UV-B irradiation and allelopathy by Sargassum thunbergii aff ects the activities of antioxidant enzymes and their isoenzymes in Corallina pilulifera*
Ming LIU , Jiqiang ZHAO , Yujuan PANG , Lipei ZHANG , Fuhua BIAN ,**, Lixia LI ,**
1 College of Life Sciences, Yantai University, Yantai 264005, China 2 Yantai Jien Biotechnology Co., Ltd., Yantai 264006, China
Keyword: Corallina pilulifera; antioxidant system; isoenzymes; UV-B radiation; allelopathy
1 INTRODUCTION
Enhanced UV-B (280-320 nm) irradiation resulting from ozone depletion is a signif icant global problem(Roleda, 2009; Karsten and Holzinger, 2014).Enhanced exposure to UV-B radiation is harmful to all living creatures and particularly to photosynthetic organisms for which light is indispensable (Bano et al., 2017; Luengo Escobar et al., 2017). Tidelands,which are the intertidal zones between the marine and terrestrial ecosystems, are regarded as one of the most sensitive areas of the biosphere with respect to environmental changes (Temmerman et al., 2013;Luan et al., 2020). Living in this special geographical area, intertidal macroalgae are inevitably exposed to stressful conditions. During low tide, marine macroalgae are exposed to more sunlight and more UV-B irradiation compared to when they are submerged at high tide (Van de Poll et al., 2001; Zhao and Li, 2014). This could lead to destructive eff ects on chloroplasts and DNA, which in turn would inf luence algal development and distribution(Holzinger et al., 2018).
During photosynthesis, photosystem II (PS II) uses the energy absorbed from light to split water into oxygen, which increases intracellular oxygen concentration and may increase the production of reactive oxygen species (ROS), especially when the organism is exposed to stressful conditions (Gill and Tuteja, 2010; de Wit et al., 2016). The antioxidant system, which includes superoxide dismutase (SOD;EC1.15.1.1), ascorbate peroxidase (APX;EC1.11.1.11), peroxidase (POX; EC1.11.1.7),catalase (CAT; EC1.11.1.6), and glutathione reductase(GR; EC1.6.4.2), is a signif icant component of the protective mechanism activated during plant stress.As a major scavenger, SOD converts the O2·radical into H2O2and O2using several isoenzymes, including cytosolic copper and zinc SOD (Cu/Zn SOD),chloroplast iron SOD (Fe-SOD), and mitochondrial manganese SOD (Mn-SOD) (Bowler et al., 1994).POX enzymes are heme proteins that catalyze H2O2-dependent oxidation and often exist in multiple molecular forms (isozymes) (Zapata et al., 1998).APX is a further eff ective enzyme that utilizes ascorbic acid (AsA) to eliminate toxic H2O2via oxidizing AsA to monodehydroascorbate (MDHA)(Mittler, 2002). APX isozymes are localized in four distinct cellular compartments, including microbody membrane-bound APX (mAPX), thylakoid membrane-bound APX (tAPX), stromal APX (sAPX),and cytosolic APX (cAPX) (Asada, 1992). The antioxidant enzyme GR, which utilizes nicotinamide adenine dinucleotide phosphate (NADPH), is primarily responsible for maintaining the high redox states of ascorbate and glutathione (Foyer et al., 1994)and contributes to the regeneration of antioxidant substrates (Smerilli et al., 2019).
The overall capacity of the antioxidant system determines its ability to remove ROS, and this has been positively correlated with enhanced adaptation or resistance to stress (Lee and Shiu, 2009;Rautenberger et al., 2013), including UV-B stress.Novel results recently published by Zhao et al. (2021)suggest a dual role for ROS in the macroalga,Ulvaprolifera. In addition to inducing the antioxidant system, they found that ROS activated secondary signaling pathways under UV-B radiation. The analysis of ROS generation and properties and the action of plant antioxidant systems is not only important for understanding the physiological metabolism of plants per se, but also has signif icance for improving the stress tolerance of transgenic plants through the bioengineering of antioxidant genes (Li et al., 2020).
In addition to the stress imposed by the abiotic environment, competition for resources due to niche overlap imposes additional stress on the macroalgae inhabiting the intertidal zone. Allelopathy, due to the allelochemicals released by macroalgae, is an important mechanism to restrain other competitors(Ohsawa et al., 2001; Mulderij et al., 2007) and is an eff ective strategy for macroalgae against other phototrophic organisms (Mulderij et al., 2005).Allelopathy and interspecif ic competition among microalgae have been well studied, including quantifying the importance of allelopathy in natural systems and understanding the corresponding chemical signaling mechanisms (Strom, 2008;Corcoran et al., 2019; Zhou et al., 2019). There are also studies focused on the inhibitory eff ects of macroalgae on microalgae (Ye et al., 2014; Dong et al., 2019). However, much less attention has been paid to interspecif ic competition between intertidal macroalgae. For example, Friedlander’s team reported thatUlvacf.lactucahad an inhibitory eff ect on the growth ofGracilariaspp. which they ascribed to allelopathy (Friedlander et al., 1996). However,intertidal macroalgae are likely to be exposed to the dual stressors of UV-B radiation and allelopathy simultaneously. Indeed, we showed in an earlier study that, in a co-culture system, the competitive ability ofGrateloupiaf ilicinawas weakened, and the interspecif ic competitive balance changed in favor ofUlvapertusaunder UV-B irradiation (Li et al., 2010).
In this study, two representative macroalgae,Corallinapilulifera(Rhodophyta) andSargassumthunbergii(Phaeophyta) were co-cultured at two diff erent ratios under increasing doses of UV-B irradiation.C.piluliferaandS.thunbergiilived in the same niche within an intertidal zone will inevitably lead to ecological competition with each other. In terms of the two stress factors under investigation(UV-B and allelopathy), we hypothesized that: (1)while one stress may occupy a more dominant position, interaction between the stresses will exist,and (2) the stressors will have a signif icant impact on antioxidant enzyme activities and, due to the sensitivity and specif icity of diff erent antioxidant enzymes under the dual eff ects of UV-B and allelopathy, these enzymes and their isoenzymes will react diff erently and play diff erent roles in the stress response system. As few studies to date have considered the roles of the antioxidant system in algal-algal interactions or have distinguished the eff ects on the individual isoenzymes, we measured the activities of key antioxidant enzymes and theisoenzyme patterns ofC.piluliferaunder the dual impact of UV-B radiation and allelopathy. This study sheds light on the intrinsic biochemical mechanisms and protection strategies of intertidal macroalgae in response to environmental stressors.
Table 1 Co-cultivation ratio and UV-B treatment of C.pilulifera and S. thunbergii
2 MATERIAL AND METHOD
2.1 Algal material and culture condition
TheC.piluliferaandS.thunbergiiplants used in this experiment were collected from the mid intertidal zone in Yueliang Bay (37°53′N, 121°42′E, Yantai,China) in June 2018. Fresh algal material was quickly brought to laboratory and rinsed thoroughly with sterilized seawater. The algae were cultured in 10 aquaria (inner diameter 50 cm) containing 4.0-L sterilized nutrient enriched seawater (Guillard and Ryther, 1962). During the experiment, changes in the nutrient concentration were tracked, and an appropriate amount of f/2 nutrient was added daily to prevent nutrient limitation, and the f inal concentrations of NaNO3-N and NaH2PO4-P were controlled at 100 and 7 μmol/L, respectively. The seawater was renewed every other day and aerated continuously by a pump.
2.2 Experiment design
UV-B irradiation was provided by UV f luorescent lamps (Philips TL 40 W/12 μV, The Netherlands) in wavelength range of 300-320 nm and maximum emission at 312 nm. The radiation intensity was measured by a UV-B spectroradiometer (Beijing Normal University, Beijing). To simulate the local average UV-B level and according to the results of preliminary experiments, four levels of dosage of UV-B were set: 0.5 W/m2(Low dosage of UV-B irradiation, Luv), 1.0 W/m2(Intermediate dosage of UV-B irradiation, Iuv), 2.5 W/m2(High dosage of UV-B irradiation, Huv), and 5.0 W/m2(Extremely high dosage of UV-B irradiation, Ehuv) (Table 1).Thalli were exposed to UV-B radiation for 8 h per day (09:00-17:00) and cultured in the dark for the rest of the time. The treatment without UV-B exposure was used as a control, which was kept at 23±0.5 °C and under an illumination intensity of 70-μ mol photons/(m2·s) photosynthetically active radiation (PAR) provided by three 30-W coolf luorescent lamps.
To study the allelopathic eff ect, the same weight(40 g: normal proportion - n) and 10 times the weight ofS.thunbergii(400 g: enhanced proportion - e) were added to the aquarium with 40-gC.piluliferato simulate a competitive environment, with f ive replicates for each treatment.
Consequently, during the one-week experiment,thalli were subjected to two levels of co-cultivation(1∶1 and 1∶10) and f ive levels of UV-B, which made up 10 combinations (Table 1). All processing arrangements of this experiment were designed according to a two-factor variance analysis.
2.3 Determination of antioxidant enzyme activity
Extracts for the determination of SOD and POX activities were prepared from 2.0-gC.piluliferatissues homogenized using mortar and pestle in 10-mL extraction buff er containing phosphate buff er(50 mmol/L, pH 7.0), ethylene diamine tetraacetic acid (EDTA) (1 mmol/L), and polyvinyl pyrrolidone(PVP) (1%). The homogenates were centrifuged at 12 000×gfor 15 min and the supernatant was used for the enzyme activity assays. For the determination of APX, 0.2-g thallus was homogenized using mortar and pestle in extraction buff er containing phosphate buff er (50 mmol/L, pH 7.0), PVP (1%), EDTA(1 mmol/L), and ASA (2 mmol/L), and centrifuged at 12 000×gfor 10 min. For the GR extract, 0.2-g tissue was homogenized in buff er including 5-mmol/L MgCl2, while the other components were the same as for the APX extraction buff er. All operations were carried out at 4 °C.
Total SOD activity was measured at 560 nm using the method of Giannopolitis and Ries (1977), based on the capacity of SOD to inhibit the photochemical reduction of nitroblue tetrazolium (NBT). POX activity was determined according to Adelaide Dias and Manuela Costa (1983) by monitoring the increased rate in absorption at 470 nm due to the formation of tetraguaiacol. APX activity was determined by estimating the decrease in absorbance of the oxidized ascorbate at 290 nm according to Nakano and Asada (1981). GR activity was determined by following the oxidation of NADPH at 340 nm as described by Rao et al. (1995).
2.4 Polyacrylamide gel electropheresis (PAGE)analysis and activity staining
Enzyme extracts of the thallus were analyzed by discontinuous PAGE under non-denaturing conditions according to the method of Laemmli (1970) with some improvements. GR isoforms were resolved on non-denaturing 7.5% polyacrylamide gel at 4 °C,with a constant current of 100 V at 4 °C.Electrophoresis on 9% polyacrylamide gels was used for SOD, POX, and APX. Separation of APX isoenzymes was analyzed with native PAGE according to the steps outlined above but in addition, 2-mmol/L AsA was added to the electrophoresis buff er and the gels were pre-run for 30 min to maintain the activity of APX isoenzymes.
SOD isoenzymes were visualized using the activity staining procedure described by Beauchamp and Fridovich (1971) with some modif ications. The gel was initially incubated in 0.25-mmol/L NBT solution for 20 min. Following the addition of 0.05-mmol/L ribof lavin and 8-mmol/L EDTA, rearrangement continued for 20 min in the dark. Finally, the gel was placed under white light until white bands appeared in the violet background. Three types of SOD isoforms(Fe-, Mn-, and Cu/Zn-SOD) were further identif ied by selective inhibition with potassium cyanide (KCN)and hydrogen peroxide (H2O2).
The electrophoretic pattern of POX isoenzymes was visualized by staining the gels with benzidine.The gels were incubated in 200-mmol/L acetate buff er(pH5.0) containing 3% H2O2and 4% benzidine until brown bands appeared (Van Loon, 1971).
For APX isoenzyme identif ication, assays were performed following the method of Mittler and Zilinskas (1993). The gels were equilibrated in 50-mmol/L sodium phosphate buff er (pH 7.0) and 2-mmol/L AsA for a total of 30 min and were then immersed in a solution of 50-mmol/L sodium phosphate buff er (pH 7.8) including 28-mmol/L N, N,N, N-tetramethyl ethylenediamine (TEMED) and 2.45-mmol/L NBT for ten more minutes, until APX bands were visible against a purple-blue background.
Staining of GR isoenzymes was achieved as follows: the gels were incubated in 50 mL of Tris-HC1 (pH 7.5) containing 10 mg of 2,6-dichlorophenolindophenol, 10 mg of 3-(4,5-dimethylthiazol-2-4)-2, 5-diphenyl tetrazolium bromide, 0.5-mmol/L reduced form of nicotinamideadenine dinucleotide phosphate (NADPH), and 3.4-mmol/L oxidized glutathione (GSSG) for 15 min,until purple bands on blue background appeared at room temperature (Rao et al., 1995).
2.5 Protein determination
The protein concentration was determined according to Bradford (1976), with absorbance readings at 595 nm. Bovine serum albumin was used for the standard curve.
2.6 Thiobarbituric acid reacting substance(TBARS) determination
Frozen thallus segments (0.5 g) were homogenized with 5 mL of 1% trichloroacetic acid (TCA) using mortar and pestle and centrifuged for 10 min at 12 000×g. The content of TBARS was calculated based on the absorbance at 535 nm (Heath and Packer,1968).
2.7 Data statistics
Statistical analysis was performed using two-way and one-way ANOVA tests, and means were compared by Duncan tests at the 0.05 and 0.01 level of conf idence, respectively. The normality of the data and the homogeneity of variance met the requirements of the ANOVA. Figures were drawn using SigmaPlot 12.0, and the error bars in the graphs represent the standard deviation based on f ive replicates.
3 RESULT
3.1 SOD activities and isoenzymes
As shown in Fig.1a, when the co-culture ratio ofC.piluliferaandS.thunbergiiwas 1∶1, nLuv and nIuv treatments were eff ective in increasing the activity of SOD, which increased 16.2% and 19.1% respectively compared with the corresponding control (P<0.05).However, at these UV levels, when the total ratio was 1∶10, the activity of SOD did not increase relative to control. Moreover, UV-B exposure impaired SOD activity signif icantly at the extremely high dose(P<0.01).
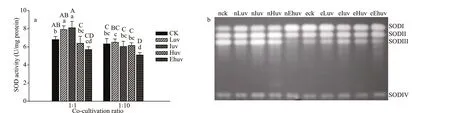
Fig.1 Activities of SOD (a) and their isoenzymes (b) in C. pilulifera under diff erent doses of UV-B radiation and co-culturing ratios
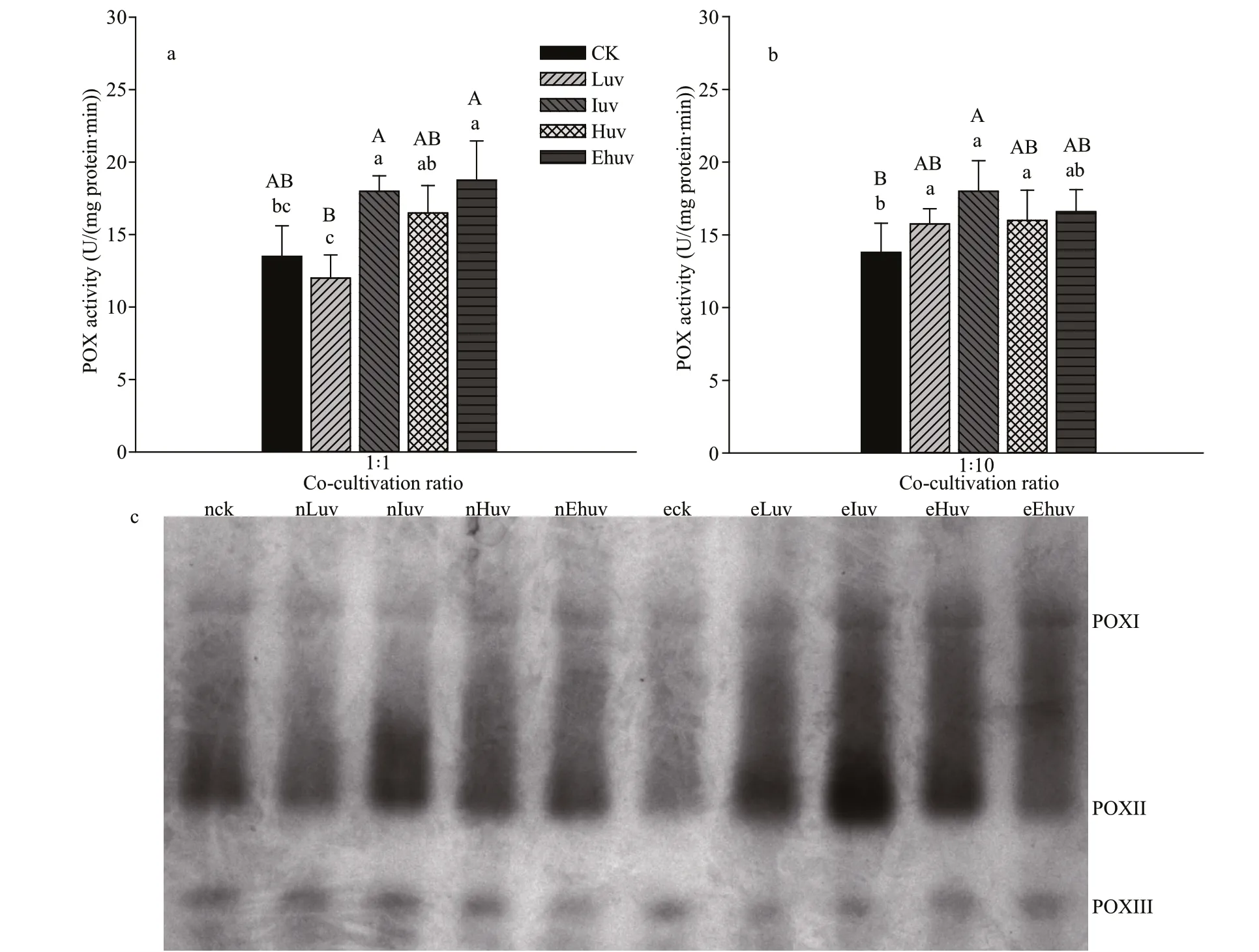
Fig.2 Activities of POX under the co-culture ratio 1∶1 (a), the co-culture ratio 1∶10 (b), and their isoenzymes (c) in C . pilulifera under diff erent doses of UV-B radiation and co-culturing ratios
In Fig.1b, SOD was presented in four unstained bands, and the application of inhibitors to the gels showed three Mn-SOD isoforms (band I-III) and one Cu/Zn-SOD isoform (band IV). The gels indicate reduced SODII and SODIII activities in the 1∶10 cocultivation treatment compared with 1∶1. However,the most obvious changes were the reduction of SODIII in both Ehuv treatments.
3.2 Activities of POX and their isoenzymes
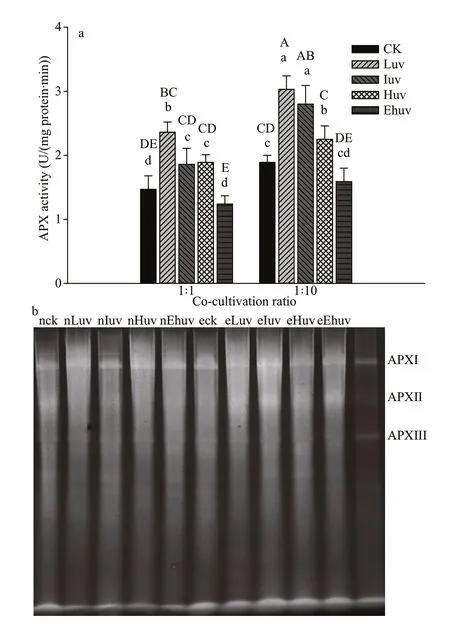
Fig.3 Activities of APX (a) and their isoenzymes (b) in C. pilulifera under diff erent doses of UV-B radiation and co-culturing ratios
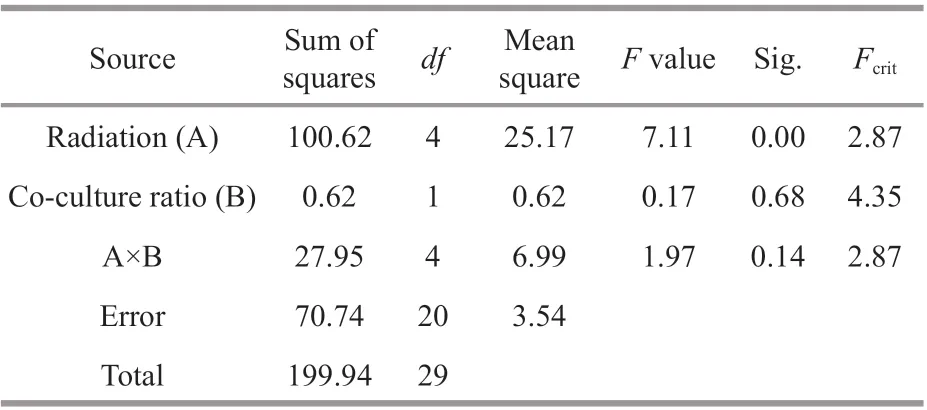
Table 2 Variance analysis of POX activities
As shown in Fig.2a & b, the activities of POX diff ered depending on the UV-B irradiation, but not co-culture proportions. Two-way ANOVA test showed that both the interaction eff ect (F=1.97<Fcrit=2.87)and the allelopathic eff ect (F=0.17<Fcrit=4.35) did not aff ect POX activity, while only UV-B irradiation had a signif icant inf luence on POX activities (Table 2).Consequently, the signif icant diff erence of UV-B treatment on POX activity was analyzed under the coculture ratios of 1∶1 and 1∶10, respectively. The diff erence among Ehuv, Iuv, and corresponding control was signif icant when the co-culture ratio was 1∶1 (P<0.05), while the diff erence between Iuv and control was highly signif icant (P<0.01) when the coculture ratio increased to 1∶10 but that in Ehuv was no longer signif icant, indicating reduced POX due to increased co-culture. Compared with SOD, co-culture did not negatively aff ect POX at lower UV.
The POX isozymes showed as three bands (Fig.2c),of which POXII had the maximum activity, and the POXIII isoenzyme had the weakest activity. When the co-culture ratio was 1∶1, the activity of POXII was obviously enhanced by the intermediate (nIuv) UV-B treatment, while in the 1∶10 co-culture, the POXII isoenzyme showed an even stronger band at this UV level.
3.3 APX activities and isoenzymes
The activity of APX increased signif icantly under the Luv and Iuv treatments (P<0.01) (Fig.3a). Under these UV levels, when the co-culture ratio was 1∶10,APX showed higher overall activity than when the co-culture ratio was 1∶1 (P<0.01). However, APX activities decreased under higher UV-B doses,especially under Ehuv, which was 15.6% and 15.8%lower than the corresponding control under the coculture ratio 1∶1 and 1∶10, respectively.
According to the electrophoresis gel for APX(Fig.3b), APX isoenzymes resolved into three electrophoresis bands. Compared with the control group, there was no distinct change in the activity of APX isoenzymes when the co-cultivation ratio was 1∶1. However, when the co-cultivation ratio was 1∶10 the APXII isoenzyme activity was enhanced signif icantly, but the activity of APXI was only enhanced in low and intermediate UV treatments.
3.4 Activities of GR enzymes and their isoenzymes
GR activity was positively inf luenced by the compounding eff ect of UV-B radiation and allelopathy(Fig.4a). Compared with controls, the total activity of GR increased signif icantly in the Huv treatments(P<0.01). When the co-culture ratio increased to 1∶10,the total activity of GR remained elevated, so that GR in both the Huv and Ehuv treatments was highly signif icantly elevated relative to the controls (P<0.01)and to the 1∶1 co-culture treatment.
The isozymes of GR resolved into four bands.When the 1∶1 culture was compared with the control,GRIII and GRIV isozymes showed an increase in activity in response to the nIuv and nHuv treatments,but there was a def inite decrease in activity across all four isoenzymes under the Ehuv treatment. When the co-culture ratio was 1∶10, the activities of GRIII and GRIV were elevated under all UV treatments whereas the activities of GRI and GRII were reduced under eLuv and eIuv treatments but were elevated under eHuv and eEhuv (Fig.4b).
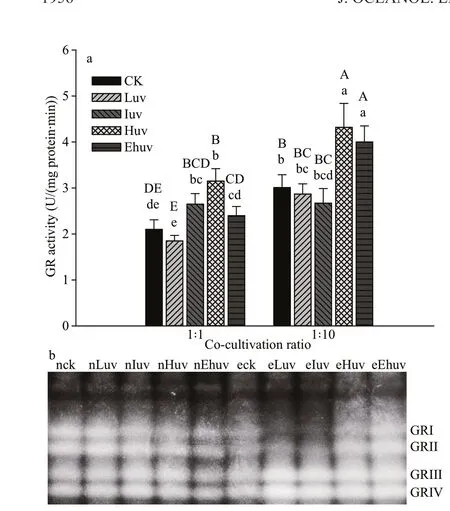
Fig.4 Activities of GR (a) and their isoenzymes (b) in C. pilulifera under diff erent doses of UV-B radiation and co-culturing ratios
3.5 TBARS concentration
TBARS concentrations changed in response to UV-B irradiation and co-culturing in a dose-dependent manner (Fig.5) and were highly signif icantly increased in comparison to the relevant control (P<0.01). When the co-culture ratio was 1∶10, the TBARS concentrations were signif icantly greater when compared with those from the 1∶1 culture at similar UV-B treatments.
4 DISCUSSION
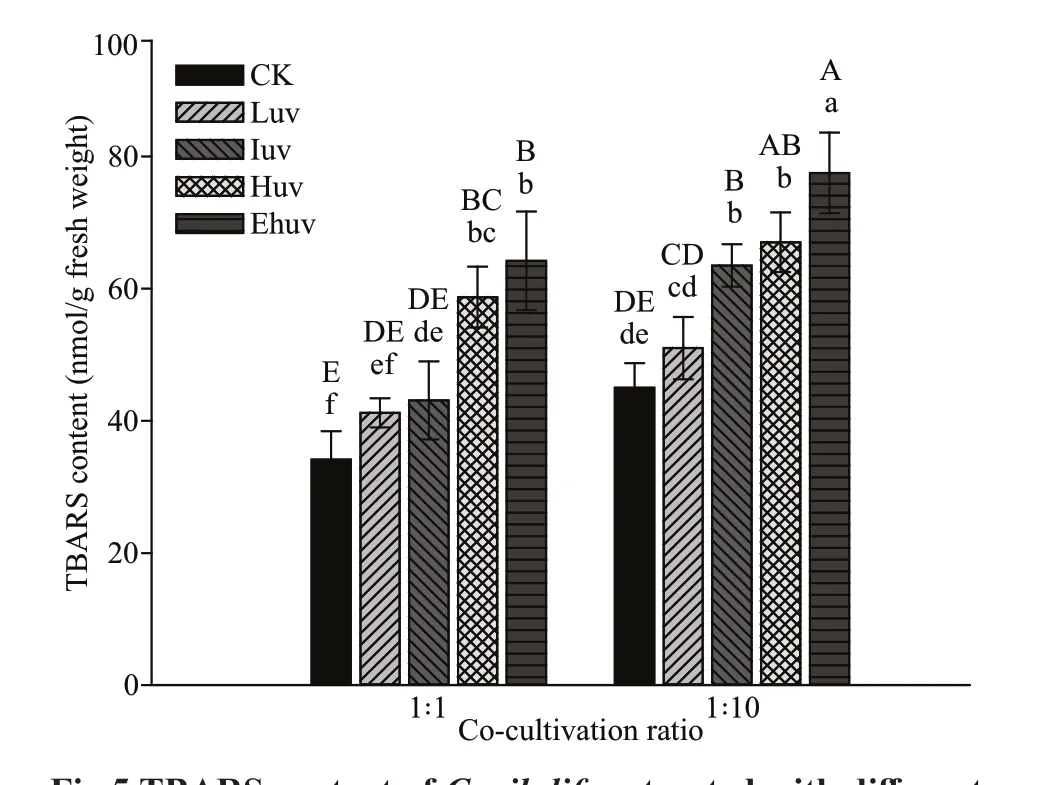
Fig.5 TBARS content of C. pilulifera treated with diff erent doses of UV-B radiation and co-culturing ratios
Sensitivity to UV-B radiation depends on plant species, cultivar, experimental conditions, and developmental stages (Caldwell et al., 1998). The species-specif ic susceptibility of the early life stages of macroalgae to UV plays an important role in the determination of zonation patterns and probably also in the shaping of community structure (Roleda et al.,2007). Many studies have focused on elucidating the consequences of increased UV-B irradiation on marine macroalgae and coastal ecosystems (Franklin and Forster, 1997; Holzinger et al., 2018). Repair and acclimation responses are naturally induced in marine algae under oxidative stress and increasing scavenging of oxygen free radicals is an important adaptive response (Shiu and Lee, 2005; Srivastava et al., 2012),indicating that the antioxidant defense mechanism against ROS plays a pivotal role in the survival of algae under stress (Schweikert and Burritt, 2012). In our study,C.piluliferainitially exhibited increased SOD activity under the lower UV-B doses, but a deleterious eff ect of UV-B and an allelopathic eff ect on the activity of SOD was observed, especially under higher UV-B doses and the higher co-culture ratio(Fig.1a). This result corresponds with the results of Wang et al. (2016), who found among the various SOD isoforms, Mn-SOD responded sensitively to the increasing generation and ROS accumulation in senescent leaves but is inconsistent with the earlier f indings of Rao et al. (1996), who showed that UV-B treatment of the model plantArabidopsisthalianapreferentially induced the Cu/Zn-SOD but failed to aff ect Mn-SOD. These diff erences are likely due to the species studied and, potentially, also the diff erent experimental conditions. Additionally, in our study,two types of SOD isoenzymes (Cu/Zn SOD, Mn-SOD) were identif ied (Fig.1b). Among these isoforms,the content of Cu/Zn-SOD (SOD IV) inC.piluliferawas at trace levels (Fig.1b). However, Bowler et al.(1994) reported that Cu/Zn-SOD is the most abundant isoform in land plants. Therefore, it seems that there is a distinction between the SOD synthesis mechanisms of higher plants and that in our red macroalga. Furthermore, these results indicate that red algae can regulate the expression of diff erent SOD subtypes in response to environmental stress at the transcriptional and post-transcriptional levels.
The activities of APX and GR under the dual eff ects of UV-B and allelopathy were signif icantly greater than those of the relevant controls. The interaction of integrated eff ects was also highly signif icant (Figs.3a & 4a). However, POX reacted quite diff erently to the other antioxidant enzymes, and only UV-B had a signif icant eff ect on its activity,while the allelopathic eff ect and interaction were not signif icant (Fig.2a & b). We speculate that diff erent properties and sensitivities of the enzymes led to the diff erent responses to stress. GR is by far the most studied and widely distributed enzyme in the ascorbic acid-glutathione cycle pathway (AsA-GSH), and can be found in both eukaryotic and prokaryotic organisms. GR is regarded as one of the most important antioxidant enzymes in plants (Noctor et al., 2012; Gill et al., 2013). Studies have shown that the activity of GR increases as the production of reactive oxygen species increases (Aguilera et al.,2002; Ding et al., 2009). Our results are consistent with previous f indings. Compared with the control,the total activity of GR increased, especially under the combination of high doses of UV-B and allelopathy(Fig.4a). As GR contributes to the regeneration of antioxidant substrates (Smerilli et al., 2019), this is perhaps not surprising. However, total activity and isoenzyme activity both decreased in the eLuv and eIuv treatments, indicating that there was a negative interaction between the stress level and enzyme activities initially (Fig.4a). The isozyme activities of GRI and GRII also decreased (Fig.4b), which was consistent with the total activity of GR. There are only two kinds of GR isozymes inArabidopsisthaliana, one located in the chloroplast and the other in the cytoplasm (Tahmasebi et al., 2012). We identif ied four bands of GR, but where the isoenzymes are located needs further exploration.
APX and POX isoenzymes exist in eukaryotic algae as well as higher plants, and their enzymatic and immunological properties are similar to those of higher plants (Shigeoka et al., 2002; Wang et al.,2007). They effi ciently catalyze the decomposition of H2O2and are used as biochemical indicators to eff ectively evaluate whether a species or an individual can resist external stress (Shigeoka et al., 2002; Shiu and Lee, 2005). In the present study, the activities of some isozyme bands of APX and POX performed similarly. Under low and medium UV-B exposure(i.e., the eLuv and eIuv treatments), APX and POX isozymes were obviously activated (Figs.2b & 3b),but the activity of two GR isozymes was signif icantly decreased (Fig.4b). There is reason to speculate there is co-regulation among the components of the ROS scavenging system, and changes in the balance of these enzymes will provide a compensating mechanism. These antioxidant enzymes cooperate with each other, and if one of them is altered, the whole antioxidant system is impacted, and this eff ect was related to the species of algae, the specif icity and sensitivity of antioxidant enzymes, and the amount of gene expression (Rautenberger et al., 2013; Li et al.,2017; Zhao et al., 2021). Consequently, it is important to analyze multiple antioxidant enzymes to gain a comprehensive picture of the antioxidant capacity of an organism (Shigeoka et al., 2002). In addition, it could be deduced that weakened bands of GR and enhanced bands of POX or APX isoenzymes may be tightly associated with resistance ofC.offi cinalisto multiple stressors. However, the TBARS content rose signif icantly as the UV-B dosage increased and notably when the co-culture ratio was 1∶10 (Fig.5).TBARS is recognized as a representative index of lipid peroxidation in plants (Costa et al., 2002).Further analysis of the content of TBARS and the enzyme activity in the diff erent treatments showed that there was a negative correlation between SOD activity and TBARS content (partial correlation coeffi cientP<0.05). However, the partial correlation coeffi cients between the activity of POX, APX, GR,and the content of TBARS did not reach statistical signif icance (P>0.05). It is possible that the intracellular reactive oxygen level inC.piluliferawas not able to be constrained by the increased activity of GR alone; multiple antioxidant enzymes, rather than one enzyme, appear to be critical.
Allelopathy is a natural phenomenon in aquatic ecosystems that involves various biochemical interactions among plants (Fistarol et al., 2004; Poulin et al., 2018). However, it is diffi cult to research allelopathy between aquatic organisms under natural conditions because of the partial or complete concealment of allelopathy by other factors such as variability of nutrients, light, pH, and temperature conditions (Keating, 1977). Therefore, this research eliminated light, pH, and temperature f luctuations because it was carried out under controlled laboratory conditions. Additionally, to provide suffi cient nutrition, nutrients were added daily to ensure the health and good growth ofC.pilulifera, so allelopathy is the most likely explanation for the detected results.
Niche overlap will aggravate interspecif ic competition, and previous studies have shown that the physiological and biochemical eff ects of allelochemicals include the destruction of cell membrane structure (Gao et al., 2017; Corcoran et al.,2019), inhibition of photosynthesis (Zuo et al., 2015;Copin and Chèvre, 2018), damage to protein and enzyme activity (Zhang et al., 2011), and interference with gene expression (Ramlall et al., 2015). To advance this research, new methods such as coupling of chemical and molecular tools and proteomics will help to elucidate the complex chemical signaling mechanisms among algae, which may aff ect the community structure in this f ield (Zhou et al., 2016).Here the impact of allelopathy on several enzymes of the antioxidation system was shown. In a previous study, we isolated a large number of unsaturated fatty acids fromS.thunbergii. Among them, hexadecenoic acid and 5E, 8E, 11E, 14E, 17E eicosapentaenoic acid have strong algacidal activity and high content(unpublished data). We speculate that the activities of SOD (Fig.1a), APX (Fig.3a), and GR (Fig.4a)increased under co-culture, and the activities of several isozymes increased simultaneously, including SODIII (Fig.1b), APXII (Fig.3b) and GRII (Fig.4b).This may ref lect some kind of regulatory stress gradient response ofC.piluliferacells to adapt to stress conditions caused by allelochemicals, similar to the “toxic excitation eff ect” described by Tóth et al.(2012). This is eff ectively the response of homeostatic mechanisms to disturbance by external stress conditions. The organism will have a compensation eff ect after the initial inhibition reaction, which may gradually exceed the control behavior, which leads to a net stimulus eff ect (Calabrese and Baldwin, 2003).However, when there was a synergistic interaction between the allelopathy and the higher dose of UV-B,the activities of the enzymes were reduced more,especially the SOD enzyme, resulting in the increase of TBARS content.
Allelopathic interactions among phytoplankton strains are well documented (Corcoran et al., 2019),and recent work has expanded to consider whole food web interactions (Franzè et al., 2018). Some allelochemicals secreted by aquatic organisms can interrupt electron transport from PSII to photosystem I (PSI) (Wu et al., 2017), induce oxidative damage and ROS oxidation (Hong et al., 2008). UV treatments caused diff erent light reactions of allelopathic substances produced by two macrophytes,PhragmitesaustralisandHydrocarismorsus-ranae, further inf luencing their allelopathic activities (Farjalla et al., 2001). The intensity and type of light can inf luence allelopathic interactions between aquatic organisms (Wang and Tang, 2016). Our results were consistent with these conclusions. In our experiment,UV-B radiation enhanced the allelopathic eff ect ofS.thunbergiionC.pilulifera. Mahmood et al. (2013)observed allelopathic induction in the rhizosphere,when plants were exposed to UV radiation. However,the specif ic biochemical mechanism of the interaction between UV-B and allelopathy, that is, whether UV-B could promote an increase of the secretion of allelochemicals or prolong the secretion time of allelopathic chemicals, has not been determined.Moreover, our study indicated that the UV-B treatment was the dominant factor with the treatment eff ect being more obvious, while allelopathy enhanced the oxidative stress injury induced by UVB.
5 CONCLUSION
The antioxidant system inC.piluliferashowed specif ic alterations under the combined eff ects of UV-B exposure and allelopathy. Dual stress conditions had signif icant eff ects on the activities of SOD, APX and GR, and the interaction eff ect was signif icant. Under stress, SOD and APX increased under lower UV-B exposure but decreased under the higher exposure. POX reacted diff erently, and only UV-B radiation had a signif icant eff ect on its activity.The results of isozyme electrophoresis were basically consistent with the results of total activity. Among them, SODIII decreased signif icantly under high UV-B treatments. When the co-culture ratio was high, the enzyme activities of POXII and APXII increased under low UV-B stress but decreased as the UV-B dose increased. Although the activity of GR increased under the high stress imposed by high UV-B and allelopathy, the activity of several of the protective enzymes was inhibited under these conditions. The obstruction of normal metabolism led to the accumulation of TBARS. Therefore, as a biochemical defense mechanism to cope with the oxidative stress caused by the combination of ultraviolet radiation and allelopathy, the antioxidation system ofC.piluliferais limited in its capacity to protect stress-induced ROS.
6 DATA AVAILABILITY STATEMENT
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
7 ACKNOWLEDGMENT
The authors are grateful to Professor Emerita Paula Jameson, University of Canterbury, New Zealand,f inancially supported by “Double Hundred” Plan for Foreign Experts in Shandong Province, China, for critical reviewing and editing of this manuscript.
 Journal of Oceanology and Limnology2022年5期
Journal of Oceanology and Limnology2022年5期
- Journal of Oceanology and Limnology的其它文章
- Comparison of three f locculants for heavy cyanobacterial bloom mitigation and subsequent environmental impact*
- Proteomic analysis provides insights into the function of Polian vesicles in the sea cucumber Apostichopus japonicus post-evisceration*
- Key physiological traits and chemical properties of extracellular polymeric substances determining colony formation in a cyanobacterium*
- Involvement of the ammonium assimilation mediated by glutamate dehydrogenase in response to heat stress in the scleractinian coral Pocillopora damicornis*
- Cyanobacterial extracellular alkaline phosphatase: detection and ecological function*
- Full-length transcripts facilitates Portunus trituberculatus genome structure annotation*
