Comparison of three f locculants for heavy cyanobacterial bloom mitigation and subsequent environmental impact*
Kaixuan LIU , Lei JIANG , Jinsheng YANG , Shuzhan MA , Kaining CHEN ,Yufeng ZHANG , Xiaoli SHI ,4,**
1 Department of Environmental Science and Engineering, Nanjing Tech University, Nanjing 211816, China
2 State Key Laboratory of Lake Science and Environment, Nanjing Institute of Geography and Limnology, Chinese Academy of Sciences, Nanjing 210008, China
3 University of Chinese Academy of Sciences, Beijing 100049, China
4 Jiangsu Collaborative Innovation Center of Regional Modern Agriculture & Environmental Protection, Huaiyin Normal University, Huaiyin 223001, China
Keyword: cyanobacterial bloom; emergency control; f locculants; lake restoration
1 INTRODUCTION
Eutrophication is characterized by excess nutrients in aquatic ecosystems, often leading to a turbid, phytoplankton-dominated state with a predominance of cyanobacteria (Ulrich et al., 2016;Waajen et al., 2016; Watson et al., 2016). Longterm in-situ observations in the f ield have shown that the daily growth rate of cyanobacteria is high during an outbreak of cyanobacteria, and the current approaches to mitigate and treat a cyanobacterial bloom do not eff ectively control its growth, resulting in a large accumulation of cyanobacteria on the leeward side of eutrophic water bodies. In some large eutrophic lakes, a high biomass of cyanobacteria can accumulate in lakeside areas. Cyanobacterial blooms can greatly harm aquatic ecosystems, especially when bloom-forming species release toxins that can cause f ish kills, are potentially toxic to humans, dogs,waterfowl, and other animals, reduce biodiversity,and cause unpleasant surface scum and malodors(Yang et al., 2020; Zhang et al., 2021). Thus, the prevention, control, and emergency management of cyanobacteria in lakeside zones of large eutrophic lakes are important measures for alleviating the ecological disasters caused by cyanobacterial blooms and reducing the impact of cyanobacterial blooms on human homeostasis and life, which are primary objectives for lake managers.
In general, cyanobacterial bloom mitigation measures can be divided into biological, physical,and chemical methods (Stroom and Kardinaal,2016). Biological methods are intended to change the ecosystem toward less favorable conditions for cyanobacteria but often fail due to high nutrient loading, high cyanobacterial biomass, and inappropriate growth conditions (Lürling and Mucci,2020). Physical methods include ultrasonic methods,adsorption methods, and separation membranes (Park et al., 2019; Jiang et al., 2020; Truttmann et al., 2020).However, these methods have high operating costs and laborious operations; the energy costs are high for full-scale operations in the f ield, and the eff ective range may be limited (Visser et al., 2016). Chemical methods are used to directly reduce the cyanobacterial biomass in water bodies via algaecides. Copper-based algaecide is commonly used but has been mostly abandoned due to its nonspecif icity and potential toxicity to other aquatic biotas (Li et al., 2011; Bishop et al., 2018). Hydrogen peroxide is considered an environmentally friendly algaecide because it can selectively suppress cyanobacterial growth without releasing any residual chemicals. However, a high dosage of H2O2is required to suppress cyanobacteria with high algal biomass and large colony size, which could cause the release of toxins (Matthijs et al.,2012; Liu et al., 2017).
In comparison with the methods mentioned above, f locculation and settling techniques have been successfully used to control phosphorus and algal biomass in many freshwater bodies worldwide.This method consists of applying a low dose of coagulants and clays, naturally or chemically modif ied, to promote the removal of phosphorus and algal biomass from the water column through f locculation and sedimentation. Coagulants based on metal, polyaluminum chloride (PAC), ferric chloride(FeCl3), and the natural coagulant cationic starch with chitosan have been widely used in small-sized stagnant water bodies, with chlorophyll-a(Chl-a)concentration normally being lower than 300 μg/L (Li et al., 2011; Aktas et al., 2013; Ma et al., 2015; Wan et al., 2015; Jin et al., 2019). However, cyanobacteria could heavily concentrate along the downwind shore in the large eutrophic lake, resulting to much larger cyanobacterial biomass than that in small lake. Our pre-experimental results showed that f locculation and sedimentation was the only method that can mitigate cyanobacterial blooms with high algal biomass, and this is an essential prerequisite for dealing with the large accumulations of cyanobacteria in the coastal areas of eutrophic water bodies. However, the precipitation effi ciency of the surface cyanobacterial scums on the sediment depends on the type and concentration of coagulants and clays applied,as well as on the concentration of algal biomass.Furthermore, the addition of various coagulants and the accumulation of high cyanobacterial biomass in the sediments would change the physicochemical parameters of the water column and sediments. And this could have a potential inf luence on the water quality and the implementation of subsequent lake restoration strategies.
In this study, we determined the doses of coagulant and ballast agents required for diff erent cyanobacterial biomasses through preliminary experiments. The Chl-aconcentration of the maximum cyanobacterial biomass was up to 1 500 μg/L, representing the heavy accumulation of cyanobacteria on the leeward side of a large eutrophic lake. Then, the effi ciency of the agents in mitigating cyanobacteria and their impacts on the major physicochemical features of the water column and sediments were evaluated, which showed potential risks for water quality deterioration that could potentially inf luence the implementation of subsequent lake restoration strategies. Our results can help lake managers develop strategies for emergencies to rapidly remove large amounts of cyanobacterial biomass from the water column on the leeward side of eutrophic water bodies.
2 MATERIAL AND METHOD
2.1 Field sampling in Chaohu Lake
With a surface area of 760 km2(Huang et al., 2018;Wu et al., 2021), Chaohu Lake is the f ifth largest freshwater lake in China and is typically eutrophic,with a location downstream of the Changjiang(Yangtze) River. ColonialMicrocystiswas collected from a surface bloom in September 2020 in Chaohu Lake. Water was collected by a 5-L Plexiglass water collector, and the sediments were collected by a column sediment sampler (inner diameter of sampling tubeΦ=84 mm) from the bottom of Chaohu Lake. All samples were stored at a low temperature, protected from light, and brought back to the laboratory on the same day they were sampled.
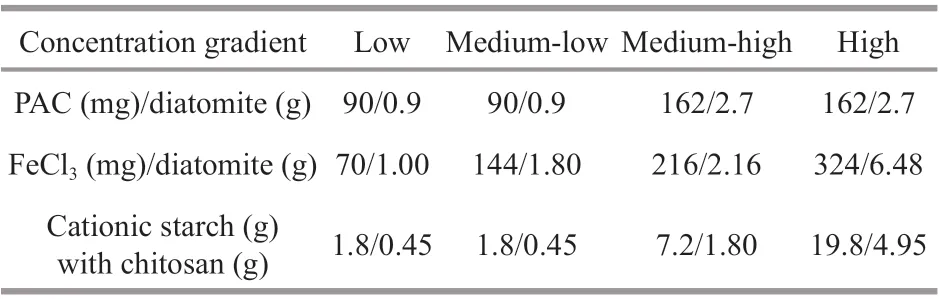
Table 1 The dosage of f locculants and Chl- a concentration
2.2 Laboratory experiment
Fig.1 Diagram of the experimental setup
2.2.1 Experiment setup
The sediments were pooled together and then homogenized with a dough mixer, and 200 mL of the sediment was transferred into plastic beakers(2 L). Lake water (f iltered with a 20-μm f ilter) was slowly poured over the sediment surface in each beaker, and then cyanobacterial scum was added to simulate cyanobacterial blooms of diff erent biomasses. The average concentrations of Chlain diff erent experimental groups from low to high were 413.30 μg/L, 637.10 μg/L, 1 001.63 μg/L, and 1 727.36 μg/L, respectively.
Flocculants, including polyaluminum chloride(PAC), ferric chloride (FeCl3), and cationic starch with chitosan, were slowly added to the beakers at triplicates per concentration, and the f locculant dosages were determined as the concentration at which the cyanobacteria f locculated and sank within a short period. Additionally, diatomite was used as a “ballast” to facilitate the settling of the f locculated complexes to the bottom for PAC and FeCl3. The f inal dosages for each treatment with the diff erent f locculants and for each group with diff erent cyanobacterial biomasses are shown in Table 1. The detailed laboratory experimental setup is shown in Fig.1.
2.2.2 Sample analysis
Water samples were taken to analyze Chl-aconcentration, dissolved oxygen (DO), pH, and specif ic nutrients concentrations, including total dissolved nitrogen (TDN) and total dissolved phosphorus (TDP). Sediment samples collected in September 2020, were freeze-dried to measure the contents of Chla, total nitrogen (TN), total phosphorus (TP), and total oxygen carbon (TOC).
Water samples were f iltered by GF/C membranes to collect Chla, which was determined by a f luorescence spectrophotometer (RF-5301PC, Shimadzu Corporation, Japan) after acetone extraction. DO and pH were measured using a multiparameter water quality analyzer (YSI 6600, Yellow Spring Instruments, USA). TDN and TDP were determined after f iltration through a 0.45-μm membrane f ilter(Millipore Corp) after persulfate oxidation. The TN and TP contents of the sediments were analyzed by peroxodisulfate oxidation and spectrophotometry.TOC was measured by a TOC meter (Shimadzu TOC 5000A) after f iltration through a GF/F (Waterman)membrane.
2.2.3 Statistical analysis
All the data in this paper were processed and graphed using Microsoft Excel and Origin 8.5 software, respectively. The diff erences in the various characteristics between the diff erent treatment groups were evaluated using IBM SPSS Statistics 20 software through one-way ANOVA.
3 RESULT
3.1 Physicochemical parameters of the water column
When f locculants and diatomite were added,the concentration of Chladropped rapidly. Of the treatments, the FeCl3treatments had the fastest rate of Chl-adecline, followed by the PAC treatment.In all the cyanobacterial biomass groups, the Chl-aconcentration dropped to almost 0 μg/L after 1 h for FeCl3treatment and after 1.5 h for PAC treatment.However, in the cationic starch with chitosan treatments, the concentration of Chlawas still as high as 159.54 and 68.89 μg/L in the high and mediumhigh groups, respectively in the end of the experiment(Fig.2).
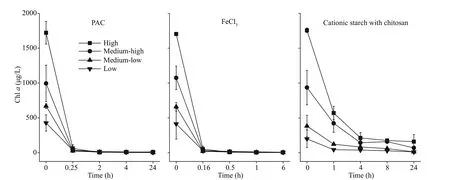
Fig.2 Chl- a content in the water column after the addition of f locculants
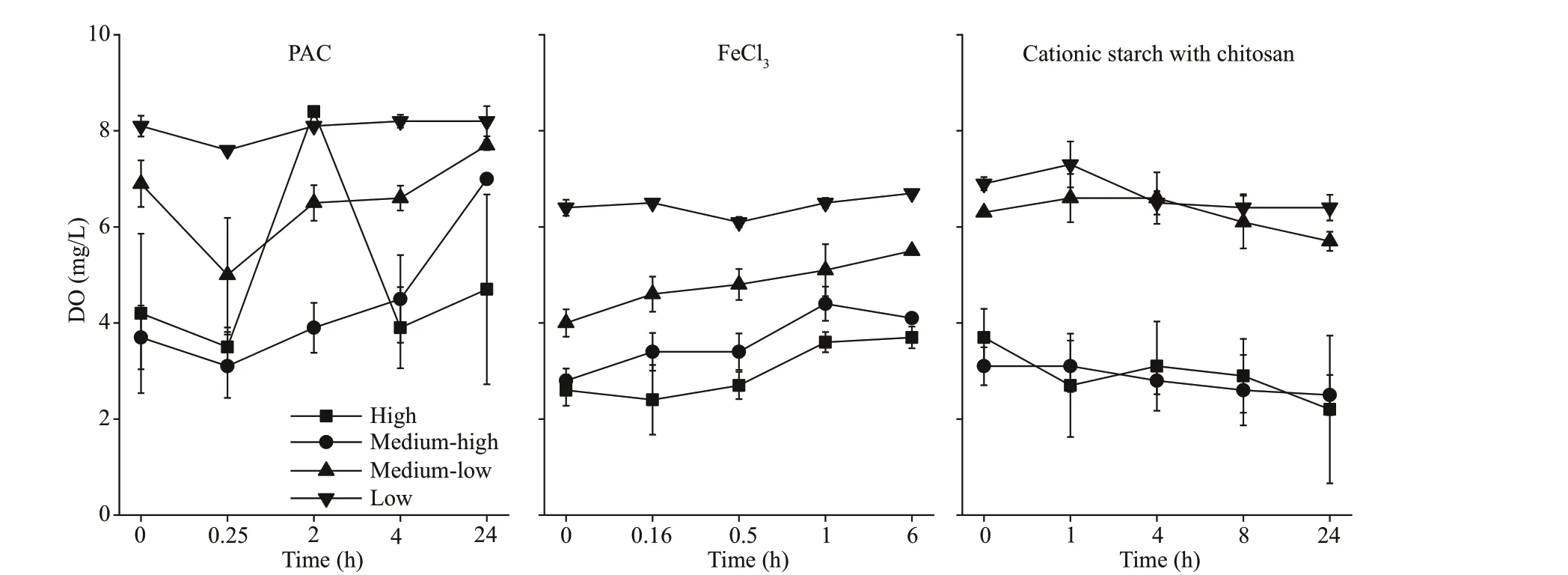
Fig.3 DO in the water column after the addition of f locculants
The DO content declined with a rise in cyanobacterial biomass, which was 7.0 and 4.7 mg/L in the PAC treatment groups and 4.1 and 3.7 mg/L in the FeCl3treatment groups after 24 h in the mediumhigh and high groups, respectively (P<0.05). In contrast, the DO contents in the cationic starch with chitosan treatment groups were 2.5 and 2.2 mg/L in the medium-high and high groups, respectively.The DO curve still showed a downward trend at the end of the experiment, which had a high probability of further developing into an anaerobic state, thus threatening the ecological balance of the aquatic environment (Fig.3).
During the experiment, the pH in the water column of cyanobacteria with diff erent biomasses was maintained between 6.9 and 7.8 after the addition of PAC and cationic starch with chitosan. However, the pH of the water column showed a sharp decrease after the addition of FeCl3(P<0.05), and the lowest pH values were 6.6, 6.4, 6.0, and 5.6 in the low, mediumlow, medium-high, and high groups, respectively(Fig.4).
3.2 Nutrients in the water column
Our experimental results show that treatment with PAC and FeCl3resulted in a marked decrease in TDN and TDP in the water. TDN decreased to approximately 0.20 mg/L and 0.28 mg/L at the end of the experiment under the PAC and FeCl3treatments, respectively,regardless of cyanobacterial concentration. After 24 h,TDN in the cationic starch with chitosan treatment decreased to 0.48 mg/L in the medium-low and lowconcentration algae groups but increased to 1.86 and 2.71 mg/L in the medium-high and high algae groups, respectively (P<0.05). Similarly, in the PAC and FeCl3treatment groups, TDP dropped rapidly to approximately 0.01 mg/L. In the cationic starch with chitosan treatment group, TDP dropped to 0.011 and 0.014 mg/L at low and medium-low concentrations of cyanobacteria, while TDP dropped to 0.04 and 0.03 mg/L at medium-high and high concentrations of cyanobacteria, respectively (P<0.05), after 24 h of treatment (Fig.5).
3.3 Chemical parameters of the sediment
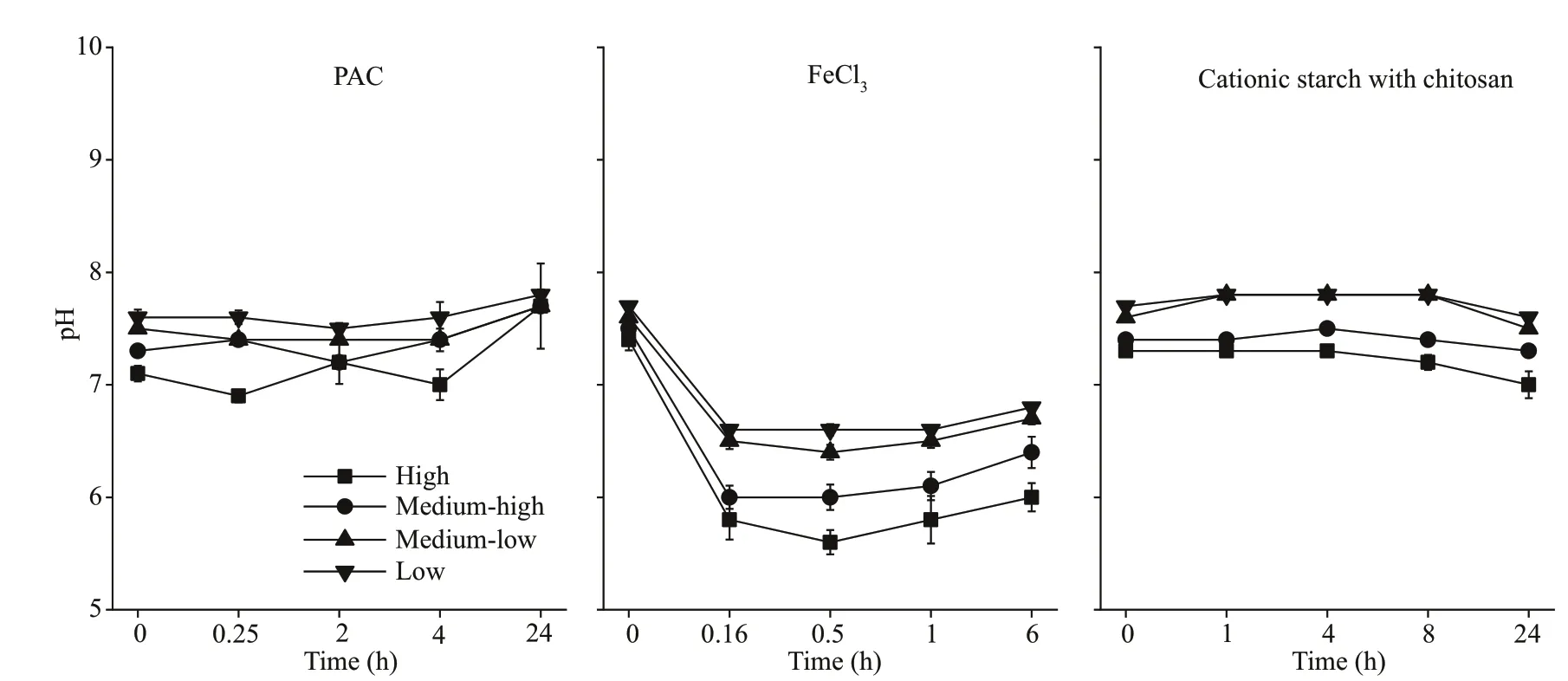
Fig.4 pH of the water column after the addition of f locculants
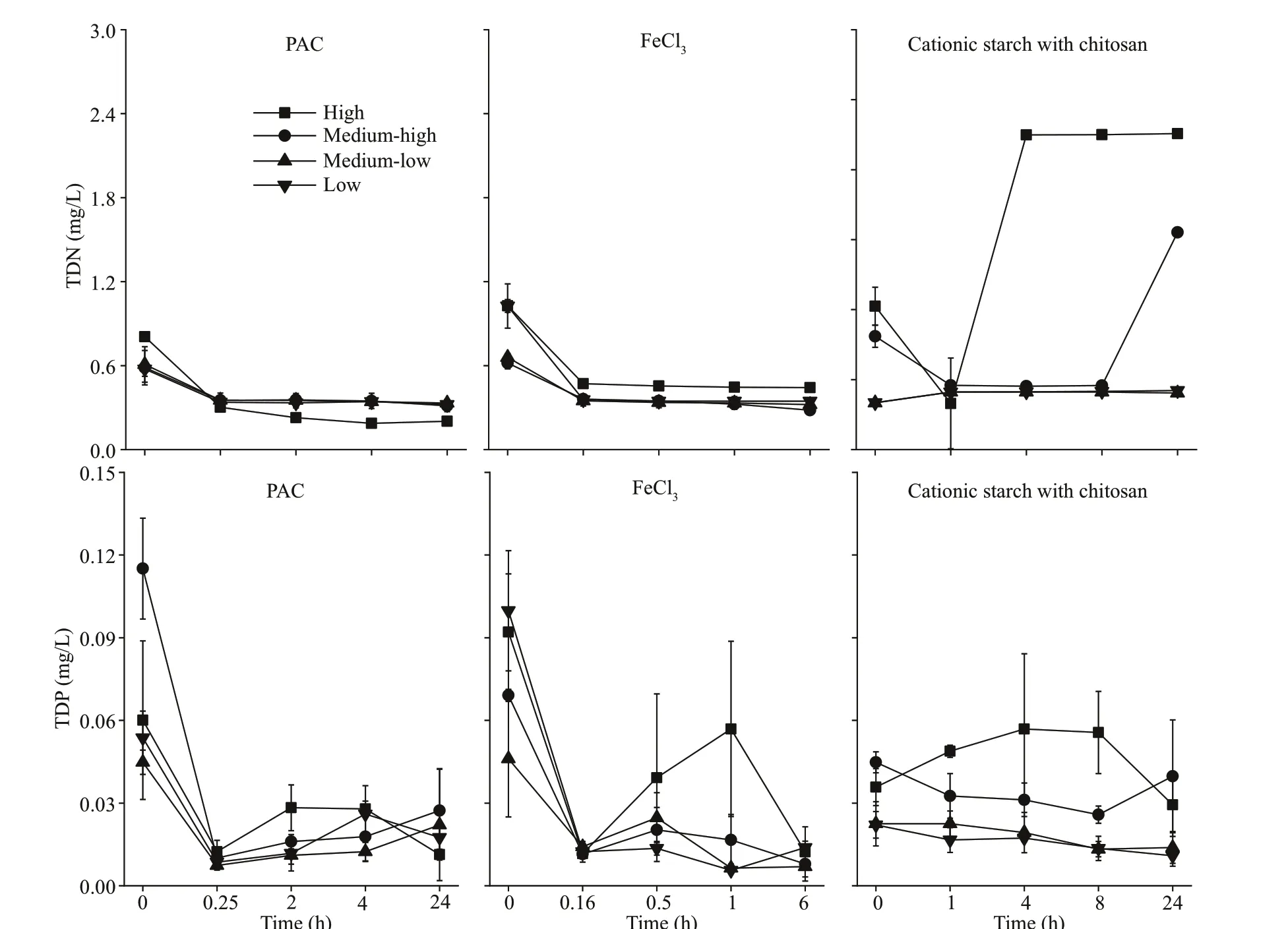
Fig.5 TDN and TDP in the water column after the addition of f locculants
The average Chl-acontent in the sediment was 1 406.64 ng/g in the control group, which increased with cyanobacterial concentrations. After PAC addition, the Chl-acontent of the sediment was 35 433.88, 59 133.19, 94 536.92, and 109 012.22 ng/g in the four cyanobacterial groups, respectively. The Chl-avalues in the FeCl3treatment were generally lower than those in the PAC treatment, which were 26 753.75, 59 260.05, 77 233.99, and 103 897.79 ng/g in the four groups, respectively. In comparison,the Chl-acontent in the sediments was lower for the cationic starch with chitosan treatments, with 14 408.64, 21 215.64, 4 755.62, and 63 232.74 ng/g in the four groups (P<0.05), respectively (Fig.6b).
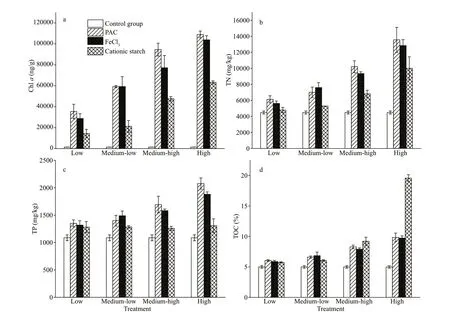
Fig.6 Variation in Chl a, TN, TP, and TOC in the sediments in the treatments with diff erent cyanobacterial biomasses
TN and TP in the sediments increased with a rise in cyanobacterial biomass after using the f locculants. Consistent with the Chl-apattern, TN and TP in the sediments were higher in the PAC treatments than in the FeCl3treatments, followed by the cationic starch with chitosan treatments.Specif ically, TN in the sediments was 6 133.84 mg/kg,10 200.45 mg/kg, and 13 553.11 mg/kg, and TP in sediments was 1 350.19 mg/kg, 1 693.06 mg/kg, and 2 075.98 mg/kg in the PAC groups in the low, mediumhigh, and high concentration groups, respectively(P<0.05) (Fig.6b & c).
The sedimentation of cyanobacteria after the addition of f locculants led to an increase in the TOC content of the sediments. The TOC content of the sediments changed slightly in the low and medium-low groups. In the medium-high and high groups, the TOC content of the sediments increased with increasing cyanobacterial biomass. Notably,in the high cyanobacterial biomass group, the TOC content in the sediment was highest for the cationic starch with chitosan treatment at 19.57%, and it was 9.84% and 9.73% in the PAC group and FeCl3group,respectively (Fig.6d).
4 DISCUSSION
4.1 Eff ect of diff erent f locculants on cyanobacterial biomass reduction
Due to the presence of negatively charged groups,such as hydroxyl groups, on the surface of microalga cells, they can be electrically neutralized with positively charged cationic electrolytes, thus reducing the repulsive eff ect between algal cells and enabling algal cells to aggregate and form f locs that can be easily separated from water (Knuckey et al., 2006).Chlais often used as an index to evaluate mitigation effi ciency since it not only determines the content of plankton but is also one of the main indicators to measure the degree of eutrophication of a water body(Tang et al., 2011).
PAC, FeCl3, and cationic starch with chitosan are reported to be effi cient f locculants that can eff ectively remove cyanobacteria from water treatment systems(Waajen et al., 2016). Our results indicate that FeCl3f locculates the fastest and has the best eff ect on cyanobacteria reduction, followed by PAC, which was not very diff erent from FeCl3in reducing cyanobacteria. Both of these f locculants can rapidly mitigate the high biomass of cyanobacteria, with the Chl-aconcentration becoming higher than 1 500 μg/L within 15 min. It is worth noting that although FeCl3has a favorable and fast f locculation eff ect, the dosage requirement is very strict. If the dosage of FeCl3is slightly in excess, the color of the water body will change because Fe3+appears yellowish or reddish brown after dissolving in water, thus having a negative impact on natural water bodies. In contrast, cationic starch with chitosan has the slowest f locculation rate,with f locculation being evident after approximately one hour. Meanwhile, when cyanobacterial biomass was high, with a Chl-aconcentration of 200 μg/L,cyanobacteria could not be fully f locculated, and some cyanobacterial biomass remained in the water column at the end of the experiment. Chitosan is regarded as an eco-friendly and nontoxic coagulant that can rapidly compromise cell membrane integrity and kill certain cyanobacteria (Li and Pan, 2015). This celldamaging eff ect can cause cyanobacterial cell lysis and reduce the possibility of f locculation and sinking.Diff erences in sensitivity to chitosan exist among diff erent cyanobacterial species, andMicrocystiswas the least sensitive among the nine cyanobacteria species tested (Barko et al., 1991).
4.2 Eff ect of diff erent f locculants on the physicochemical properties of water bodies
When cyanobacteria settled to the bottom of the water column, the physicochemical characteristics,such as DO and pH, changed with time in the four diff erent treatments. In particular, DO in the water column dropped to 2.2 mg/L in cationic starch with chitosan treatments in the high-concentration cyanobacterial group. In fact, at high biomass, algal respiration can lead to a sharp decline in the DO of water bodies, which may make the water column biologically anaerobic and lead to the development of poor sediment and water quality (Huo et al.,2015). A low DO concentration can cause f ish and shrimp death or even black odors from the water body in serious cases and aff ect the geochemical cycle (Yang et al., 2008). Therefore, when treating high concentrations of cyanobacterial blooms for emergency purposes, a combination of f locculants and oxygenators should be considered. The addition of FeCl3can generate a positively charged cationic electrolyte that can electrically neutralize negatively charged groups, such as hydroxyl groups, present on the surface of microalgal cells, resulting in a decrease in the pH of the water column (Li et al.,2015). For medium-high and high cyanobacterial biomass groups, the anaerobic decay of algae produces carboxylic or dicarboxylic acids, which cause the pH of the water column to fall between 5.5 and 6.5, lower than the normal pH level of the water column (Li et al., 2015).
4.3 Eff ect of diff erent f locculants on substrate
In our experiments for the emergency treatment of high biomass cyanobacteria, diatomaceous earth was used to settle the f locculated cyanobacterial complexes to the bottom of the water column (Li and Pan,2015). The bottom sediments, however, are usually a collection point for pollutants, such as nutrients and heavy metals, and pollutants can become a source of endogenous pollutants in natural lakes after longterm accumulation (Jin et al., 2005). In the process of algae control, the better the eff ect of the f locculant is, the greater the algal cells settle to the bottom sediments; thus, the algae control eff ect of PAC is superior in the experimental group of cyanobacterial water bodies with diff erent concentration gradients.Although temporarily eff ective in controlling high cyanobacterial blooms, if nutrients continue to accumulate in the sediments, the f locculent may eventually transform the contaminated sediment into endogenous nutrient load to the lake water body.Nutrients can be released into the overlying water column by absorption, diff usion, convection, and resuspension and become the “source” of lake water pollution (Zhong et al., 2008). The results from 18 European lakes have indicated that a TP content in surf icial sediment of 1 000 mg/kg may represent an approximate threshold above which it will perpetuate internal loading for f low diversion (Cooke et al.,1994). Our results indicate that the background TP content in the sediments was already approximately 1 000 mg/kg. This value increased after the cyanobacterial scum was f locculated and sedimented,reaching more than 2 000 mg/kg. Considering the longterm management of eutrophic lakes, endogenous pollution can be reduced by physical methods such as substrate dredging and underwater aeration (Caille et al., 2003; Liu et al., 2015).
Flocculation and sedimentation are eff ective in removing large cyanobacterial biomass from the water column in a relatively short time when an emergency situation occurs in certain lake areas.However, long-term prevention and control strategies are essential for lake managers. In fact, cyanobacterial biomass mitigation is only the f irst step; lake restoration should be subsequently implemented to achieve macrophyte-dominated clear water ecosystems, particularly in coastal lake regions. The physiochemical characteristics of sediment exert an important inf luence on macrophyte productivity and species composition. Lake sediment is a recognized site for the accumulation of organic carbon. Its TOC content can not only indicate the input of organic matter to the lake but can also ref lect the storage status of organic matter and the status of lake productivity characteristics (Meyers, 1994). In addition, TOC content is related to bulk density, nutrient content, and nutrient availability. Macrophyte nutrition in highly organic sediments may also be disrupted by the presence of phytotoxic compounds produced during anaerobic decomposition. Adding low levels of labile organic matter to the sediments resulting from the precipitation of algal detritus may provide nutritional benef its to submerged macrophytes, particularly if on coarse-textured sediments (Kiørboe, 1980). However,the accumulation of large amounts of refractory organic matter with potentially inhibitory properties in sediments can generally be expected to diminish sediment nutrient availability and the associated growth of rooted submerged macrophytes (Barko et al., 1991). In a previous study, the growth ofHydrillaverticillataandMyriophyllumspicatumdecreased almost linearly with increasing sediment organic matter up to a concentration of approximately 20%(Barko et al., 1986). The maximum TOC content in sediments can reach approximately 20% during cationic starch with chitosan treatment, which would be diffi cult to follow with lake restoration measures.
5 CONCLUSION
The f locculants PAC, FeCl3, and cationic starch with chitosan were tested for their eff ectiveness in controlling cyanobacteria blooms at four diff erent concentrations, and the subsequent environmental impacts on the water column and sediments were also evaluated. Our results indicate that PAC and FeCl3are effi cient f locculants that rapidly mitigated cyanobacterial blooms with Chl-aconcentrations higher than 1 500 μg/L within 15 min. In comparison,cationic starch with chitosan could treat only cyanobacterial blooms with Chl-aconcentrations less than 200 μg/L. However, treatment with FeCl3and cationic starch with chitosan could increase risks for water quality deterioration. For example,the addition of FeCl3caused a decline in the pH value. DO in the water column dropped to 2 mg/L with cationic starch of chitosan treatment in the high cyanobacterial biomass group. Additionally,the cell lysis of cyanobacteria caused by cationic starch with chitosan could result in an increase in DTP and DTN. Thus, PAC is the optimum f locculant for mitigating heavy cyanobacteria blooms under emergency in comparison with FeCl3and cationic starch with chitosan. However, the high TOC content in the sediments due to the settling of cyanobacterial biomass could threaten the success of lake restoration achieved by planting submerged macrophytes.Hence, cyanobacteria could still recover over time in eutrophic water bodies, requiring repeated use of PAC. This would bring other environmental and ecological problems, which requires further study.
6 DATA AVAILABILITY STATEMENT
The datasets generated and/or analyzed during the current study are available from the corresponding author on request.
 Journal of Oceanology and Limnology2022年5期
Journal of Oceanology and Limnology2022年5期
- Journal of Oceanology and Limnology的其它文章
- Proteomic analysis provides insights into the function of Polian vesicles in the sea cucumber Apostichopus japonicus post-evisceration*
- Key physiological traits and chemical properties of extracellular polymeric substances determining colony formation in a cyanobacterium*
- Involvement of the ammonium assimilation mediated by glutamate dehydrogenase in response to heat stress in the scleractinian coral Pocillopora damicornis*
- UV-B irradiation and allelopathy by Sargassum thunbergii aff ects the activities of antioxidant enzymes and their isoenzymes in Corallina pilulifera*
- Cyanobacterial extracellular alkaline phosphatase: detection and ecological function*
- Full-length transcripts facilitates Portunus trituberculatus genome structure annotation*
