 DISCUSSION
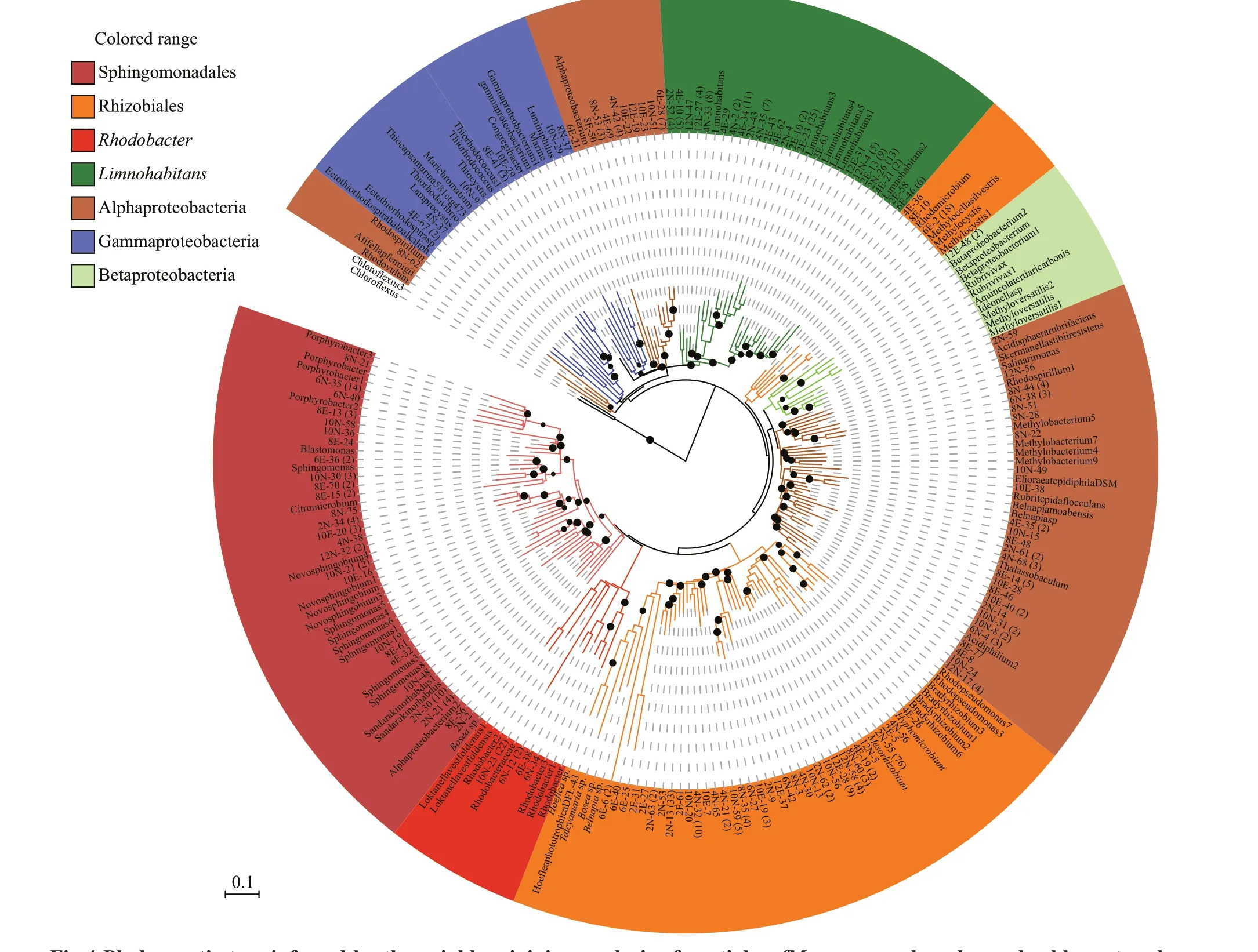
Fig.4 Phylogenetic tree inferred by the neighbor-joining analysis of partial pufM sequences based on a backbone tree by using the ARB software package (http://www.arb-home.de)
The higher concentrations of both Chlaand PC in Site N than that in Site E of Taihu Lake conf irmed that cyanobacterial blooms occurs in Site N, and there are relatively low cyanobacterial biomass in Site E. PC is an accurate probe for monitoring cyanobacteria for signif icant correlation between PC and cyanobacterial biomass (Brient et al., 2008). In particular, PC was not displayed in the dbRDA results due to signif icantly correlation between PC and Chla(Pearson correlation,R=0.993,P<0.001). The well correlated relationship between PC and Chlaalso convinced dominance of cyanobacteria in total phytoplankton.Our results demonstrated the high abundance ofpufMgene and their positive correlations with DOC in the investigated regions of Taihu Lake. The abundance of AAPB in Taihu Lake was comparable with that in the Waihai and Chaohai areas of Dianchi Lake, a eutrophic lake (Tian et al., 2018) and mesotrophic lake Vlkov (Kolářová et al., 2019), but was considerably higher than that in oligotrophic saline lakes in the Tibetan plateau (Jiang et al., 2009). The ratio of AAPB to the total bacteria in Taihu Lake was similar to that in glacial lakes in Northern Europe (Mašín et al., 2012) and lakes in Northern Québec (Fauteux et al., 2015) (Table 4). Moreover, the result that ratio of AAPB to the total bacteria was higher in winter than other seasons was consistent to previous study (Zhang and Jiao, 2007), further indicating less dependent of some AAPB on temperature. The result that AAPB abundance was signif icantly positively related to DOC in Taihu Lake was similar to the observation that has been noted in lakes in three distinct regions in Northern Québec (Fauteux et al., 2015), in the alpine lake Gossenköllesee located in Tyrolean Alps, Austria(Čuperová et al., 2013), and in freshwater lake Vlkov(Kolářová et al., 2019). AAPB may rely on labile DOC excreted by phytoplankton and contribute to refractory DOC production (Jiao et al., 2007, 2010).In addition, AAPB utilize light energy under natural conditions to maintain much higher growth rates than many other bacterioplankton groups (Ferrera et al.,2017a), and may be even higher under elevated water temperatures in the absence of grazers (Sato-Takabe et al., 2019). Therefore, we suggested that AAPB may contribute signif icantly to the alteration and regeneration of carbon forms in these ecosystems.
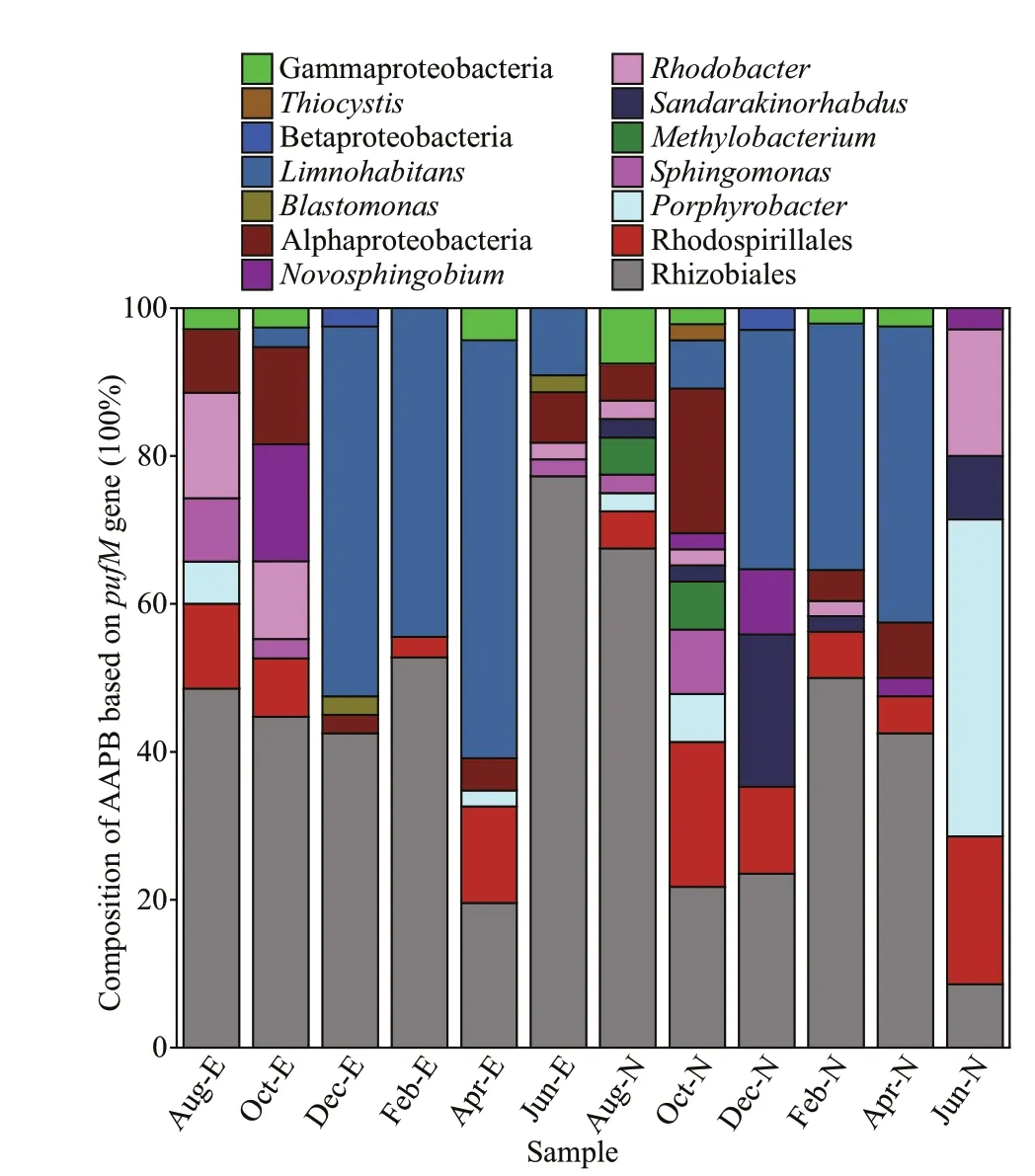
Fig.5 Community compositions of AAPB based on pufM sequences in the two sites in Taihu Lake
The phylogenetic analyses of thepufMsequences revealed that AAPB in both sites of Taihu Lake were dominated by Rhizobiales-like sequences in Alphaproteobacteria, followed byLimnohabitanslike sequences in Betaproteobacteria. This result was diff erent from that in most German freshwater lakes including the alkaline to pH neutral lakes Stolp,Stechlin, and Fuchskuhle NE basin whereRhodoferaxrelatedpufMsequences in Betaproteobacteria are predominant (Salka et al., 2011). AlphaproteobacterialikepufMsequences were observed to be dominant in Taihu Lake in our preliminary study (Shi et al., 2010)and other freshwater lakes, such as southwestern basin of Lake Grosse Fuchskule in Germany (Salka et al., 2011) and Lugu Lake, Erhai Lake, and Chenghai Lake in China (Tian et al., 2018). These results further indicated lake specif ic diversity of AAPB community.Some Alphaproteobacteria AAPB may possess the genomic potential for anoxygenic phototrophy and carbon f ixation via the Calvin-Benson-Bassham cycle and for sulf ite and thiosulfate oxidation (Graham et al., 2018; Imhoff et al., 2018). Thus, the eff ect of the predominance of Alphaproteobacteria-likepufMsequences on the primary production in these lakes should be considered, especially in the calculation of the carbon budget in these lakes.
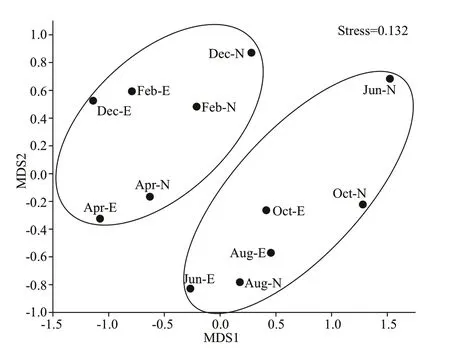
Fig.6 Nonmetric multidimensional scaling plot based on Bray-Curtis dissimilarity
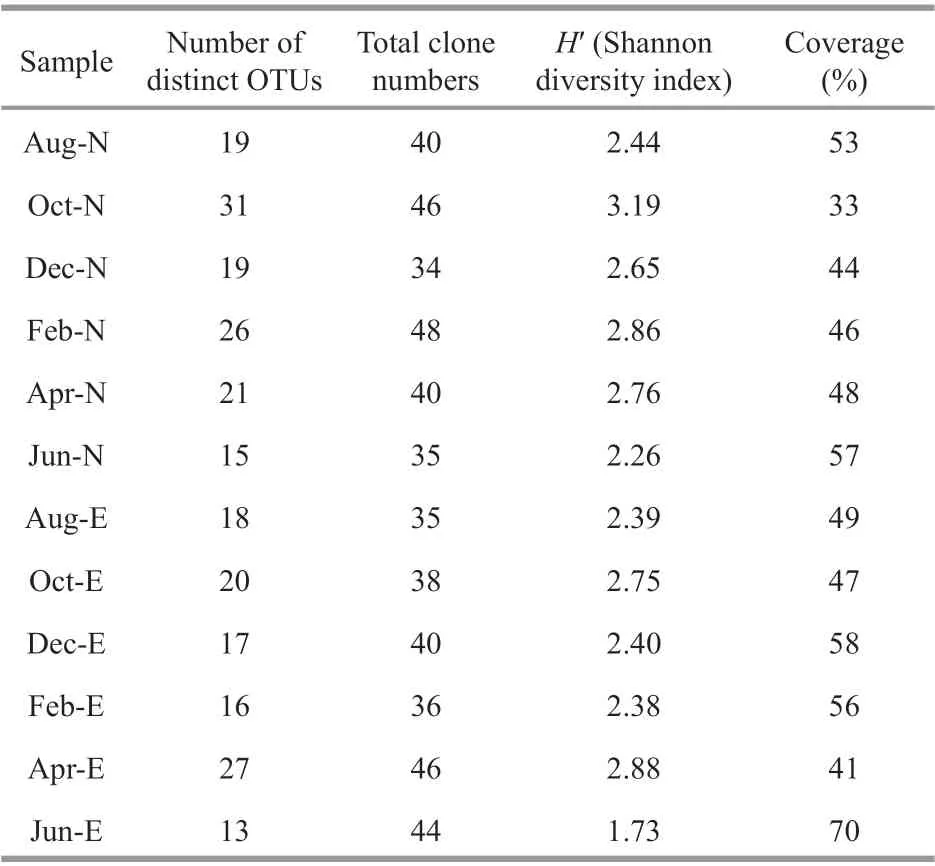
Table 2 Properties of the distribution of phylotypes in both Sites N and E
SandarakinorhabdusandMethylobacteriumlikepufMsequences were detected in Site N only.Porphyrobacter-likepufMsequences was dominant in Site N in June when Chl-aconcentrations exhibited a peak value of 65 μg/L, which indicated serious cyanobacterial blooms. Bacteria affi liated toSandarakinorhabdus,Methylobacterium, andPorphyrobacterare dominant bacteria attached to cyanobacterial phycosphere (Berg et al., 2009; Shi et al., 2009a, 2009b). Therefore, these AAPB mayoriginate from cyanobacterial phycosphere, which is a special microenvironment that feeds some unique bacterial f lora (Shi et al., 2009b). These results indicated inf luence of cyanobacterial blooms on certain AAPB communities. Furthermore,SandarakinorhabdusandPorphyrobacterin cyanobacterial phycosphere participated in forming the phosphorous cycling copathway as their functional links to cyanobacteria(Zhu et al., 2021). Dominance ofMethylobacteriumthat could utilize single-carbon compounds was observed in small cyanobacterial aggregates (Cai et al., 2014). Moreover, association between AAPB and cyanobacteria have also been observed in Tama River,Japan (Sato-Takabe et al., 2020), and global ocean (Jiao et al., 2007). AAPB have high effi ciency in organic carbon utilization (Koblížek et al., 2007; Cepáková et al., 2016); thus, the presence of these functional bacteria in cyanobacterial phycosphere may accelerate nutrient cycling and facilitate cyanobacterial growth.Future studies on the ecological roles of these AAPB attached to cyanobacteria should be conducted.
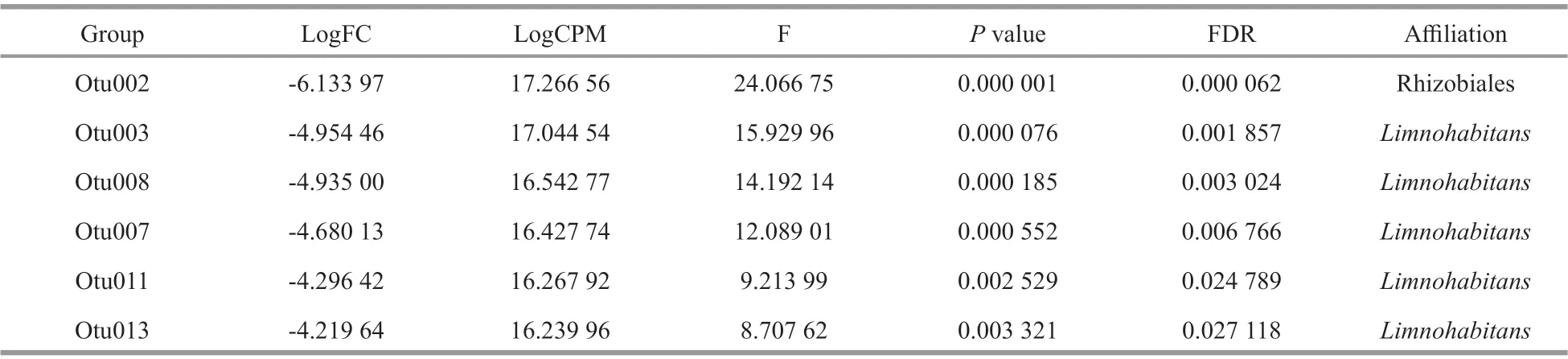
Table 3 EdgeR analysis revealed pufM sequences that were signif icantly diff erent between warm and cold seasons ( P< 0.05,FDR-corrected)
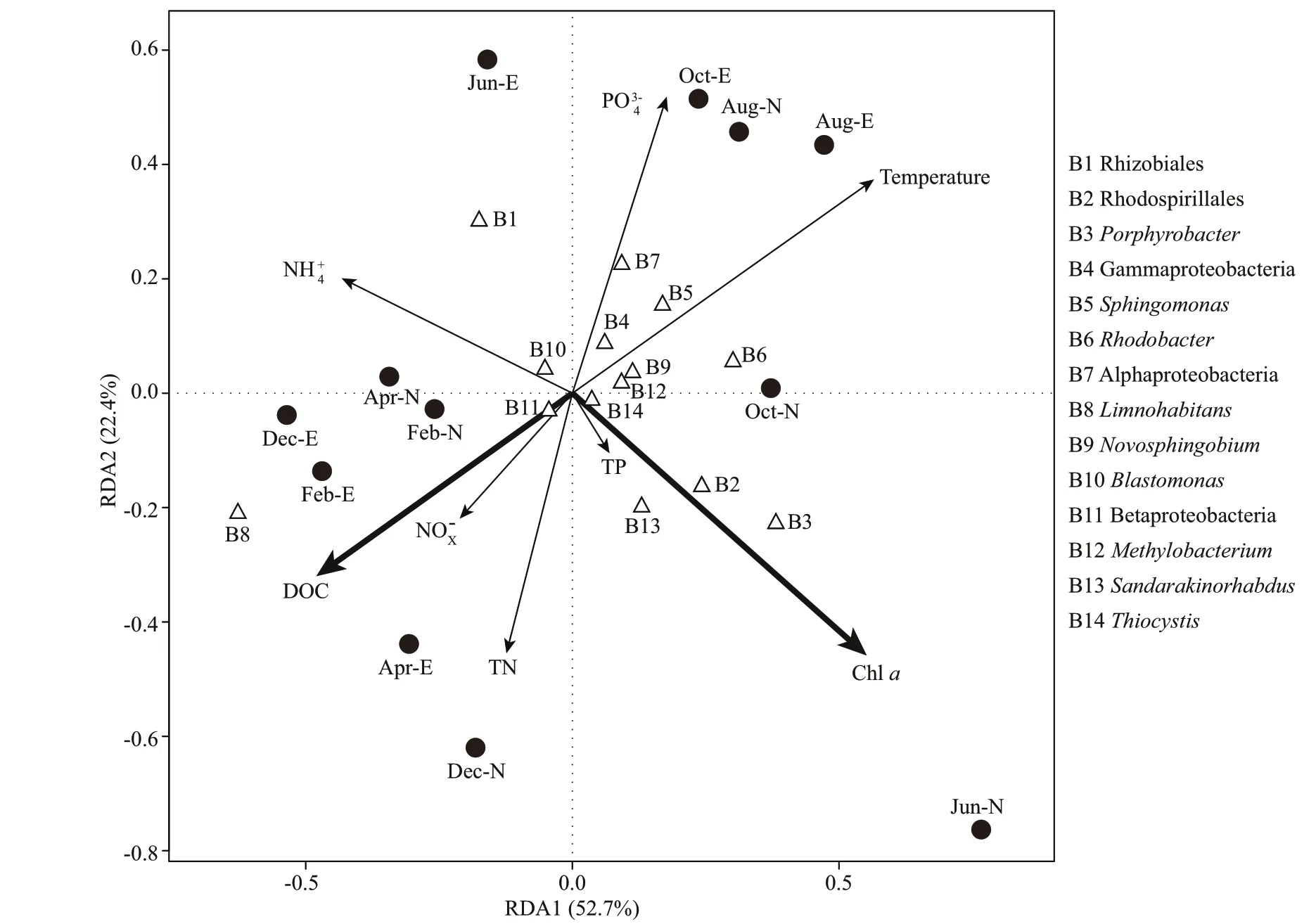
Fig.7 Distance-based redundancy analysis ordination plot showing the relationship of the samples, AAPB, and water environmental parameters
Our results demonstrate the high diversity ofpufMin Taihu Lake. Rarefaction curves indicated that diversity was not fully captured in most communities,and our clone libraries were incomplete. Future studies involving high-throughput sequencing based onpufMshould also be conducted. No obvious separation was observed between the samples in the two sites. This may be due to sample scale limitation;more samples are needed in future for accurately analysis of AAPB distribution pattern in these two sites. However, NMDS analysis revealed a separation between warm seasons (June, August, and October)and cold seasons (December, February, and April).Rhizobiales andLimnohabitans-likepufMsequences were found to contribute to the diff erence between the two seasons, especially enriched in cold seasons.Rhizobiales associated with plant shoot and root systems having photosynthetic systems may provide additional energy for nitrogen f ixation, and promote plant growth and stem nodulation (Fleischman and Kramer, 1998; Giraud and Fleischman, 2004).Limnohabitanswas considered a ubiquitous photoheterotrophic bacterium in various freshwater habitats (Kasalický et al., 2018).Limnohabitans-likepufMsequences are predominant in ultraoligotrophic high-altitude lakes (Central Pyrenees), where Chlais lower than 5 μg/L when temperature is lower than 15 °C (Caliz and Casamayor, 2014).Limnohabitansis also assumed a photoautotroph and ammonia oxidizer(Zeng et al., 2012). Therefore, these genotypes specif ically enriched in cold seasons indicated separated ecological niches might exist between them and cyanobacteria.
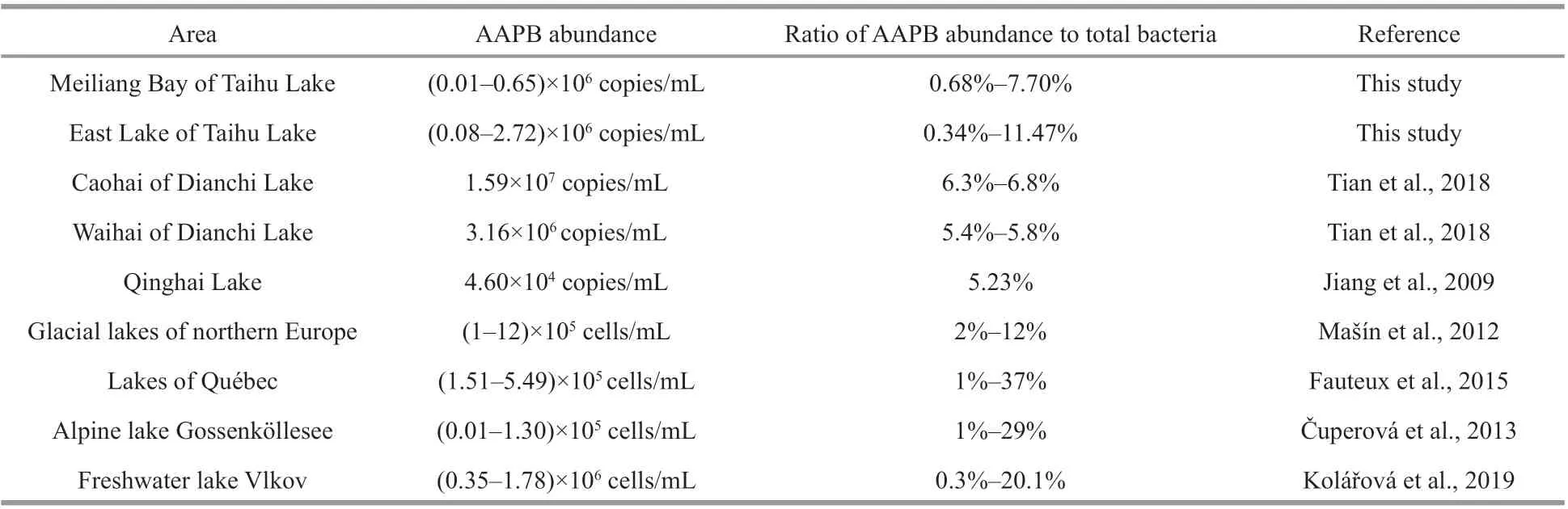
Table 4 Comparison of AAPB abundance and ratios of AAPB abundance to total bacteria in Taihu Lake and other lakes
DbRDA results showed that Chlaand DOC were the most signif icant variables associated with the community structure of AAPB indicating inf luence of cyanobacterial blooms on AAPB composition. Furthermore, certain AAPB genotypes were signif icantly correlated with Chla, DOC, or temperature. These results suggested that diverse AAPB ecotypes were present in Taihu Lake and they may be inf luenced by diff erent environmental factors.Some AAPB may share a similar ecological niche with phytoplankton or be specif ically stimulated by cyanobacterium-derived DOC because they are mixotrophic (Yurkov and Beatty, 1998; Graham et al., 2018). Specif ic clades ofpufMwere signif icantly associated with high abundances ofSynechococcusand chlorophyll in coastal regions of the Pacif ic Ocean (Ritchie and Johnson, 2012). Taxonomic specif ic response of AAPB to light and predation were also observed (Ruiz-González et al., 2020). The close correlation between some AAPB and Chlamay indicate that they coexist in a tightly linked nutrient cycle (Kolber et al., 2001). Given the rapid growth and photoheterotrophic characteristics of AAPB, they can form large sinks for dissolved organic matter (Li et al., 2017) and inf luence carbon and nitrogen cycle,especially the microbial loop in aquatic systems(Koblížek, 2015). Furthermore, mixed AAPB may have higher DOC and nitrogen removal effi ciencies(Zhang et al., 2020). Therefore, the ecological role of the diverse AAPB in biogeochemical cycles in these large shallow eutrophic lakes should be considered in future.
5 CONCLUSION
In summary, this study revealed the presence of abundant and diversepufMsequences in Meiliang Bay and East Bay of Taihu Lake. The abundance ofpufMsequences was inf luenced by DOC, and the ratio ofpufMsequences to total bacteria peaked in winter in both sites. Alphaproteobacteria-likepufMsequences were predominant in Taihu Lake. A clear separation between the samples collected in the two sites was not observed, but a separation between warm seasons (June, August, and October) and cold seasons (December, February, and April) was revealed. The community structure of AAPB in the investigated samples was signif icantly inf luenced by Chlaand DOC, and certain AAPB genotypes were signif icantly correlated with temperature, Chlaor DOC, respectively. These results indicated thatpufMsequences were abundant and diverse in Taihu Lake,and some ecotypes were inf luenced by cyanobacterial blooms. Thus, their ecological role in carbon and nutrient cycling, especially in these lakes, should be considered.
6 DATA AVAILABILITY STATEMENT
The data supporting the conclusions are presented in the main article.
7 ACKNOWLEDGMENT
We are grateful to Mingyong DU, Shiming LIU,Mingbo SUN, and Yinping WANG from Nanjing Institute of Geography and Limnology for their assistances on sample collection, and Mengyu QIAN for determination of concentration of chlorophyllaand phycocyanin. Of the authors, Limei SHI conducted the experiments and drafted the manuscript, Yuanfeng CAI performed data analyses. Xiaoli SHI and Min ZHANG reviewed and edited the manuscript. Qingfei ZENG helped in sample collection. Fanxiang KONG designed the study. Ping XU revised the English language. All authors have read and approved the f inal manuscript.
 Journal of Oceanology and Limnology2022年5期
Journal of Oceanology and Limnology2022年5期
- Journal of Oceanology and Limnology的其它文章
- Comparison of three f locculants for heavy cyanobacterial bloom mitigation and subsequent environmental impact*
- Eff ect of light intensity on bound EPS characteristics of two Microcystis morphospecies: the role of bEPS in the proliferation of Microcystis*
- Tidal water exchanges can shape the phytoplankton community structure and reduce the risk of harmful cyanobacterial blooms in a semi-closed lake*
- Eff ect of random phase error and baseline roll angle error on eddy identif ication by interferometric imaging altimeter*
- Estimating the evolution of sea state non-Gaussianity based on a phase-resolving model*
- Sodium acetate can promote the growth and astaxanthin accumulation in the unicellular green alga Haematococcus pluvialis as revealed by a proteomics approach*
