Key physiological traits and chemical properties of extracellular polymeric substances determining colony formation in a cyanobacterium*
Zhipeng DUAN , Xiao TAN , , Qingfei ZENG
1 Key Laboratory of Integrated Regulation and Resource Development on Shallow Lakes, Ministry of Education, College of Environment, Hohai University, Nanjing 210098, China
2 College of Hydrology and Water Resources, Hohai University, Nanjing 210098, China
3 State Key Laboratory of Lake Science and Environment, Nanjing Institute of Geography and Limnology, Chinese Academy of Sciences, Nanjing 210008, China
Abstract Colony formation of cyanobacteria is crucial for the formation of surface blooms in lakes.However, the underlying mechanisms of colony formation involving in physiological and cell surface characteristics remain to not well be established. Six cyanobacterial Microcystis strains (including both unicellular and colonial ones) were employed to estimate the inf luences of their physiological traits and the composition of extracellular polymeric substances (EPS) on colony or aggregate formation. Results show that raising the number of the photosynthetic reaction center and light-harvesting antenna in the PSII and reducing the growth rate were the major physiological strategies of Microcystis to produce excess EPS enhancing colony formation. Tightly bound EPS (T-EPS) was responsible for colony formation, which approximately accounted for 50% of the total amount of EPS. Five f luorescent components (protein-,tryptophan-, and tyrosine-like components and two humic-like components) were found in the T-EPS,although the amounts of these components varied with strains. Importantly, colonial strains contained much higher tyrosine-like substances than unicellular ones. We suggest that tyrosine-like substances might serve as a crosslinking agent to connect other polymers in EPS (e.g., proteins or polysaccharides) for colony formation. Our f indings identif ied key physiological traits and chemical components of EPS for colony formation in Microcystis, which can contribute to a better understanding on the formation of Microcystis blooms.
K eyword: colony formation; physiological properties; extracellular polymeric substances (EPS)composition; cyanobacterial blooms
1 INTRODUCTION
Cyanobacterial blooms globally threaten freshwater ecosystems that supply drinking water,recreation, and support f isheries (Harke et al.,2016). One of the key traits of most bloom-forming cyanobacteria is colony formation that enforces the formation of surface cyanobacterial scums through enhancing vertical and horizontal migration (Xiao et al., 2018) and resisting to predation from zooplankton(Gerphagnon et al., 2015). The f loating accumulation of cyanobacterial colonies provides better access to carbon dioxide and light while, at the same time,shading to other phytoplankton in the water column below (Sukenik and Kaplan, 2021).
Extracellular polymeric substances (EPS) are critical for the colony formation of cyanobacteria(Stal, 2017; Xiao et al., 2018). In the past decades,studies focused on colony formation have made great eff orts to identify the culturing conditions that inf luence the production and chemical composition of EPS in cyanobacteria (Yang et al., 2008; Wilson et al., 2010; Li et al., 2013; Duan et al., 2018; Xiao et al., 2019). Despite that, knowledge on how EPS production and EPS chemical characteristics of cyanobacteria depended on their physiological traits and impacted the colony formation is limited (Duan et al., 2018). Cyanobacterial cells in colonies commonly invest abundant photosynthates into EPS compared with unicellular ones (Zhang et al., 2011). Excessive investment in EPS has been shown to negatively correlate with growth rates, although this relationship was derived mainly from laboratory experiments using unicellular strains under diff erent culturing conditions(Yang et al., 2008; Li et al., 2013; Xu et al., 2016a).However, considerably physiological variations existed between unicellular and colonial strains.More specif ically, in comparison to unicells, colonies contained higher pigment content, more eff ective photosystems, and higher affi nity for inorganic carbon(Shen and Song, 2007; Zhang et al., 2007; Wu and Song, 2008), which might enhance their capacities to harvest light and accumulate photosynthates. It implies that the negative correlation between EPS production and growth rate may be absent in colonial strains (Duan et al., 2018). Therefore, it is worthy to estimate the relationships among photosynthetically energy acquisition and allocation for growth and EPS production or colony formation, using multiple strains with diff erent morphological characteristics.
Colony formation is not only associated with EPS content but relies heavily on EPS composition (Duan et al., 2019). Initially, polysaccharides were found in cyanobacterial EPS, which contained various mono-saccharides, and varied with morphospecies or strains (Forni et al., 1997). Although polysaccharides are dominant in cyanobacterial EPS by weight(e.g.,c.~75% of the total extracellular polymers in cyanobacterial EPS), other biomolecules (e.g.,protein, lipid, and DNA) existed as well (Pereira et al.,2009; Helm and Potts, 2012). With the development and application of f luorescence excitation-emission matrices (EEMs) in semi-quantifying dissolved organic matters (Stedmon and Markager, 2005; Murphy et al.,2014), various protein- and humic-like components were identif ied in cyanobacterial EPS (Xu et al., 2013a, c;Xu et al., 2014; Duan et al., 2019). Despite the rapidly collected information on the chemical composition of EPS, however, key chemical components involved in colony formation remain to be distinguished (Xiao et al., 2019). EPS extraction is a major challenge in the assays of EPS composition because it is easy to cause cell lysis and contamination (Xu et al., 2013c). It is responsible for the uncertainties in chemical assays of EPS composition (Pereira et al., 2009; Xu et al.,2013c; Liu et al., 2014). Recently, a thermal method for EPS extraction has been developed, which can eff ectively improve EPS yield and minimize cell lysis through stabilizing the cellular osmotic pressure and external pH (Duan et al., 2020). It may contribute to a more accurate assay of EPS chemical composition,especially the identif ication of colony-involved components.
A cosmopolitan cyanobacterium,Microcystis,often exists as large colonies in natural waters (Xiao et al., 2018), and forms harmful cyanobacterial blooms all over the world (Harke et al., 2016).Moreover,Microcystiscontains numerous strains that have a diversity of morphological characteristics(large colonies, small colonies, and unicellular forms). Therefore, the mechanisms underlying colony formation of cyanobacteria can be investigated on an intraspecif ic level. In this study, sixMicrocystisstrains(including both colonies and unicells) were employed.It aims to i) examine the interspecif ic variation in physiological and extracellular properties; ii) estimate the linkage between the properties and colony formation using structural equation modelling (SEM),EEMs, and Fourier Transform Infrared Spectrometer(FTIR) analyses; and iii) identify the key components in EPS involved in colony formation. The f indings of this study provide new insights into colony formation of cyanobacteria prospected from the physiological and extracellular properties.
2 MATERIAL AND METHOD
2.1 Preparation of Microcystis strains
SixMicrocystisstrains with diff erent morphological characteristics were employed to assess the interspecif ic variation of physiological and extracellular properties (Table 1). TM1 and TM2(large colonies) were isolated from Meiliang Bay of Taihu Lake, China (Duan et al., 2018). FACHB908 and FACHB1294 (small colonies) were purchased from the Freshwater Algae Culture Collection at the Institute of Hydrobiology (FACHB), Chinese Academy of Sciences. TM1(u) was separated from TM1, but has lost its ability to form colonies.FACHB469 (unicells) was also purchased from FACHB. These strains were pre-cultured andactivated separately in BG-11 medium (Stanier et al., 1971) for 6 days. The culture condition was set at 25±0.5 °C under 45 μmol photons/(m2·s) light intensity with a light to dark cycle of 12 h∶12 h.After acclimation, all strains were grown in 300-mL Erlenmeyer f lasks containing 200 mL of fresh BG-11 with three replicates for each strain. The starting cell concentration was set at 5×104cells/mL. These f lasks were gently mixed and randomly repositioned every day. For the assessments of physiological traits, extracellular properties, and size distribution,all strains were harvested at the exponential phase after being cultured for 10 days. Average growth rate(μ) was calculated following the equation (Xu et al.,2010):μ=ln(Nt-N0)/Δt, whereN tandN0represent the cell concentrations (cells/mL) at the end and the start of the experiment, respectively; and Δtindicates the experimental period (day). The cell concentration was determined via cell counting under a light microscope using a hemocytometer. For colonial strains, before cell counting, colonies were dispensed into single cells using Milli-Q water according to the method of Duan et al. (2021).
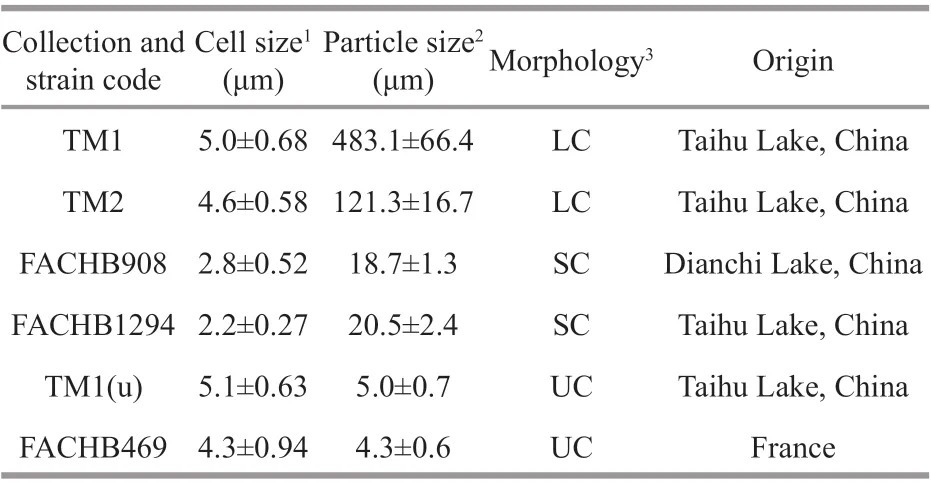
Table 1 Details of the six Microcystis strains
2.2 Microscopic analysis and quantifying cell size
Morphological characteristics of these strains were examined, and images were taken using a microscope(Carl Zeiss, Germany) equipped with a camera(AxioCam ICc 3). For cell size, the microscopic images were analyzed by using UTHSSA ImageTool v3.00 software (Duan et al., 2018).
2.3 Photosynthetic activity measurement
Cyanobacterial cultures (2 mL) were taken at the exponential phase and kept them in darkness for 20 min before the measurement (Duan et al., 2017).Photosynthetic activity or maximum quantum yield(Fv/Fm) was recorded in triplicate using a FluorPen f luorometer (AquaPen-C AP-C 100, Photon Systems Instruments, Czech Republic) at room temperature.Illumination in the equipment was provided by a PIN photodiode with 667- to 750-nm bandpass f ilters, with the saturating light of 3 000 μmol photons/(m2·s).
2.4 Particulate size distribution (PSD) analysis for colony size
A particle analyzer (Mastersizer 2000, Malvern Instruments Ltd., UK) was used to measure the particle size distribution. Particulate size of D50 indicated the average colony size (Li et al., 2014). The dispersant was NaCl solution (8.6 mmol/L). A refractive index of 1.40 and a light absorption index of 0.1 was used for all the strains (Li et al., 2014).
2.5 EPS extraction and analysis
In this study, EPS indicates soluble EPS (S-EPS)and bound EPS. Generally, the bound EPS of microorganisms is double-layered (Sheng et al.,2010), including loosely bound EPS (L-EPS) and tightly bound EPS (T-EPS). S-EPS and L-EPS were extracted according to Yang et al. (2008) and Xu et al. (2013a), respectively. A thermal extraction method was applied to extract T-EPS (Duan et al., 2020).All the EPS samples were f iltered (0.45 μm, Xinya Purif ication Materials Co., China) and were stored at 4 °C before EEM, FTIR, and biochemical assays.
2.6 EEM and FTIR analyses
EEM spectra were assayed by a f luorescence spectrometer (Hitachi F-7100, Tokyo, Japan) with a 700-voltage xenon lamp at 25 °C after instrumentspecif ic correction. Parameters of the spectrometer for EEM measurements were set according to Xu et al.(2013a). The blank scans were conducted using NaCl solution (8.6 mmol/L). Beforehand, non-f luorescence of the blank solutions was verif ied. Raman and Rayleigh scatterings of water were eliminated by subtracting the EEM spectra of Milli-Q water as blank or were f iltered using interpolation (Bahram et al., 2006). The measurement of EEM spectra was conducted in quadruplicate. Normalization of f luorescent intensity was performed according to Murphy et al. (2010).
The EPS samples for FTIR analysis were dried overnight by lyophilization. FTIR spectra were recorded using an attenuated ref lectance (ATR)-FTIR spectrometer (Bruker Tensor 27, Ettlingen, Germany)with a DLaTGS detector and a KBr beamsplitter. Each sample was placed in an ATR cell with a platinum single crystal, and then scanned from 4 000/cm to 400/cm at a resolution of 2/cm. Data were analyzed using OPUS 7.2 software.
2.7 Chemical assays and statistical analyses
Phycocyanin (PC) and chlorophylla(Chla) were extracted and measured following a previous study(Duan et al., 2021). Polysaccharide and protein in EPS samples were determined by the anthrone-sulfuric acid method (Li et al., 2013) and Bradford’s method(Bradford, 1976), respectively. All measurements were performed in triplicate. The contents of pigment and EPS were divided by cell volume and cell surface area, respectively, to eliminate the potential interferences from cell size. The cell volume and cell surface area were calculated based on the cell diameter.
EEM spectra were decomposed using PARAFAC analysis coupled with DOMFluor toolbox (http://www.models.life.ku.dk/) in MATLAB 12.0(Mathworks, Natick, MA) following a tutorial(Stedmon and Bro, 2008). Data are presented as means±standard deviation (SD). Pearson correlation analysis was employed to estimate the relationships among physiological characteristics, extracellular polysaccharide and protein content, and particulate size ofMicrocystisstrains. Structural equation modelling (SEM) was conducted in Amos 21.0.0 to evaluate eff ects of physiological and extracellular properties on colony formation. Principal component analysis (PCA) was performed using the “prcomp”function in the “stats” package of R to identify the potential correlation between EPS chemical composition and colony formation. Signif icant diff erences in photosynthetic activity (Fv/Fm), growth rate, and cellular pigment and EPS content among diff erent strains were analyzed with one-way ANOVA in SPSS 22.0 after testing for homoscedasticity. The signif icance level for comparative purposes was def ined atP<0.05, unless mentioned otherwise.
3 RESULT
3.1 Intraspecif ic variations in morphological and physiological traits
The cell size of the six strains varied from 2.2 μm to 5.0 μm in diameter (Table 1). TM1 and TM2 formed large colonies with the average colony sizes at 483.1 and 121.3 μm, respectively. The particulate size distribution of FACHB908 and FACHB1294 were bimodal with peaks at around 2.5 and 70 μm,which indicated the cell diameter and colony size,respectively. It revealed that culture suspensions of both strains contained unicells and small colonies.TM1(u) and FACHB469 were unicells (Fig.1a).
These strains diff ered from pigment content(Fig.1c-d). The large colony strains (TM1 and TM2)contained much more Chla(7-10 fg/μm3) and phycocyanin (~65 fg/μm), whereas the unicellular strains (TM1(u) and FACHB469) have less pigment(~3.5-fg/μm3Chlaand ~25-fg/μm3PC). However,the unicells and small colonies (FACHB906 and FACHB1294) grew much faster than the large colonies (Fig.1f). SimilarFv/Fmvalues (~0.42) were found among these strains (P>0.05), indicating a comparable photosynthetic effi ciency (Fig.1e).
3.2 Intraspecif ic variations in extracellular properties
Cells of the large colonial strains contained high EPS content, especially the massive bound EPS (Land T-EPS) (Fig.2). Specif ically, polysaccharide content in EPS of the large colonies (TM1 and TM2)was approximately 3 times higher than those of the unicells (TM1(u) and FACHB469). T-EPS was the main EPS fraction (>50% of total EPS). There was no diff erence in EPS polysaccharide content between the small colonies strain FACHB908 and the unicellular strain FACHB469 (Fig.2a), where both strains contained less extracellular polysaccharides(12.1 fg/μm2for FACHB908 and 13.3 fg/μm2for FACHB469). Large colonies strains contained more extracellular protein as well (Fig.2b). However,protein content in EPS of the unicellular strain TM1(u) was dramatically higher than those of the small colonies (FACHB908 and FACHB1294). The unicellular strain FACHB469 contained the lowest extracellular proteins (2.6 fg/μm2).
3.3 Eff ects of physiological and extracellular properties on colony formation
Extracellular polysaccharide content (particularly the polysaccharides in T-EPS) was negatively correlated with growth rate, but positively with Chlaand phycocyanin contents (RPearson>0.9,P<0.05).Extracellular protein content was positively related to pigment content as well. Particulate size of the strains was negatively related to the growth rate(RPearson=-0.75,P<0.05), but positively associated with Chlaand phycocyanin contents (RPearson>0.95,P<0.01). Non-signif icant correlation was observed between photosynthetic activity (Fv/Fm) and extracellular polysaccharides, extracellular proteins,and particulate size (Table 2).
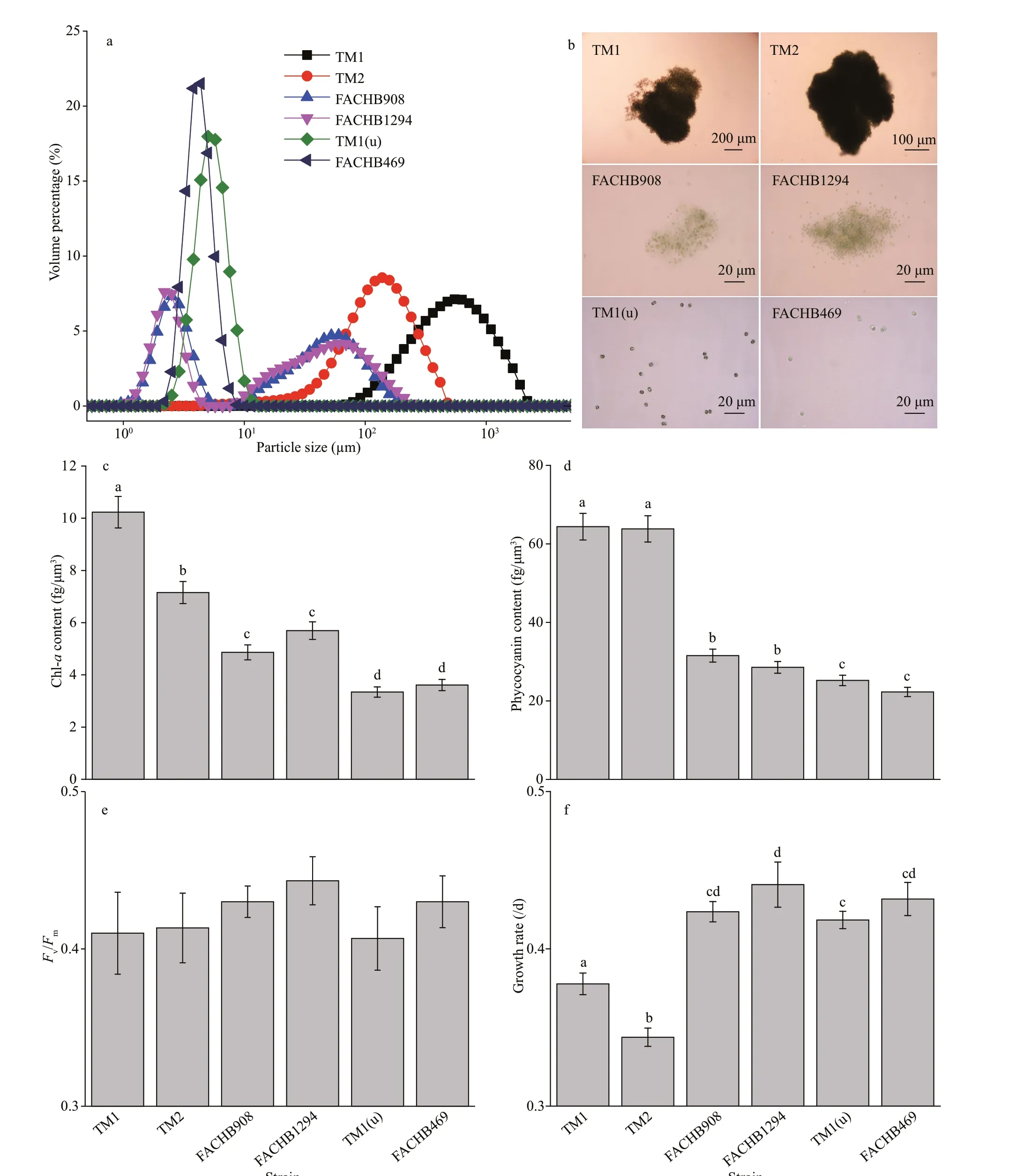
Fig.1 Particulate size distribution curves (a) and microscopic images (b) of the six strains; interspecif ic variations in Chl a(c) and phycocyanin (d) content, photosynthetic activity ( F v/ F m) (e), and growth rate (f)
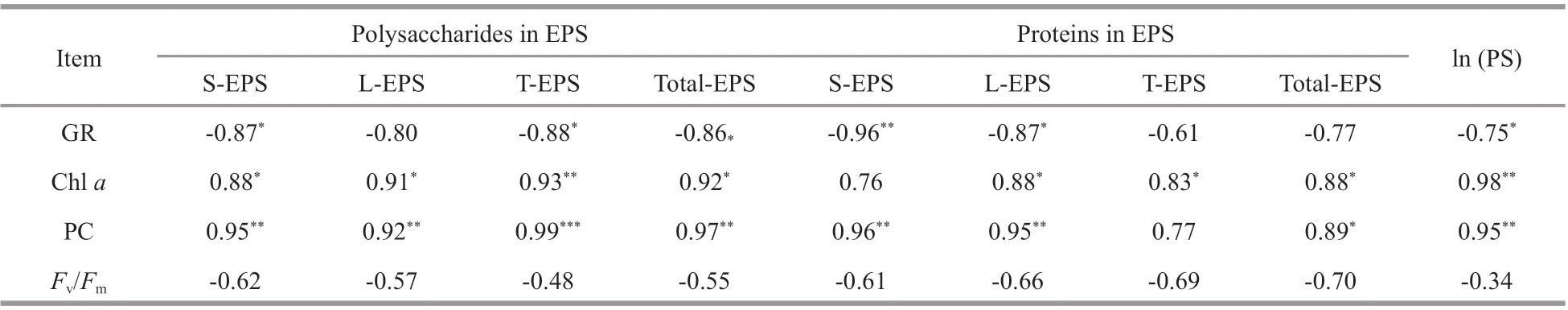
Table 2 Pearson coeffi cients ( R Pearson) among physiological characteristics, extracellular polysaccharide and protein contents,and particulate size
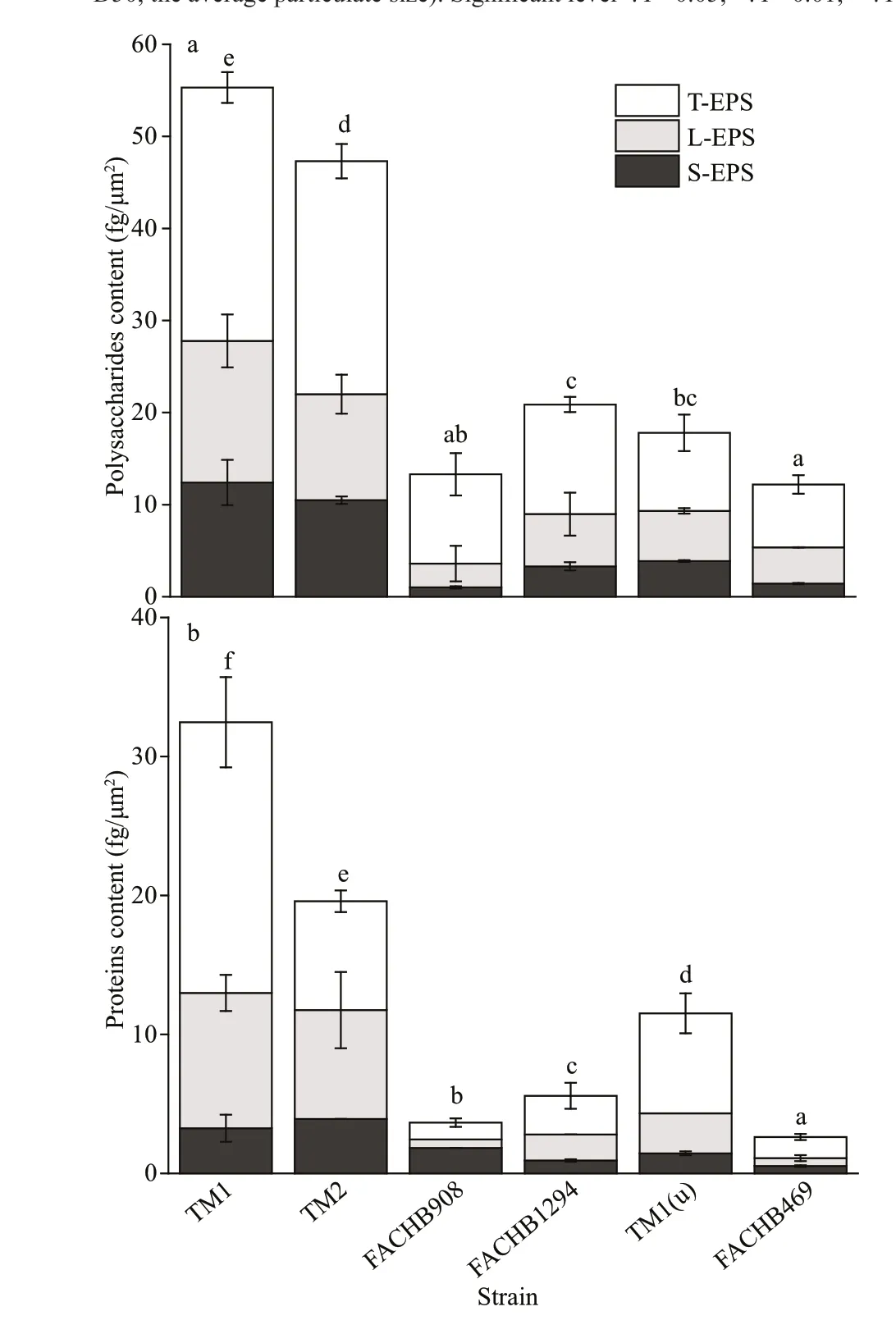
Fig.2 Polysaccharide and protein contents in EPS fractions of the six strains
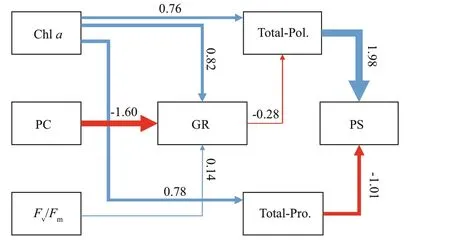
Fig.3 The eff ect of physiological and extracellular properties on the colony formation as estimated using structural equation modelling
Eff ects of physiological and extracellular properties on colony formation were further estimated by structural equation modeling (Fig.3). Chlapositively aff ected growth rate and enhanced extracellular polysaccharide and protein accumulation, whereas phycocyanin mainly negatively responded to growth rate with a standardized path coeffi cient at -1.60.Polysaccharides negatively responded to growth rate as well, indicating that phycocyanin could enhance polysaccharide accumulation indirectly.Polysaccharide accumulation was the main contributor to particulate size with a standardized path coeffi cient at 1.98. Conversely, extracellular protein negatively correlated with particulate size.
3.4 Intraspecif ic variation in extracellular composition estimated using EEM coupled with PARAFAC
To examine the potential key components in EPS facilitating colony formation ofMicrocystis, EEM was employed to characterize EPS composition in all the fractions (Supplementary Figs.S1-S3). Assignments of the peaks identif ied in the EEM contours are summarized in Supplementary Tables S1 & S2 shows the identif ied peaks in diff erent EPS fractions and strains. As for S-EPS, both large colonial strains(TM1 and TM2) and unicellular strains (TM1(u)and FACHB469) contained tryptophan- and humiclike substances, while only humic-like substances were observed in S-EPS of the small colonial strains(FACHB908 and FACHB1294). Tryptophan-like substances were identif ied in all samples of the L-EPS fraction, but additional humic-like substances were observed in the small colonial strains (Supplementary Table S2). Humic- and tyrosine-like substances were identif ied in the T-EPS fraction of colonial strains,while tryptophan- and humic-like substances were in unicellular strains except for the tyrosine-like substances of TM1(u).
The PARAFAC analysis was conducted to decompose the f luorescence EEM spectra and to semi-quantitatively identify the f luorophore composition using PARAFAC-derived compounds.A f ive-component model was appropriate according to the residual and split-half analyses of the PARAFAC (Supplementary Fig.S4), and the f ive components are shown in Supplementary Fig.S5.Specif ically, component 1 (C1, excitation/emission(Ex/Em)=(230, 280)/336) and component 3 (C3, Ex/Em=(220, 272)/292) were attributed to tryptophanlike and tyrosine-like substances, respectively (Xu et al., 2013a; Duan et al., 2019). Component 2 (C2,Ex/Em=280/346) was identif ied as protein-like substances (Chen et al., 2003). Component 4 (C4,Ex/Em=(260, 370)/452) and component 5 (C5, Ex/Em=(230, 280, 360)/444) were attributed to humiclike substances (Xu et al., 2014; Xiao et al., 2019).
The main f luorescent components in S-EPS were C2 and C4, whereas C1 and C2 dominated in L-EPS of the large colonies and unicellular strains (Fig.4a).T-EPS was more complex and composed of the f ive PARAFAC-derived components. Specif ically, C3 and C5 dominated in T-EPS of the colonial strains, but a weak signal of them in the unicells, indicating C3 and C5 may play an essential role in colony formation ofMicrocystis. Plots of PCA showed that f luorescent component composition in S-EPS and L-EPS failed to distinguish colonies and unicells, indicating colonies and unicells shared some identical components in both EPS fractions (Fig.4b). In the PCA plot of the T-EPS, however, colonies and unicells were separated under the f irst PCA axis (PC1) that explained 74.0%of the total variation (Fig.4b). Fluorescent C3, C4,and C5 negatively contributed to PC1, but they were positively related to colony formation (especially the C3). In comparison, C1 and C2 positively contributed to PC1, but negatively related to colony formation.
3.5 Intraspecif ic variations in FTIR spectra of the tightly bound EPS
To further explore functional groups of organic molecules in the tightly bound EPS (T-EPS), FTIR was conducted. The FTIR spectra of T-EPS samples are illustrated in Fig.5; the dominant bands with their assignments are included in Supplementary Table S3. The FTIR spectra of T-EPS extracted from colonial strains were highly overlapping, which signif icantly diff ered from those of the unicellular ones, especially the FACHB469. The band around 3 394/cm (hydrogen bonded N-H and O-H stretching vibrations) and 3 182/cm (stretching vibration of hydrogen bonded-CONH2) (Griffi ths and de Haseth,2006) were observed merely in T-EPS of colonies.The peaks at 2 918/cm and 2 848/cm pertaining to the methyl-metal stretching vibration (from 2 810/cm to 3 050/cm) (Nakamoto, 2009) were much stronger in T-EPS of colonial strains, but weak or disappeared in unicellular ones. Similarly, the peaks at 1 469/cm and 1 418/cm were found in T-EPS of colonial strains,which assigned to stretching vibration of -CH2or -COO-groups (Xu et al., 2017; Wang et al.,2018). The bands of 1 644/cm (C=O/C=N stretches in δ-lactam or proteins) (Xu et al., 2013c) and 1 039/cm (stretching vibration of -CO or -OH in polysaccharides) (Duan et al., 2019) appeared in all the T-EPS samples, indicating there were common proteins or polysaccharides in T-EPS. The f ingerprint region included a band at 646/cm (assigned to ring vibrations from aromatic amino acids) (Wang et al.,2018) in T-EPS of colonial strains, which was redshift in that of the unicellular FACHB469.
4 DISCUSSION
In this study,Microcystisstrains diff ered profoundly in their EPS protein and polysaccharide contents,particularly in the tightly bound EPS (Fig.2). This EPS fraction has been recognized to greatly contribute to colony formation (Li et al., 2013), especially at the early stage of cell adhesion (Xu et al., 2014). This can explain the positive eff ect of polysaccharide content in EPS on colony formation (Fig.3). However, it should be noted that the relationship between EPS content and colony size is not in a straight line (Duan et al., 2018), and EPS composition dramatically impactsMicrocystiscolony size and morphology as well (Duan et al., 2019; Xiao et al., 2019).
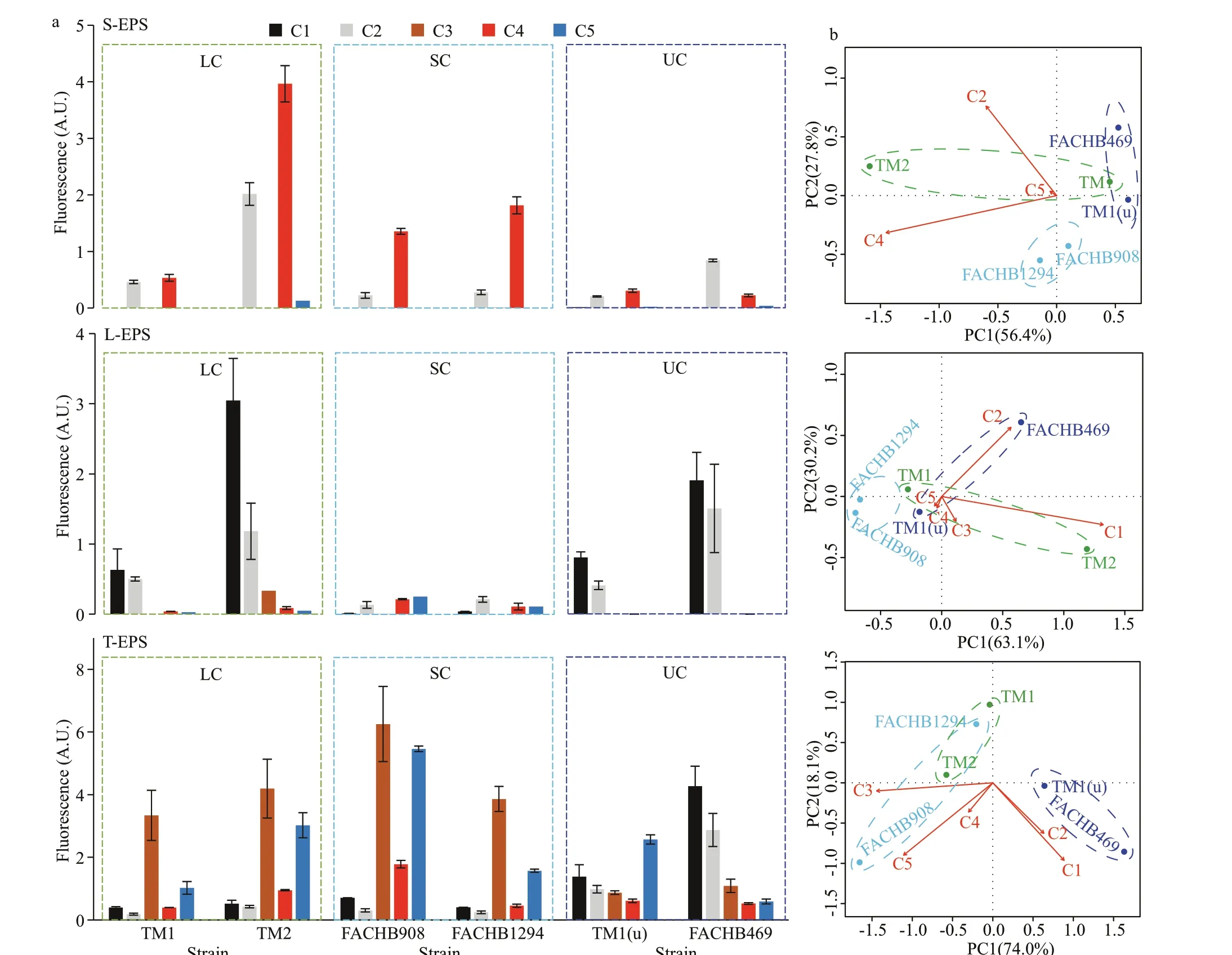
Fig.4 Fluorescent component composition in the EPS fractions of Microcystis strains as estimated by EEM coupled with PARAFAC analysis based on the f ive PARAFAC-derived components (a); principal component analysis (PCA) plots of the variation in the f luorescent composition of the EPS fractions (b)
The f luorophore composition in tightly bound EPS(T-EPS) was more complex compared to the soluble and loosely bound EPS, and it could be used to identify the colonial strains (Fig.4). Specif ically, tyrosinelike substances in the T-EPS were key components for colony formation, because they were observed mainly in the colonial strains rather than the unicells(Fig.4). Tyrosine-like substances have also been found frequently and abundantly in various morphospecies ofMicrocystiscolonies (i.e.,M.ichthyoblabe,M.aeruginosa, andM.novacekii) collected from Taihu Lake, China (Xu et al., 2013b; Duan et al., 2019). In contrast, tyrosine-like substances were much lower in T-EPS of the unicellular strains, where tryptophan- and protein-like substances dominated (Fig.4 and Table 1;see also Xu et al., 2013a). The existence of aromatic substances (e.g., tyrosine and tryptophan) in T-EPS was also conf irmed by the FTIR spectra (Fig.5).
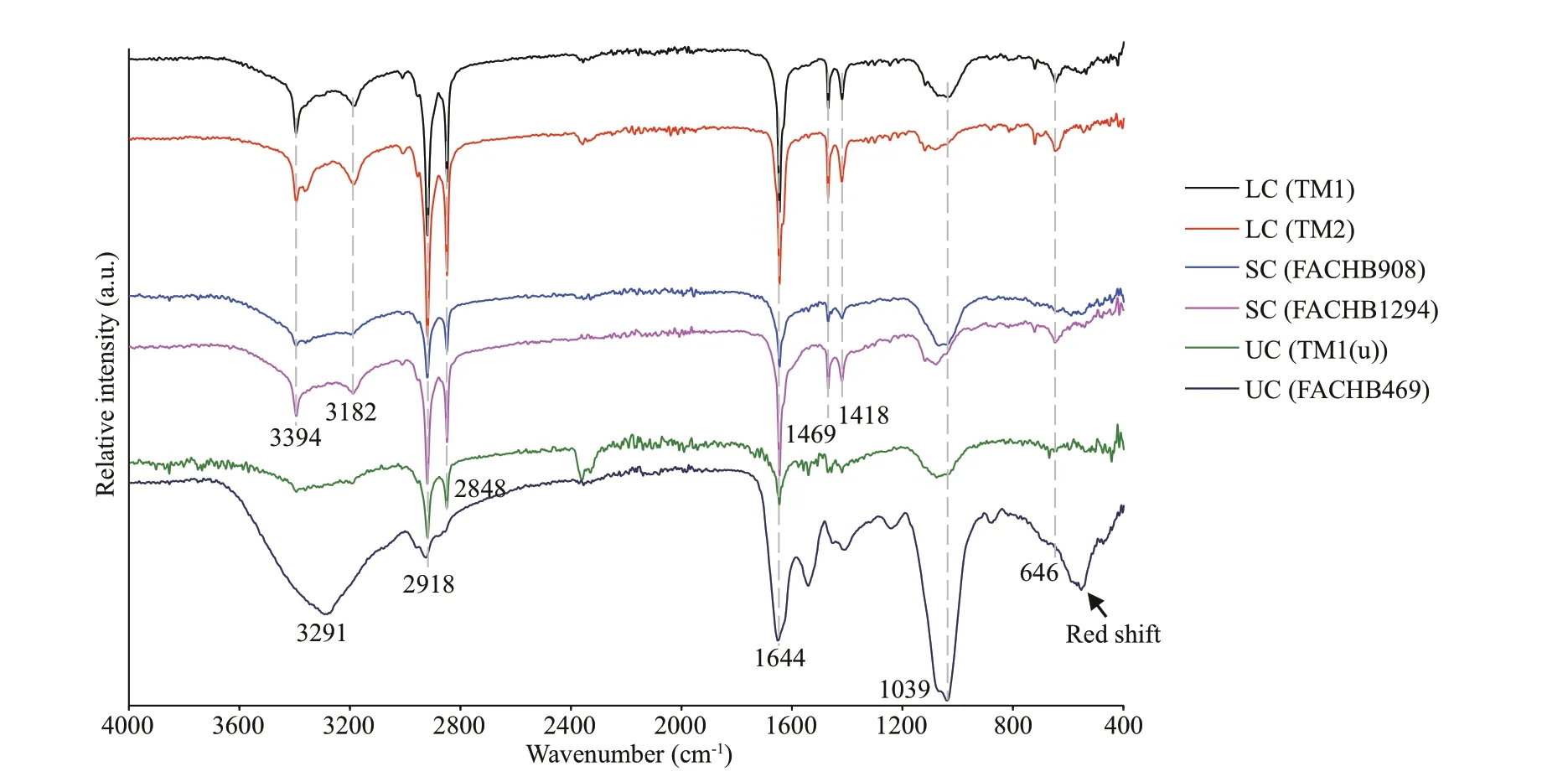
Fig.5 FTIR spectra of the tightly bound EPS extracted from the six Microcystis strains
Tyrosine is highly reactive due to the tyrosine residues. They are easy to form multi-tyrosine crosslinking in vitro via multiple physiologically and photochemically relevant reactions (e.g.,enzymatic, photo-initiated), which may contribute to cell aggregation (Fig.6). These reactions can also naturally occur in tyrosine residues or additional phenol moieties that may be added via tyramine derivatization to non-tyrosine-containing substrates(i.e., polysaccharides and humic-like substances) by bio- or photo-chemical reactions (Sof ia et al., 2002;Partlow et al., 2016). It indicates that tyrosine-like substances could be a critical crosslinking agent for EPS assemblage. Alternatively, tyrosine-like substances were associated with cation captures (e.g.,Fe3+) in EPS (Xu et al., 2013b), which can form a“bridge eff ect” of cations to bind mucilaginous EPS and cells (Xu et al., 2016b). It is consistent with the metal-binding groups that were detected abundantly in T-EPS of colonial strains, but signif icantly weak in that of the unicellular FACHB469 (Fig.5). Humiclike substances often occurred in T-EPS (C4 and C5 in Fig.4), which may contribute to colony formation as well (Duan et al., 2019; Xiao et al., 2019). However,the unicellular strain (TM1(u)) also contained higher humic-like substances in its T-EPS; for example, the humic-like C5 was nearly two-fold higher than that of the colonies strain of TM1 (Fig.4). It indicates that humic-like substances could hardly gather cells by themselves.
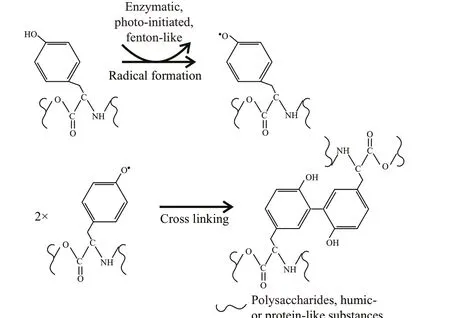
Fig.6 Mechanisms of tyrosine cross-linking reactions
The large colonial strains invested large amounts of EPS production for colony formation,while the unicells were devoted to proliferation(Figs.1-2). Pigment content commonly ref lects the photosynthetic potential of light energy acquisition and transformation (Glazer, 1984). In photosystems(PS) ofMicrocystis, phycocyanin is the main lightharvesting pigment connecting to the reaction center(RC), which is composed of Chla-protein complex or P680 in PSII (P700 in PSI is also a Chla-protein complex) (Glazer, 1984; Grossman et al., 1993).Light photons captured by phycocyanin protein are transported into RC for photochemical reactions (e.g.,ATP production and carbon f ixation). Similar to Zhang et al. (2007, 2011), the colonial strains contained much more Chlaand phycocyanin compared to the unicellular ones (Fig.1c-d). It indicates that the colonial strains contained a higher number of RCs in their photosystems. Moreover, colonies may be equipped with more eff ective photosystems. For example, Zhang et al. (2011) found that theFv/Fmof colonies was much higher than that of the unicells,especially under high light intensities (>300 photons/(m2·s)). However, in this study, all strains displayed a similar maximal quantum yield of PSII (Fv/Fm),implying comparable photosynthetic effi ciencies among the strains. Therefore, enriching the RC number was a signif icant strategy of the colonial strains to promote carbon accumulation. Excessive carbon accumulation in cells facilitated EPS secretion(Fig.2; see also Myklestad, 1995), and then colony formation (Yang et al., 2008).
Growth rate was negatively related to colony formation (Table 2; see also Li et al., 2013) through down-regulating carbon allocation into EPS production(Duan et al., 2021). It indicates that the large colonial strains might enhance EPS content via reducing growth rate as well (Fig.1f). However, it should be noted that colony formation can elevate phycocyanin content but frustrate growth rate as well, owing to the strong eff ect of “self-shading” (Wilson et al., 2006).More phycocyanin content facilitates light acquisition of the inner cells of colonies (Zhang et al., 2011).This may be responsible for the signif icant negative relationship between phycocyanin and growth rate,and the indirectly positive eff ects of phycocyanin on extracellular polysaccharide production (Fig.3; Table 1). Therefore, increasing phycocyanin in the colonial strains could partly compensate for the negative eff ect of “self-shading” on light harvesting. Nevertheless, it off ers new insights into the eff ect of photosynthetic energy acquisition and reallocation on colony formation (Fig.3).
5 CONCLUSION
This study estimated the linkages between colony formation and the variations in physiological traits and extracellular characteristics in sixMicrocystisstrains. The chemical components of EPS involved in colony formation were identif ied. Our data revealed that increasing in photosynthetic reaction centers and reducing growth rate could be the main strategies ofMicrocystisto enhance EPS production, then enlarging colony size. Although f ive PARAFAC-derived f luorescent components were identif ied in the tightly bound EPS (T-EPS), tyrosine-like substances acted as a crosslinking agent and connected polysaccharides and humic- or protein-like substances for EPS assemblage and thus enhancing colony formation.This study provides a better understanding of how physiological and extracellular properties contribute to colony formation in cyanobacteria.
6 DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during this study are available from the corresponding author on reasonable request.
 Journal of Oceanology and Limnology2022年5期
Journal of Oceanology and Limnology2022年5期
- Journal of Oceanology and Limnology的其它文章
- Comparison of three f locculants for heavy cyanobacterial bloom mitigation and subsequent environmental impact*
- Eff ect of light intensity on bound EPS characteristics of two Microcystis morphospecies: the role of bEPS in the proliferation of Microcystis*
- Community structure of aerobic anoxygenic phototrophic bacteria in algae- and macrophyte-dominated areas in Taihu Lake, China*
- Tidal water exchanges can shape the phytoplankton community structure and reduce the risk of harmful cyanobacterial blooms in a semi-closed lake*
- Eff ect of random phase error and baseline roll angle error on eddy identif ication by interferometric imaging altimeter*
- Estimating the evolution of sea state non-Gaussianity based on a phase-resolving model*
