Involvement of the ammonium assimilation mediated by glutamate dehydrogenase in response to heat stress in the scleractinian coral Pocillopora damicornis*
Jia TANG , Wenqi CAI , Zhicong YAN , Zhongjie WU , Qianxi YANG , Zhi ZHOU ,**
1 State Key Laboratory of Marine Resource Utilization in South China Sea, Key Laboratory of Tropical Hydrobiology and Biotechnology of Hainan Province, Hainan Aquaculture Breeding Engineering Research Center, Hainan University, Haikou 570228, China
2 Hainan Academy of Ocean and Fisheries Sciences, Haikou 570228, China
Abstract Glutamate dehydrogenase (GDH) plays an important role in the ammonium assimilation and nitrogen metabolism by catalyzing the reversible oxidative deamination of L-glutamate to α-ketoglutarate. In the present study, the potential functions of GDH in response to heat stress were explored in the scleractinian coral Pocillopora damicornis (designated as PdGDH). The cDNA of PdGDH contained an open reading frame of 1 611 bp encoding a polypeptide of 536 amino acids, which exhibited the highest sequence identity to GDH of Stylophora pistillata (96% identity), and the deduced PdGDH protein was predicted to contain one GdhA domain (from Val95 to Tyr525). The recombinant protein of PdGDH (rPdGDH) was expressed in Escherichia coli BL21 (DE3)-Transetta, and its catalytic activity was measured under diff erent temperatures, pH conditions and epigallocatechin-3-gallate (EGCG, a GDH inhibitor) concentrations. The purif ied rPdGDH only used reduced coenzyme nicotinamide adenine dinucleotide (NADH) as coenzyme,and its highest activity was observed at 35 ℃ and pH 7.5, respectively. The rPdGDH activity was negatively correlated with the concentration of EGCG, and was inhibited by more than half (65%, P< 0.05) at 10 -4 mol/L EGCG. No signif icant alteration of PdGDH mRNA expression was detected at 12 h after exposure to heat and ammonium ( P >0.05). Furthermore, the activities of NADH-GDH in the scleractinian coral P.damicornis increased signif icantly at 12 h after the heat and ammonium stress, and the NADH-GDH activity in the heat stress group (32.66 U/mg, P< 0.05) was signif icantly higher than that in the heat and ammonium stress group (11.26 U/mg). These results collectively suggested that PdGDH, as a homologue of glutamate dehydrogenase in the scleractinian coral P. damicornis, could respond to heat stress at the protein level,which would have ability to further promote ammonium assimilation to increase the heat acclimatization of the coral-Symbiodiniaceae symbiotic association.
K ey word: scleractinian coral; glutamate dehydrogenase; ammonium assimilation; global warming; heat response
1 INTRODUCTION
Scleractinian corals f lourish in the oligotrophic tropical and subtropical marine environment due to the subtle coral-Symbiodiniaceae symbiosis (Tong et al., 2017). In the symbiotic association, the coral hosts absorb and assimilate inorganic nutrients from seawater environment, and further transfer these nutrients to symbiotic Symbiodiniaceae. In exchange,the symbionts provide its photosynthetic products as organic nutrients to the coral hosts (Wang and Douglas, 1999; Rosic et al., 2014). However, the symbiosis has been threatened by the rise of sea surface temperature owing to global warming(Douglas, 2003; Claar et al., 2020), and the threatening process was aff ected by the assimilation and exchange of nitrogen nutrients in the symbiotic association(Rädecker et al., 2015; Morris et al., 2019).
The coral-Symbiodiniaceae symbiotic association assimilates ammonium as preferred nitrogen resource,and the resulting nitrogen cycling plays an important role in the maintenance of the symbiosis. It has been proposed that ammonium can be assimilated mostly by the coral hosts through glutamine synthetase and/or glutamate dehydrogenase (GS/GDH) pathway, and slightly by symbiotic Symbiodiniaceae through glutamine synthetase/glutamine:2-oxoglutarate aminotransferase (GS/GOGAT) pathway (Rädecker et al., 2015; Su et al., 2018). The symbiotic Symbiodiniaceae need more nitrogen resource than the coral hosts, and therefore the symbionts are under nitrogen limitation, which can regulate symbiont density to maintain their symbiosis (Muscatine et al.,1989; Yellowlees et al., 2008; Rädecker et al., 2015).It has been also reported that elevated nitrogen availability disrupts the nitrogen limitation of symbiotic Symbiodiniaceae, and ultimately causes coral bleaching (Wiedenmann et al., 2013;Wooldridge, 2013). Furthermore, high temperature has been considered to be able to disrupt the symbiosis via increasing the ammonium assimilation rates of coral hosts and regulating the nitrogen limitation of symbiotic Symbiodiniaceae (Rädecker et al., 2015).However, the molecular mechanism underlying the process is still not explored well in scleractinian corals.
GDH widely exists in most organisms and catalyzes the reversible oxidative deamination of L-glutamate to α-ketoglutarate and ammonia using NAD(P)H as coenzyme, which is the primary pathway of ammonia assimilation (Dudler et al., 1987; Hudson and Daniel,1993). GDH is classif ied into three types according to their coenzyme specif icity, including NADPHspecif ic, NADH-specif ic, and nonspecif ic enzymes,and diff erent types of GDH show distinct regulatory and kinetic properties (Catmull et al., 1987; Dudler et al., 1987). Several GDHs have been also identif ied in cnidaria, such as coralAcroporaformosaand sea anemoneAnthopleuraxanthogrammica, and play an important role in the nitrogen metabolism in cnidariandinoflagellate symbiosis (Male and Storey 1983;Catmull et al., 1987; Dudler and Miller 1988).Furthermore, our previous study showed that the catalytic activity of NADH-GDH was induced by acute heat stress in the scleractinian coralP.damicornis(Tang et al., 2020). The detailed activity characteristics and heat stress response mode of GDH in scleractinian corals is still unclear, in spite of its important role in the nitrogen metabolism in the symbiotic association.
Pocilloporadamicornisis a scleractinian coral belonging to the family Pocilloporidae, and native to the tropical and subtropical regions of the Indian and Pacif ic Oceans. It is a bleaching-sensitive coral and commonly used as a model species for studying experimental biology and physiology of scleractinian corals (Hill et al., 2014; Cunning et al., 2018). This study focuses on the following critical questions:(1) identify the homologue of GDH presented in the scleractinian coralP.damicornis(designated as PdGDH); (2) explore the eff ects of temperature, pH,green tea catechin, and epigallocatechin-3-gallate(EGCG) on PdGDH activity; (3) investigate the expression and activity changes of PdGDH in response to heat and ammonium stress. These results pave a new way to further understand the ammonium assimilation and nitrogen cycling mechanisms under stress response and environmental acclimatization of scleractinian corals.
2 MATERIAL AND METHOD
2.1 Coral
Pocilloporadamicorniscolonies were collected from a fringing reef in Wenchang (Hainan Province,China), and then cultured in 500-L f low-through tanks with natural seawater (temperature: 26 ℃; salinity:35). Cultured corals were illuminated with two white and two blue f luorescent bulbs (Philips T5HO Activiva Active 54 W) in a 12-h∶12-h light-dark cycling to acclimatize for one month in the laboratory condition.
2.2 Heat and ammonium stress experiment
Thirty-f ive coral nubbins were employed in the heat and ammonium stress experiments. Specially,f ive untreated coral nubbins were sampled at 0 h as the blank group, and the remaining thirty nubbins were randomly divided into three groups: control,heat, and heat_NH4(n=10 per group). The coral nubbins in the control and heat groups were placed in seawater at 26 °C and 32 °C, respectively, whereas the coral nubbins in the heat_NH4group were placed in ammonium seawater at 32 °C. The ammonium seawater was prepared by adding ammonium chloride(NH4Cl) to f iltered seawater at a f inal concentration of 10 μmol/L. Five nubbins were sampled from three groups (control, heat, and heat_NH4) at 12 and 24 h after treatment. The obtained samples were used for subsequent RNA extraction and NADH-GDH activity assay.
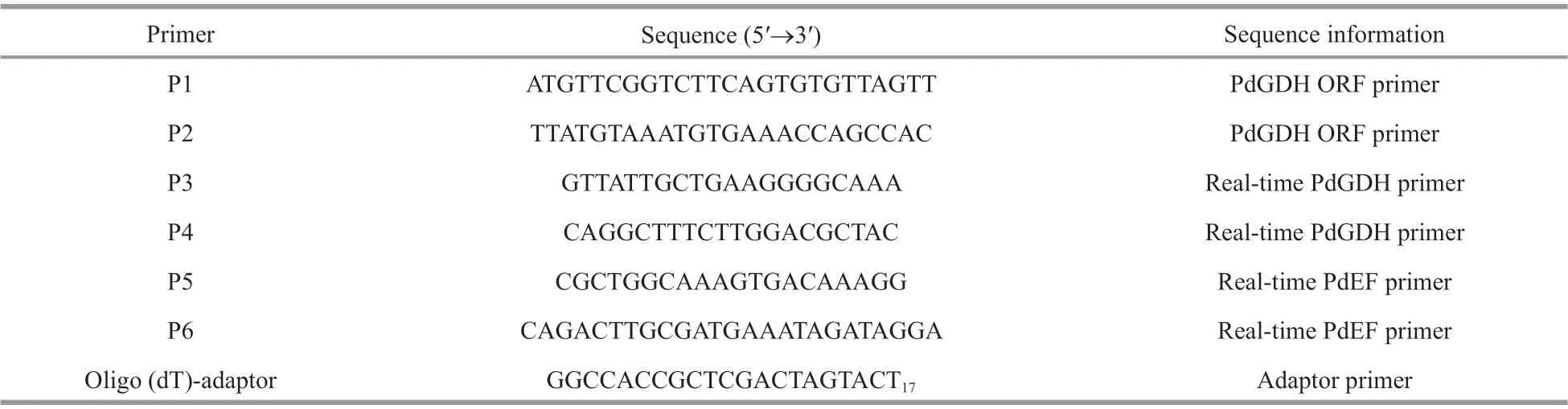
Table 1 Sequences of the primers used in the experiment
2.3 RNA extraction and cDNA synthesis
The sampled coral nubbins were grinded in liquid nitrogen, followed by mixing with 1-mL Trizol reagent (Invitrogen), and then the harvested homogenates were centrifuged at 5 000×g, 4 ℃ for 5 min to remove the calcium carbonate skeleton. Total RNA was extracted according to the protocol described by Stefanik (Stefanik et al., 2013), and reversed into cDNA using M-MLV kit (Promega,USA) with oligo (dT)-adaptor as primer (Table 1)(Jiang et al., 2013). The synthesized cDNA libraries were diluted to 1∶100 and stored at -80 ℃ for subsequent gene cloning and quantitative real-time PCR.
2.4 Cloning and sequence analysis of PdGDH
Transcriptome libraries of the scleractinian coralP.damicornisafter ammonium stress were previously sequenced and assembled (Yuan et al., 2017), and one transcript (No. TR73996_c2_g2_i1) was revealed through BLAST search to be homologous to GDHs identif ied previously in other animals. The transcript contained a completed open reading frame (ORF),which represented the whole-length coding region of PdGDH. Two specif ic primers (Table 1) were designed to clone the PdGDH ORF. After PCR amplif ication,the product fragment (1 611 bp) was purif ied using the MiniBEST Agarose Gel DNA Extraction Kit(TaKaRa, Japan) and cloned into pMD19-T simple vector (TaKaRa, Japan). After being transformed into the competent cells ofEscherichiacoliDH5α(Transgen, China), three positive clones were identif ied through PCR screening and sequenced on an ABI 3730 XL Automated Sequencer (Applied Biosystems) to verify the sequence.
The theoretical isoelectric point and molecular weight of the deduced PdGDH protein were calculated with ExPASy (https://web.expasy.org/compute_pi/). The possible signal peptide and domain of PdGDH protein were predicted by SignalP 4.1 Server (http://www.cbs.dtu.dk/services/SignalP/)and Conserved Domain Database at NCBI (http://www.ncbi.nlm.nih.gov/Structure/cdd/cdd.shtml/),respectively. Homologous molecules for PdGDH were searched using BLAST algorithms in NCBI GenBank(http://www.ncbi.nlm.gov/blast/). Multiple alignment of amino acid sequences of PdGDH and other GDHs was analyzed by ClustalX program, and secondary structure of PdGDH was predicted using Protein Data Bank (https://www.rcsb.org/), following by the visualization using ESPript (http://espript.ibcp.fr/ESPript/ESPript/) (Robert and Gouet, 2014).Phylogenetic analyses of PdGDH and other GDHs were performed using the neighbor-joining (NJ)method in the MEGA X software, and a bootstrap analysis was performed based on 1 000 bootstrap replications.
2.5 Quantitative real-time PCR and gene expression analysis
Quantitative real-time PCR was carried out to examine the relative expression level of PdGDH in coral nubbins at 12 h after heat and ammonium stress in accordance with a previous protocol (Zhou et al.,2012). A PdGDH fragment was amplif ied using two specif ic primers, and the fragment of elongation factor (PdEF) was used as endogenous control. All primers used in the present study were shown in Table 1. Three replicates were conducted for each coral nubbin sample. The 2-ΔΔCtmethod was used to calculate the relative mRNA expression levels of PdGDH in corals (Livak and Schmittgen, 2001).
2.6 Recombinant expression and purif ication of PdGDH
The sequence encoding PdGDH mature peptide was amplif ied using specif ic primers (Table 1) with the vector pMD19-T-PdGDH as templates, and then inserted intopEASY-E1 expression vector (TransGen,China). Next, thepEASY-E1-PdGDH construct was transformed intoE.coliBL21 (DE3)-Transetta(TransGen, China), and the positive transformants were cultured in 100 mL of lysogeny broth (LB)medium with 100-mg/mL ampicillin at 37 ℃ with shaking at 200 r/min. When the culture media reached OD600of 0.6, isopropyl β-D-thiogalactopyranoside(IPTG) was added at the f inal concentration of 1.0 mmol/L to induce the expression of PdGDH recombinant protein (rPdGDH) for 4 h. The bacteria were collected, sonicated, and centrifuged to remove the insoluble debris. The rPdGDH with His-tag was purif ied through a Ni2+chelating Sepharose column(Sangon Biotech, China), and the purif ied protein sample was analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The purif ied rPdGDH was refolded in gradient urea-TBS buff er (50-mmol/L Tris, 50-mmol/L NaCl, 1-mmol/L EDTA . 2Na, 1-mmol/L reduced glutathione, 2-mmol/L oxidized glutathione, 133-mmol/L glycine, a gradient urea concentration of 8-, 6-, 4-, 2-, 1-, and 0-mol/L urea in each gradient, pH 7.4, each gradient at 4 °C for 12 h). The concentration of purif ied rPdGDH was quantif ied using bicinchoninic acid (BCA) Protein Assay kit (Sangon Biotech, China).
2.7 Activity assay of rPdGDH at diff erent temperature, pH conditions, and EGCG concentrations
The activity of rPdGDH was measured at diff erent temperature and pH conditions according to the description of Yang et al. (2005). Brief ly, 10 μL of rPdGDH was added to 800 μL of reaction mixture(100-mmol/L Tris-HCl, 2-mmol/L α-ketoglutarate,0.2-mmol/L NAD(P)H, and 10-mmol/L NH4Cl), and then absorbance at 340 nm was measured at 0 min and 5 min. To evaluate the eff ects of temperature and pH on the rPdGDH activity, it was measured at temperatures ranging from 20 to 35 ℃ at 5-℃ intervals(pH 7.5), and pH ranging from 6.5 to 9.5 at 0.5 pH intervals (at 35 ℃). Temperature and pH were changed by water bath and adding hydrochloric acid respectively, and the pH value was detected by pH meter (METTLER TOLEDO, FiveEasy Plus, FE28-Micro). One unit of enzyme was def ined as that the amount which catalyzes the consumption of 1 nmol/L of NAD(P)H per minute, and the specif ic activity was standardized to per mg protein and expressed as U/mg.
The inhibitory eff ect of diff erent concentrations of EGCG on rPdGDH activity was also evaluated according to the above-mentioned methods. To avoid the inf luence of EGCG color on the experimental results, each concentration of EGCG required a control tube. Specially, EGCG was added into the reaction mixture (35 ℃, pH 7.5) at f inal concentrations of 0,10-4, 10-5, and 10-6mol/L, and then 10 μL of rPdGDH was added to the reaction mixture. Similar process was performed in the control tube, expect that rPdGDH samples were replaced with equal volumes of phosphate buff ered saline (PBS, 377-mmol/L NaCl, 2.7-mmol/L KCl, 8.09-mmol/L Na2HPO4, 1.47-mmol/L KH2PO4,pH 7.4). The relative activities of rPdGDH at diff erent concentrations of EGCG were determined as the ratio of OD340to that at 0-mol/L EGCG.
2.8 Activity assay of NADH-GDH after heat and ammonium treatment
NADH-GDH activity was measured in the coral nubbins at 0, 12, and 24 h after heat and ammonium stress. Coral tissue homogenates were prepared with the Waterpik water jet and pre-cooled PBS (4 °C), and the homogenates were centrifuged at 5 000×g, 4 °C for 15 min to obtain the supernatants for the determination of NADH-GDH activity. Coral NADHGDH activity was also determined according to the above-mentioned methods, except for replacing 10-μL rPdGDH with 200-μL coral supernatant.Moreover, the concentration of total protein in the supernatant was quantif ied using BCA method, and NADH-GDH activity was normalize to per mg protein and expressed as U/mg.
2.9 Statistical analysis
All data were expressed as mean±standard deviation (SD). Statistical diff erences were assessed through one-way analysis of variance (ANOVA)followed by multiple comparison (S-N-K). The above operations were all performed using software IBM SPSS 20.0 (IBM Inc.), and signif icant diff erences were considered atP<0.05.
3 RESULT
3.1 Molecular characteristics of PdGDH
The ORF of PdGDH with the length of 1 611 bp was cloned and identif ied, and it was the same with the sequence of XM_027194020 (GenBank ID)which was from the genome annotation ofP.damicornis(Cunning et al., 2018). PdGDH encoded a polypeptide of 536 amino acid residues,and its theoretical molecular weight and isoelectric point were 59.27 kDa and 7.57, respectively. No signal peptide was predicted by SignalP software. The deduced PdGDH protein contained a GdhA domain(from Val95 to Tyr525) and a NAD(P)H binding site(Gly290, Asn291, Val292, Glu312, Arg313, Arg364,Asn365, Gly385, Ala386, and Asn387).
The results of amino acid sequence alignment showed that PdGDH shared 96% identity with GDH fromStylophorapistillata(XP_022804879.1), 87%with that fromAcroporamillepora(XP_029200822.1),and 90% with that fromOrbicellafaveolata(XP_020616550.1). The alignment also revealed eight conserved cysteine residues (Cys46, 92, 126,152, 234, 297, 307, and 357). Furthermore, the predicted secondary structure of PdGDH protein was composed of 17 α-helix, 10 β-sheet, 7 3/10-helix,1 α-turns, and 6 β-turns (Fig.1).
3.2 Phylogenetic analysis
A phylogenetic tree was constructed based on the polypeptide sequences of PdGDH and GDHs from other animals. Two distinct clades were observed in the tree. The PdGDH was f irstly clustered with GDHs from cnidariaS.pistillata(XP_022804879.1),O.faveolata(XP_020616550.1),A.millepora(XP_029200822.1),Nematostellavectensis(XP_032237771.1),Exaiptasiadiaphana(KXJ18637.1),Actiniatenebrosa(XP_031562890.1),andDendronephthyagigantea(XP_028408260.1) to form the f irst clade. All the GDHs from vertebrates were clustered into the second clade, includingThalassophryneamazonica(XP_034040172.1),Scleropagesformosus(XP_029110146.1), andGadusmorhua(XP_030234570.1) (Fig.2).
3.3 The expression changes of PdGDH mRNA after heat and ammonium treatment
The quantitative real-time PCR was employed to determine the expression level of PdGDH mRNA in coral nubbins at 12 h after heat and ammonium stress.The expression level of PdGDH mRNA did not change signif icantly in both heat and heat_NH4groups, in comparison with that in the control group.However, the expression level of PdGDH mRNA in the heat_NH4group (1.21-fold,P<0.05) was signif icantly higher than that in the heat group (Fig.3).
3.4 Expression and purif ication of rPdGDH
To further reveal the possible function of PdGDH,its recombinant protein rPdGDH was expressed in theE.coliBL21 (DE3) which received an IPTG induction. A specif ic band with a molecular weight of~60 kDa was exhibited through Ni2+chelating Sepharose column purif ication and 12% SDS-PAGE analysis, which was consistent with the predicted molecular weight of rPdGDH (Fig.4). The concentration of purif ied rPdGDH was measured to be 0.3 mg/mL.
3.5 Eff ects of heat and pH on rPdGDH activity
The activities of rPdGDH were determined at diff erent temperatures (20, 25, 30, and 35 °C) and pH(6.5, 7.0, 7.5, 8.0, 8.5, 9.0, and 9.5). The rPdGDH only uses NADH as coenzyme, and its activity increased steadily with increasing temperature, with the highest level at 35 °C (785.98 U/mg). Signif icant diff erences were observed in all pairwise comparisons(P<0.05) (Fig.5). In addition, the highest activity was observed at pH 7.5 (785.98 U/mg), which was signif icantly higher than those in all other pH(P<0.05). The activities of rPdGDH at pH 6.5(124.44 U/mg), 7.0 (130.23 U/mg), 8.0 (121.54 U/mg),8.5 (101.29 U/mg), and 9.0 (75.24 U/mg) were also signif icantly higher than that at pH 9.5 (26.05 U/mg,P<0.05) (Fig.6).
3.6 Inhibitory eff ect of EGCG on rPdGDH activity
The inhibitory eff ect of EGCG on rPdGDH activities was investigated at the concentrations of 10-4, 10-5, and 10-6mol/L (35 ℃, pH 7.5). The relative activity of rPdGDH decreased as EGCG concentration increased, and began to decrease signif icantly at 10-5-mol/L EGCG (70.0%,P<0.05), in comparison with that without EGCG. The lowest activity of rPdGDH was detected at 10-4-mol/L EGCG (35.0%,P<0.05), and was less than 50% of that without EGCG(Fig.7).
3.7 Temporal activities of NADH-GDH after heat and ammonium stress
The NADH-GDH activity in theP.damicornisnubbins was determined at 0, 12, and 24 h after heat and ammonium stress. The NADH-GDH activities increased signif icantly only at 12 h in the heat and heat_NH4groups (32.66 U/mg and 11.26 U/mg,respectively,P<0.05), in comparison with that in the control group. Besides, the NADH-GDH activity in the heat group at 12 h was signif icantly higher than that in the heat_NH4group (2.90-fold,P<0.05)(Fig.8).
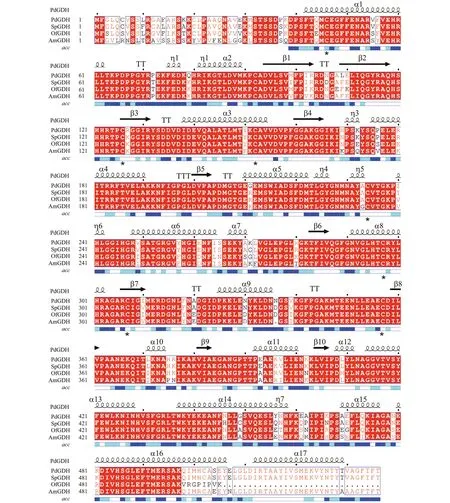
Fig.1 Multiple sequence alignment of PdGDH with other glutamate dehydrogenases from Stylophora pistillata (SpGDH,XP_022804879.1), Acropora millepora (AmGDH, XP_029200822.1), and Orbicella faveolata (OfGDH, XP_020616550.1)in the NCBI GenBank
4 DISCUSSION
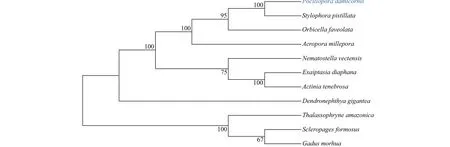
Fig.2 A phylogenetic neighbor-joining tree based on sequence alignment of glutamate dehydrogenases from diff erent animals
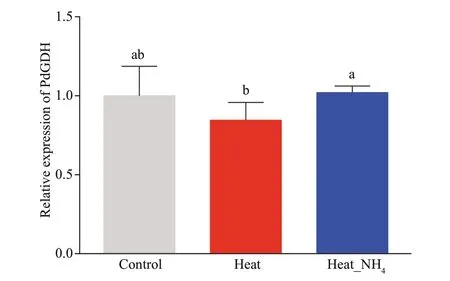
Fig.3 Temporal variability in the expression of NADHGDH in the coral nubbins at 12 h of heat and ammonium stress
The nitrogen cycling between coral hosts and symbiotic Symbiodiniaceae is vital to the stability of the symbiotic relationship between the two partners(Pernice et al., 2012). GDH is involved in the crucial nitrogen cycling through catalyzing the assimilation of ammonium, which is the main nitrogen resource for the symbiotic association (Yellowlees et al., 1994).In the present study, a GDH gene was identif ied from the scleractinian coralP.damicornis(PdGDH), and its ORF encoded a polypeptide of 536 amino acid residues. The amino acid sequence of PdGDH protein shared the highest identity (96%) with that of GDH from coralS.pistillata(XP_022804879.1). Structure analysis predicted that PdGDH had one GdhA domain(from Val95 to Tyr525), which contained 10 amino acid residues for binding NAD(P)H. Phylogenetic tree showed that PdGDH was f irst clustered with GDHs from other cnidarians, and then clustered with GDHs from vertebrates. Furthermore, the catalytic activity of rPdGDH was determined to rely on NADH not NADPH, and reduced gradually with EGCG concentration, with more than 50% inhibition at 10-4-mol/L EGCG. The present study demonstrated that PdGDH used only NADH as co-factor. Further research is needed on the types of GDH inP.damicornis, because the activity of GDH in coralAcropora latistellahas shown both NADH- and NADPH-specif ic (Dudler et al., 1987). EGCG also has the ability to specif ically inhibit rPdGDH activity,and this can provide an inhibitor choice for related research in the future (Li et al., 2007; Zhang et al.,2016). Together, these results suggest that PdGDH, as a homologue of glutamate dehydrogenase, is able to catalyze the reversible oxidative deamination of L-glutamate to α-ketoglutarate and mediate the ammonium assimilation in the scleractinian coralP.damicornis.
The rise of sea surface temperature caused by carbon dioxide emission is the main global threat to the coral-Symbiodiniaceae symbiosis, and can impair the coral growth and lower coral resilience (Anthony et al., 2011). Although ammonium assimilation plays important roles in the nitrogen cycling between coral hosts and symbiotic Symbiodiniaceae (Morris et al.,2019; Xiang et al., 2020), little is explored about the eff ect of environmental changes on this assimilation process. To reveal the potential eff ect in scleractinian coral, the catalytic activities of rPdGDH were detected f irstly under diff erent temperature and pH conditions.In the present study, the highest activity of rPdGDH was observed at 35 ℃ and pH 7.5. The rPdGDH activity increased with the elevation of temperature,and the similar rise of GDH activity in vitro at high temperature was also observed inPycnoporuscinnabarinus(Piumi et al., 2014). Furthermore, the rPdGDH activity reached highest level at pH 7.5.Similar result was reported that GDH activity was higher at pH 7.3 than other pH conditions in coralS.pistillata(Rahav et al., 1989), and the optimal pH for GDH activity was 7.0 in sea anemoneA.xanthogrammica(Male and Storey, 1983). Thesignif icant changes of PdGDH activity at diff erent temperature and pH conditions might due to the changes in protein conformation and substrate affi nity(Su et al., 2018). The higher activity of rPdGDH at high temperature and low pH would contribute to the coral host’s need for more inorganic nitrogen for the repair of heat and acidif ication damage and acclimatization of ocean warming and acidif ication.These results suggested that PdGDH could keep higher catalytic activities at high temperature and lower pH, which would play an important role in the physiological acclimation of the scleractinian coralP.damicornisto ocean warming and acidif ication.
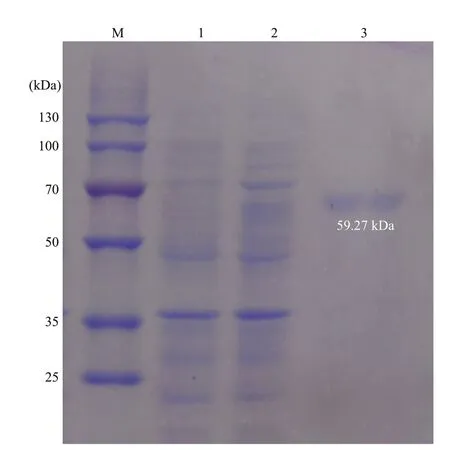
Fig.4 SDS-PAGE analysis of rPdGDH
To further understand the potential function of PdGDH in the coral acclimatization to environmental changes such as ocean warming, the changes of NADH-GDH activity and PdGDH mRNA expression were investigated in the scleractinian coralP.damicornisafter heat stress. In the present study,elevated temperature increased NADH-GDH activity signif icantly at 12 h, but no signif icant diff erence in the PdGDH mRNA expression level was observed after heat stress, in comparison with that in the control group. It demonstrated that heat stress induced the expression and activity of NADH-GDH encoded by PdGDH in the scleractinian coralP.damicornis. Our previous study showed that NADH-GDH activities increased signif icantly inP.damicornisafter heat stress (Tang et al., 2020). Combined with the observation that rPdGDH activity increased at high temperature, we speculated that coralP.damicornismight increase the NADH-GDH activity and ammonium assimilation to maintain the coral-Symbiodiniaceae symbiosis and resist heat stress. It could be also supported the report that more nitrogen availability from ammonium assimilation of coral hosts contributes to the maintenance of the coral-Symbiodiniaceae symbiosis under heat stress (Yuan et al., 2017). The result collectively suggested that heat stress could induce the NADH-GDH activity at the protein level, rather than PdGDH mRNA expression level, which might increase ammonium assimilation and improve the heat acclimation of the scleractinian coralP.damicornis.
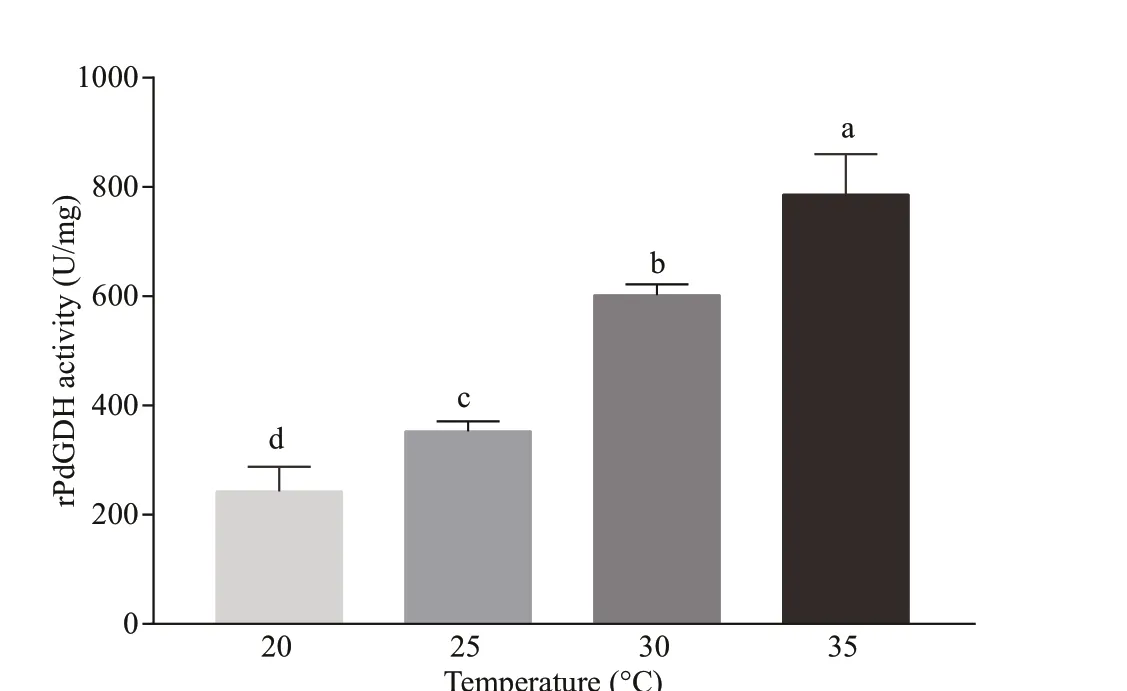
Fig.5 The relative activities of rPdGDH at temperature 20,25, 30, and 35 ℃ (pH 7.5)
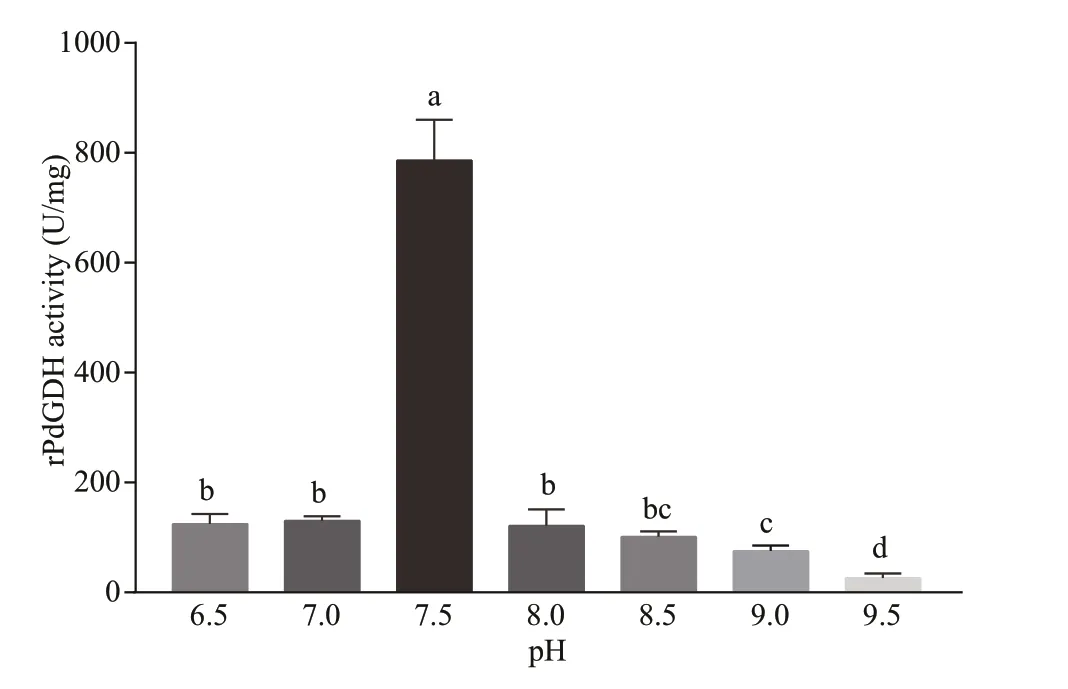
Fig.6 The relative activities of rPdGDH at pH 6.5, 7.0, 7.5,8.0, 8.5, 9.0, and 9.5 (35 ℃)
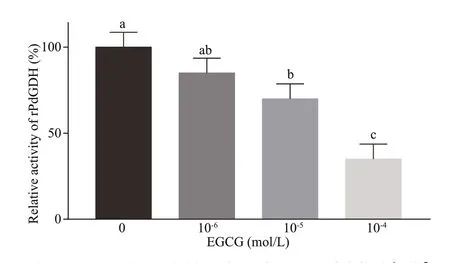
Fig.7 Ta h nd er 1 e0l- a6 tmi voe la/L c t(i3v5i t i℃e s,o p f H rP 7d.5G)DH at EGCG 10- 4, 10- 5,
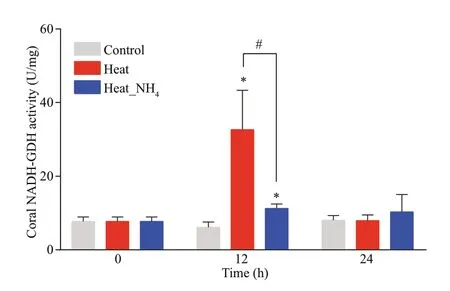
Fig.8 Temporal variability in the activities of NADH-GDH in the coral nubbins at 0, 12, and 24 h of heat and ammonium treatment
To further verify the regulation of heat acclimatization of coral by ammonium assimilation,the NADH-GDH activity and PdGDH mRNA expression were also detected in the scleractinian coralP.damicornisafter heat and ammonium treatment. The NADH-GDH activity in the heat_NH4group decreased signif icantly at 12 h, in comparison with that in the heat group, but was still signif icantly higher than that in the control group. In addition, the PdGDH mRNA expression level in the heat_NH4group was signif icantly higher than that in the heat group, but showed no signif icantly diff erence in comparison with that in the control group. This was also manifested in some observation that GS activity(another ammonium assimilation-related enzyme) in coral hosts could be triggered under elevated ammonium (Su et al., 2018). The elevation of ammonium concentration could meet the elevated substrate needs of NADH-GDH in coral hosts after heat stress, and more ammonium would be assimilated to be provided to symbiotic Symbiodiniaceae, which might explain the observation that the NADH-GDH activity in the heat_NH4group was signif icantly lower than that in the heat group. It also revealed that ammonium assimilation and nitrogen cycling could be optimized dynamically to maintain the symbiosis between coral hosts and symbiotic Symbiodiniaceae.To sum up, all results suggested that PdGDHmediated ammonium assimilation could be involved in the response of the scleractinian coralP.damicornisto heat stress, which would be able to balance the coral-Symbiodiniaceae symbiosis and improve the coral acclimatization to high temperature.
5 CONCLUSION
A GDH was identif ied in the scleractinian coralP.damicornis, which was able to catalyze the reversible oxidative deamination of L-glutamate to α-ketoglutarate and ammonia with NADH as coenzyme. Furthermore, PdGDH-mediated ammonium assimilation was involved in response to heat stress in scleractinian coralP.damicornis. More researches are needed to unravel the mysterious symbiosis between corals and Symbiodiniaceae in the future.
6 DATA AVAILABILITY STATEMENT
All data generated and/or analyzed during this study are available from the corresponding author upon request on reasonable request.
7 ACKNOWLEDGMENT
The authors are grateful to all of the laboratory members for their continuous technical advice and helpful discussions.
 Journal of Oceanology and Limnology2022年5期
Journal of Oceanology and Limnology2022年5期
- Journal of Oceanology and Limnology的其它文章
- Comparison of three f locculants for heavy cyanobacterial bloom mitigation and subsequent environmental impact*
- Proteomic analysis provides insights into the function of Polian vesicles in the sea cucumber Apostichopus japonicus post-evisceration*
- Key physiological traits and chemical properties of extracellular polymeric substances determining colony formation in a cyanobacterium*
- UV-B irradiation and allelopathy by Sargassum thunbergii aff ects the activities of antioxidant enzymes and their isoenzymes in Corallina pilulifera*
- Cyanobacterial extracellular alkaline phosphatase: detection and ecological function*
- Full-length transcripts facilitates Portunus trituberculatus genome structure annotation*
