Tidal water exchanges can shape the phytoplankton community structure and reduce the risk of harmful cyanobacterial blooms in a semi-closed lake*
Wenxu ZHENG , , Renhui LI , , Wenli QIN , Binbin CHEN , Min WANG ,Wanchun GUAN , Xiaoling ZHANG , Qiao YANG , Min ZHAO ,**, Zengling MA ,**
1 Zhejiang Provincial Key Laboratory for Subtropical Water Environment and Marine Biological Resources Protection, Wenzhou University, Wenzhou 325035, China
2 National and Local Joint Engineering Research Center of Ecological Treatment Technology for Urban Water Pollution, Wenzhou University, Wenzhou 325035, China
3 Department of Marine Biotechnology, School of Laboratory Medicine and Life Science, Wenzhou Medical University, Wenzhou 325035, China
4 Marine Science and Technology College, Zhejiang Ocean University, Zhoushan 316022, China
5 School of Petrochemical Engineering and Environment, Zhejiang Ocean University, Zhoushan 316022, China
Keyword: estuary; harmful cyanobacterial blooms; phytoplankton community; water exchange; tidal movement; environmental parameters
1 INTRODUCTION
In estuaries, tides play a vital role in aquatic ecosystems by bringing in seawater with nutrients,salt, and microorganisms, and mixing with freshwater(Choi et al., 2017). Such complex processes can result in high-productivity areas that act as habitats, and feeding areas for many species, such as f ish, mammals,and birds (Wu et al., 2021; Yi et al., 2021). Moreover,estuarine islands are vital for the maintenance of biodiversity in the estuarine ecosystem because they provide a transfer point for migratory birds and mammals, and greatly contribute to the protection of freshwater (Li et al., 2021). Lakes play an important role in the control of f looding, water supply, and recreational activities, and they are also the most vulnerable ecological systems that can be disturbed by many natural factors and anthropological activities(Xu et al., 2021a).
Lakes in estuarine islands are a kind of extraordinary natural lake system isolated from the surrounding water. They are aff ected by the exchange of tidal water and play an important role in the island ecosystem balance. Normally, their elevations are higher than the outside saline water, but the fresh water in the lakes can exchange with the outside brackish water as a result of the tidal movement. The exchange will result in water disturbance, physicochemical alteration, salinity invasion, nutrient input, and biological exchange; and these processes may inf luence the phytoplankton community structure in regional ecosystems (Ramond et al., 2021). Therefore, lakes in estuarine island are ideal places to study the eff ects of tide-driven water exchange on the phytoplankton community structure due to their special geographical location, small area,and semi-isolated status. In addition, eutrophication,freshwater salinization, and bio-invasion are becoming heavy environmental stresses for the estuarine island lakes because of anthropogenic and natural causes(Cordeiro et al., 2020; Mo et al., 2021).
Phytoplankton plays a central role in aquatic ecosystems in driving biogeochemical cycles through their nutrient uptake and primary biomass production for supporting the aquatic food webs (Cardinale, 2011;Striebel et al., 2012; Worden et al., 2015). However, if a particular phytoplankton species grows excessively and forms algal blooms, such a massive quantity of algal cells will hinder the sunlight across the water surface and deplete dissolved oxygen, consequently suff ocating the aquatic organisms and destroying the balance of the ecosystem (Paerl and Huisman, 2008;Huisman et al., 2018; Pyo et al., 2021). Phytoplankton community responds rapidly to the changes in the environmental conditions of the habitat by altering the factors such as species composition, dominant species, and cell abundance (Griffi ths et al., 2016;Zhang et al., 2021a). Growth of phytoplankton is restricted by both biotic and abiotic factors, which include predation, water temperature, salinity, light,nutrient supply, and others (Yu et al., 2008; Zhang et al., 2021b). The phytoplankton community structure of estuaries is mainly aff ected by extreme rainfall events and nutrient loading (Van Meerssche and Pinckney, 2019; Vizzo et al., 2021). Moreover,hydrography conditions such as tides and coastal freshwater streams play a key role in structuring the estuarine phytoplankton community (Kasai et al.,2010; Paerl et al., 2014; Flores-Melo et al., 2018).Therefore, f ield investigation on the characteristics of a phytoplankton community can provide a visual result of the environmental inf luence (Rao et al.,2021). In China, harmful algal blooms formed by cyanobacteria (CyanoHABs) have occurred in the lakes, and even in the reservoirs of drinking water as a result of eutrophication and anthropological activities(Guan et al., 2020; Huang et al., 2020). In addition, in recent years, more CyanoHABs capbable of causing various degrees of marked visible discoloration of water in lakes located in Jiangxin Islet are reported.Such a phenomenon has also aroused public concerns.
Tidal backf low may bring extreme shifts to estuarine island lakes for the following reasons.(1) The invasion of brackish water might favor phytoplankton species that are diff erent from those of freshwater due to environment preferences (Kim et al., 2021; Xu et al., 2021b). (2) The water exchange might exacerbate eutrophication because the frequent water turbulence is a trigger to the release of the nutrients trapped in surface of the sediment (Baek et al., 2020; Wirtz and Smith, 2021). (3) A decrease in water transparency caused by the water turbulence can limit light availability for phytoplankton and impede their growth (Domingues et al., 2005). (4) Intrusion of salinity may become the main threat to the salt intolerant algal species by aff ecting photosystem II (PSII) performance and extracellular osmotic pressure (Corsi et al., 2010). (5) Invasive organisms brought by the seawater might cause unpredictable impacts on the phytoplankton habitat through biotic factors, such as predation, competition, and parasitism(Bailey, 2015). However, to our best knowledge,there has been a paucity of studies focusing on the eff ects of water exchange (caused by tides) on the phytoplankton community structure and algal bloom outbreak in the estuary island lakes.
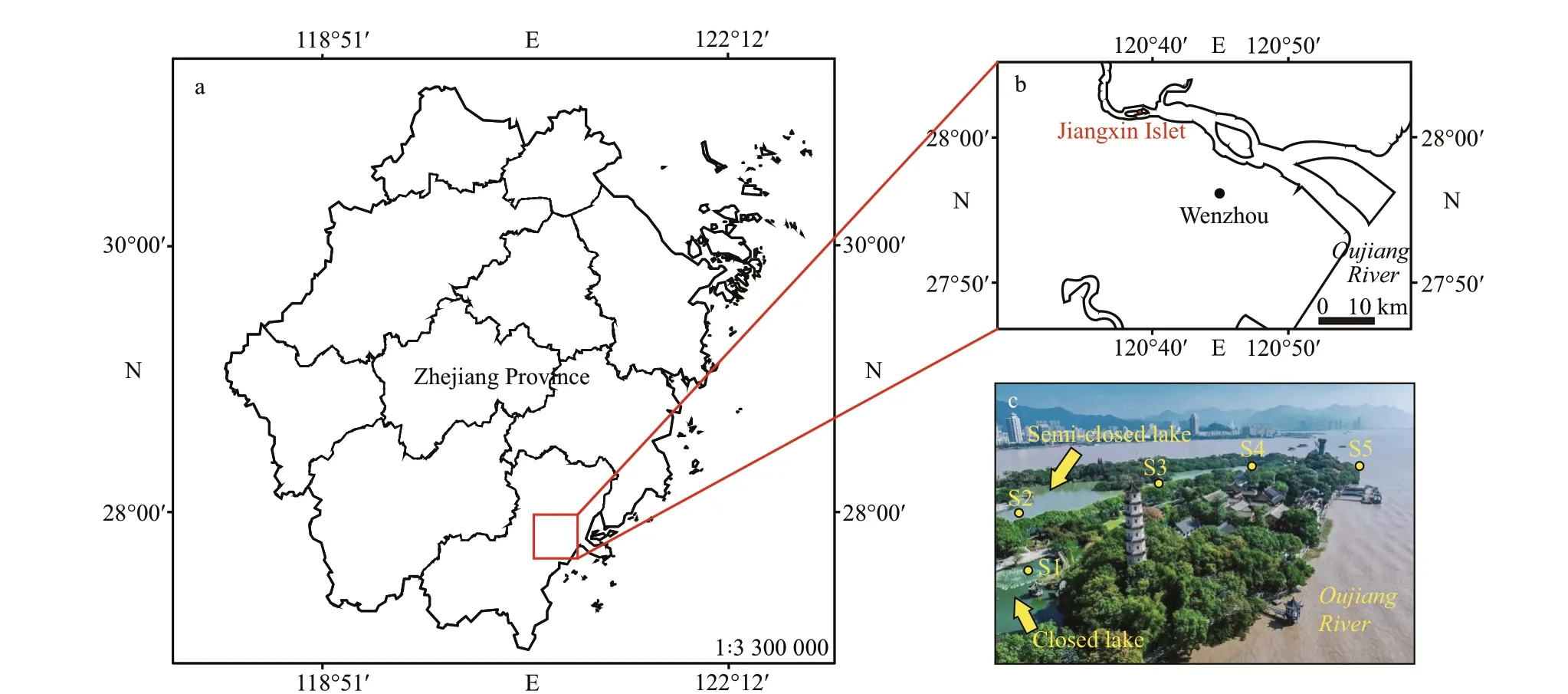
Fig.1 The location of Oujiang River in Zhejiang Province, China (a), the map of Oujiang River estuary (b), and the enlarged map of the Jiangxin Islet, showing Bibo Lake, the semi-closed Gongqing Lake, and the sampling sites (c)
The Oujiang River originates from the western area of Zhejiang Province and f lows into the East China Sea (ECS) and along a large number of islands situated in the river channel. Situated in the middle reaches of the Oujiang River is the Jiangxin Islet.There are two types of lakes in the Jiangxin Islet; a semi-closed lake that can exchange water with the Oujiang River during the tidal period, and a closed lake isolated from the other sources of water, and its water source comes mainly from rainfall. Normally,the elevation of the semi-closed lake, Gongqing Lake, is higher than the water around the islet, and the outside water will rise and f low into the lake during a period of astronomical tides. The closed lake, Bibo Lake, is adjacent to Gongqing Lake and it is completely isolated. Therefore, these two lakes are the ideal research objects for assessing the inf luences of tidal backf low on the phytoplankton community and the outbreak of algal blooms.
Compared with the characteristics of the phytoplankton community in a completely closed lake,it is reasonable to speculate that the phytoplankton community structure can be shaped by the exchange of water and concomitant physicochemical alterations in a semi-closed lake and the changes may favor the control of harmful cyanobacterial blooms. To test the hypothesis, a nearly one-year bi-weekly f ield investigation was carried out in the semi-closed Gongqing Lake and nearby closed Bibo Lake from June 29, 2020 to June 14, 2021, during which the changes in phytoplankton community structure and physicochemical parameters were determined.
2 MATERIAL AND METHOD
2.1 Study area and sampling station setting
The Jiangxin Islet (28°03′N, 120°63′E) is in the midstream of the Oujiang River which f lows through the northern area of Wenzhou City (Fig.1b). The annual averaged discharge of the Oujiang River is approximately 470 m3/s, and the maximum discharge can reach 23 000 m3/s, for example, during the f lood period in July 1959. The lunar semi-diurnal tide that dominates the estuary during the spring has a tidal range of approximately 6 m (Li et al., 2017; Xu and You, 2017). The semi-closed lake, Gongqing Lake,has a water area of 7.4×104m2in water volume of 88 800 m3and average depth of 1.2 m, and it lies in Jiangxin Islet and connects to the Oujiang River with an exit (Fig.1c). The brackish water outside the islet f lows into the lake through the exit during the astronomical tidal period of the lunar calendar, and after that, the water in the lake returns into the Oujiang River. The closed lake, Bibo Lake beside the Gongqing Lake, has water area of 0.4×104m2in water volume of 2 000 m3and average depth of 0.5 m (Fig.1c). To investigate the inf luence of water exchange on the structure of the phytoplankton community at diff erent distances to the exit, f ive sampling sites were selected at diff erent places (Fig.1c).
2.2 Field sampling and data collection
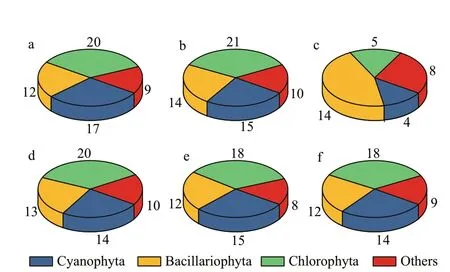
Fig.2 Composition of phytoplankton species in the closed(a) and semi-closed (b) lakes on the Jiangxin Islet and in the Oujiang River near the exit (c); d, e, and f show the phytoplankton species composition in semiclosed Gongqing Lake at the sampling sites from the farthest to the closest from the exit
The water sampling was carried out on the 14thand 29thevery month of the Chinese lunar calendar,the days before the astronomical tide. Samples for quantitative analysis of the phytoplankton species were collected from 0.5 m below water surface every station and stored in 1.0-L hydrochloric acidwashed opaque polypropylene bottles. Samples were preserved with Lugol’s iodine solution and sit for at least 24 h before analysis. Samples for the analysis of phytoplankton taxonomy were collected by horizontal trawling with 25#plankton net at surface water; and the collected plankton was stored in 100-mL transparent plastic bottles. Water samples for nutrient analysis were stored in a cooler with ice packs, then transported to laboratory and processed within 10 h. Water temperature, dissolved oxygen,and pH were measured in situ using a HACHHD40d portable multi-parameter water quality analyzer(HACH, USA). Salinities were measured in situ using a DDBJ-350F portable conductivity meter (INESA Scientif ic Instrument, China), and transparency was measured with Secchi disc.
2.3 Determination of nutrients in surface water
Total nitrogen (TN) and total phosphorus (TP)concentrations were determined using the acidic peroxydisulfate method reported by D’Elia et al.(1977) and Gales et al. (1966), respectively.
2.4 Determination of chlorophyll- a concentration in surface water
Chlorophyllawas collected by filtering 200 mL of water (from surface samples) through a Whatman GF/C filter (47-mm-diameter and 1.2-μm-pore-size)and the f ilter was then extracted overnight in the dark with absolute methanol at 4 ℃. After centrifugation at 5 000×gfor 5 min, the absorption spectra of the supernatants were measured with a spectrophotometer(UV 530; Beckman Coulter, USA). The concentration of chlorophyllawas calculated according to equation of Porra (2002).
2.5 Identif ication of phytoplankton species and determination of diversity indices
Identif ication and quantif ication of phytoplankton species were performed under a Zeiss Axiolab 5 phase contrast microscopes (Carl Zeiss, Germany).Phytoplankton cells were identif ied and counted using Groove-type 0.1-mL counting slide with a mold-type grid on its bottom under a working magnif ication of 400×. For each sample, a minimum of 400-500 cells were counted from randomly selected transects at multiple magnif ications; and all the samples were counted 2-3 time to improve the accuracy and reproducibility. In addition, to characterize the changes in the phytoplankton community structure,species abundance, species richness, and three other diversity indices were determined. The dominance index (Y) that emphasizes the role of the important species was calculated according to the equation of Mcnaughton (1967). The species richness (R) was calculated according to equation of Margalef (1958).The species diversity (H) was calculated according to equation of Shannon and Weaver (1949). The species evenness (E) was calculated according to equation of Pielou (1967).
Phytoplankton community composition (cell density) at the species level was visualized using non-metric multidimensional scaling (NMDS) based on Bray-Curtis dissimilarities between each pair of samples. Analysis of similarity (ANOSIM) was used to reveal diff erences in the phytoplankton communities among the sampling sites. The NMDS and ANOSIM analyses were performed using PRIMER v.7.0.21.Graphic displays were performed using Origin 2019(OriginLab, USA), Adobe Illustration 2020 (Adobe,USA), and Adobe Photoshop 2020 (Adobe, USA).
3 RESULT
3.1 Phytoplankton species composition and dominant species
In the closed lake (Bibo Lake), 58 species from 7 phyla were identif ied, and they were Cyanophyta,Bacillariophyta, Chlorophyta, Euglenophyta,Pyrrophyta, Chrysophyta, and Haptophyta (Fig.2a).Among them, Chlorophyta was the most diverse,consisting of 20 species whereas Cyanobacteria and Bacillariophyta were the second and third most diverse, having 17 and 12 species, respectively(Fig.2a). In the semi-closed lake (Gongqing Lake),60 species were identif ied, of which 21, 15, and 14 species belong to Chlorophyta, Cyanophyta, and Bacillariophyta, respectively (Fig.2b). In the Oujiang River, 31 species were identif ied (Fig.2c), and the most diverse group was diatoms (Bacillariophyta),consisting of 14 species. In addition, in the semiclosed lake, the number of phytoplankton species showed a decreasing trend as 57, 54, and 53 species were found in site S2 (farther away from the lake exit)(Fig.2d), S3 (Fig.2e), and S4 (closer to the lake exit)(Fig.2f), respectively.
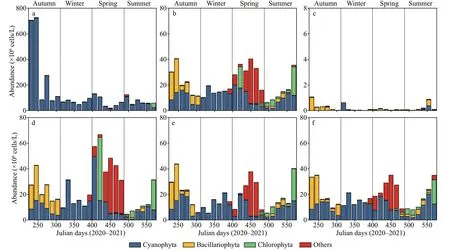
Fig.3 Phytoplankton abundance in the closed (a) and semi-closed (b) lakes on the Jiangxin Islet and in the Oujiang River near the exit (c); d, e, and f show the phytoplankton species composition in the semi-closed Gongqing Lake at the sampling sites from the farthest to the closest from the exit
In the closed lake, the 5 dominant species (Y≥0.02)were all f ilamentous cyanobacteria, and the most dominant species wasPlanktothrixagardhii, in dominance of 0.36 (Table 1). In the semi-closed lake and the Oujiang River, the number of dominant species increased, and they belonged to diff erent taxonomical groups, i.e., cyanobacteria, diatoms, green algae.
3.2 Phytoplankton abundance
In the closed lake, the monthly abundance of total phytoplankton species was between 1.90×107and 7.26×108cells/L, with an annual average abundance of 1.35×108cells/L during the study period (Fig.3a).The abundance of total phytoplankton species was the highest in autumn and lowest in spring. In addition,during the entire study period, the abundance of cyanobacteria accounted for more than 96% of thetotal phytoplankton (Fig.3a). In the semi-closed lake, the monthly abundance of total phytoplankton species was between 6.22×106and 4.05×107cells/L,on annual average abundance of 2.10×107cells/L(Fig.3b). Cyanobacteria, diatoms, and green algae appeared throughout the year on average abundance of 1.08×107, 3.55×106, and 2.24×106cells/L (Fig.3b),respectively. In the Oujiang River, the monthly abundance of total phytoplankton species was between 1.17×104and 1.07×106cells/L, on annual average of 2.04×105cells/L (Fig.3c). The average abundance of total phytoplankton species in the closed lake was 6 times higher than in the semi-closed one, and nearly 5 800 times higher than in the Oujiang River(Fig.3a-c). In other words, the average dominance of cyanobacteria in the closed lake was 0.96, and those in the semi-closed lake and the Oujiang River were 0.51 and 0.22, respectively.
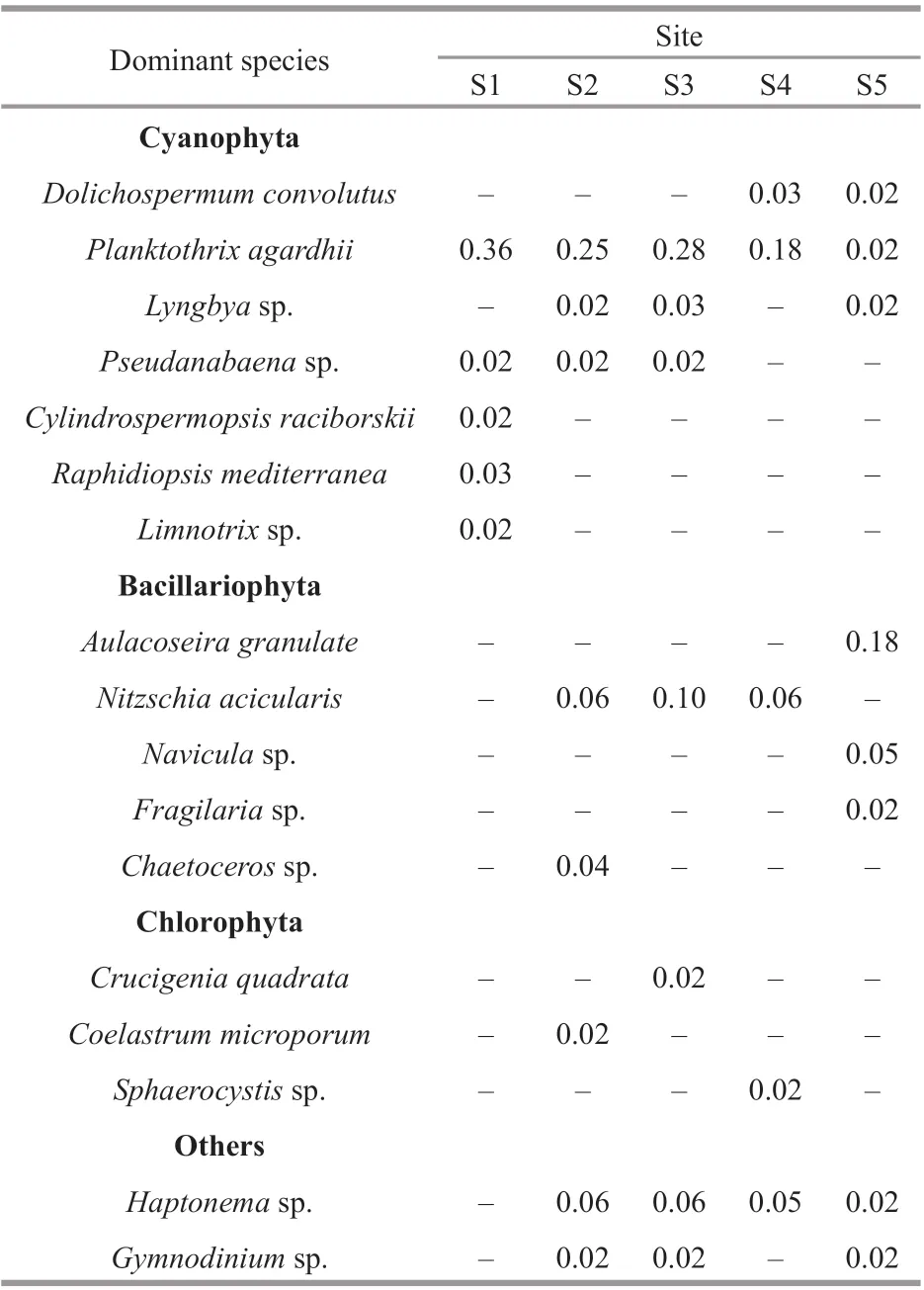
Table 1 Dominant phytoplankton species and their dominance ( Y) at diff erent sites
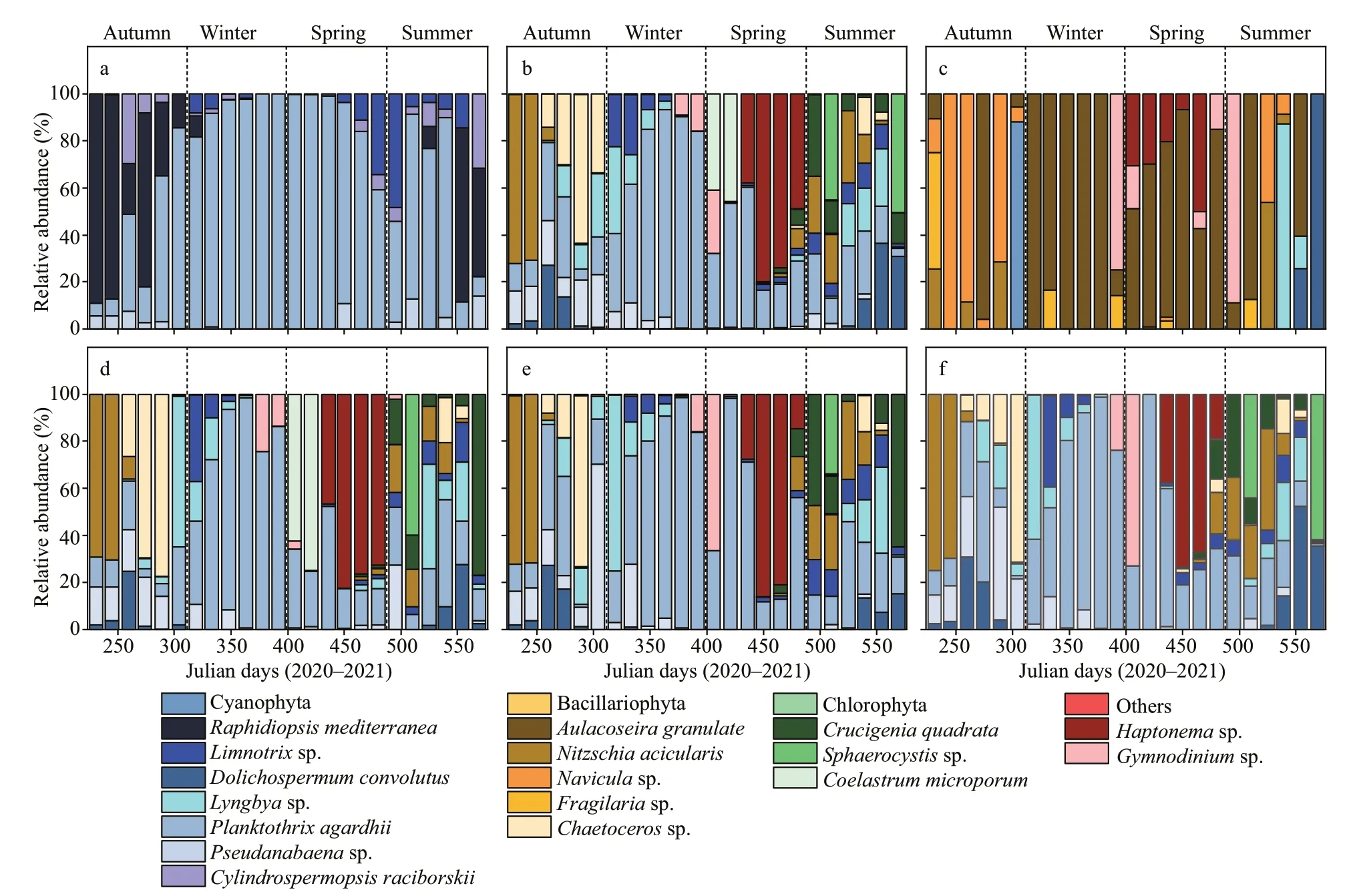
Fig.4 Relative abundance of dominant phytoplankton groups in the closed (a) and semi-closed (b) lakes situated in the Jiangxin Islet and that in the Oujiang River near the exit (c); d, e, and f show the phytoplankton species composition in the semi-closed Gongqing Lake at the sampling sites from the farthest to the closest from the exit
Sites S2 (Fig.3d), S3 (Fig.3e), and S4 (Fig.3f)in the semi-closed lake showed similar patterns in phytoplankton abundance, and the annual averages were 2.52×107, 1.93×107, and 1.84×107cells/L(Fig.3d-f), respectively. The total phytoplankton population consisted of cyanobacteria, green algae,diatoms, and other species, and their abundance exhibited a decreasing trend from S2 to S4 (Fig.3d-f).
3.3 Relative abundance of diff erent phytoplankton groups
In the closed lake, cyanobacteria were the most dominant group during the entire study period(Fig.4a). The relative abundance of diatoms, green algae, and other species accounted for only 0.79%,5.17%, and 1.14%, respectively. Filamentous cyanobacteriumP.agardhiiwas the most dominant species from September 2020 to February 2021(Julian day 304-465), and peaked at 1.20×108cells/L and a relative abundance of 98.8% on January 26,2021 (Julian day 392).
In the semi-closed lake, cyanobacteria dominated,and the relative abundance of other algae increased signif icantly during the study period (Fig.4b).Planktothrixagardhiiwas replaced byHaptonemasp. (Haptophyta) as the most abundant species on January 29, 2021 (Julian day 436). In addition,diatoms began to dominate in summer and autumn.Among them,N.aciculariswas the most dominant species on June 29, 2020 (Julian day 231) with the highest abundance of 2.15×107cells/L, accounting for a relative abundance of 72%. In addition, the brackish water diatomChaetocerossp. began to be dominated in autumn and reached a peak abundance of 7.44×107cells/L, equivalent to a relative abundance of 63.4%. Green algae dominated in winter and summer, andCrucigeniaquadrata,Sphaerocystissp.,andCoelastrummicroporumassumed the dominant position alternately (Fig.4b).
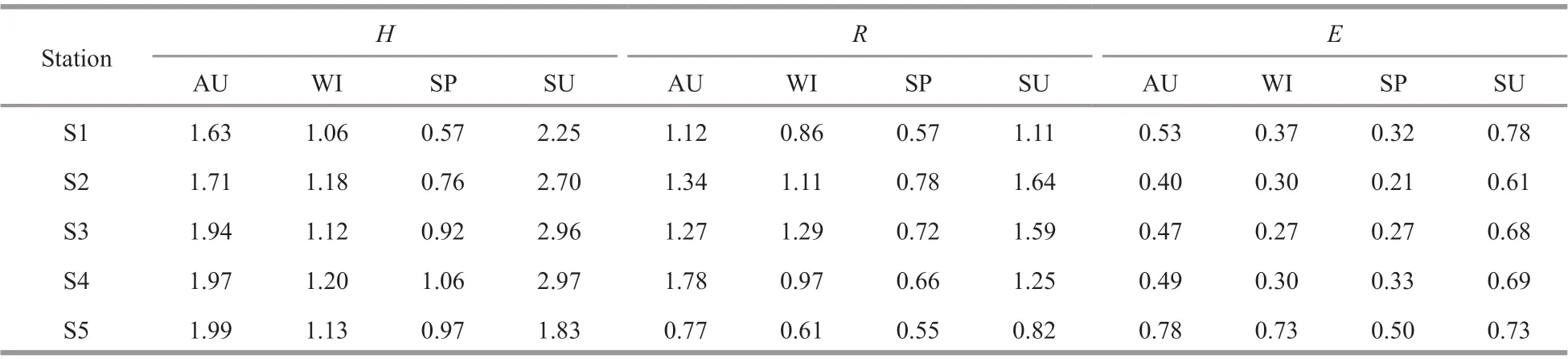
Table 2 Changes in the indices of species diversity ( H), species richness ( R), and species evenness ( E) of phytoplankton in diff erent sites and seasons
In the Oujiang River, the diatomAulacoseiragranulatewas the most dominant species during most of the study period, with the highest abundance of 4.42×105cells/L and a relative abundance of 60.5%.However, in some of the months, cyanobacteria(Dolichospermumconvolutus), diatom (Naviculasp.), euglena (Gymnodiniumsp.), and haptonema(Haptonemasp.) also became the dominant species in the Oujiang River (Fig.4c).
Furthermore, for the sampling stations S2 (Fig.4d),S3 (Fig.4e), and S4 (Fig.4f), cyanobacteria were still the dominant phytoplankton during the study period.Nevertheless, diatoms, green algae, and other algae(especiallyHaptonemasp. andGymnodiniumsp.)were the dominant groups for nearly half of the study time.
3.4 Diversity indices of phytoplankton species
The diversity index (H) of the phytoplankton species in the closed lake, the semi-closed lake, and the Oujiang River was 0.57-2.25, 0.76-2.97, and 0.97-1.99, respectively (Table 2). In addition, the diversity index showed a season-dependent pattern,with the highestHvalue in summer, followed by autumn, winter, and spring. For the sampling sites,the order of increasingHindex was S2 < S3 < S4. The richness (R) in the closed lake, the semi-closed lake,and the Oujiang River was 0.57-1.12, 0.66-1.78, and0.55-0.82, respectively, displaying a similar trend in temporal and spatial changes to that ofH. The evenness (E) of phytoplankton species in the closed,the semi-closed lakes and the Oujiang River was 0.32-0.78, 0.21-0.69, and 0.50-0.78, respectively,during the study period and it showed the same season-dependent patterns to that ofH(Table 2).
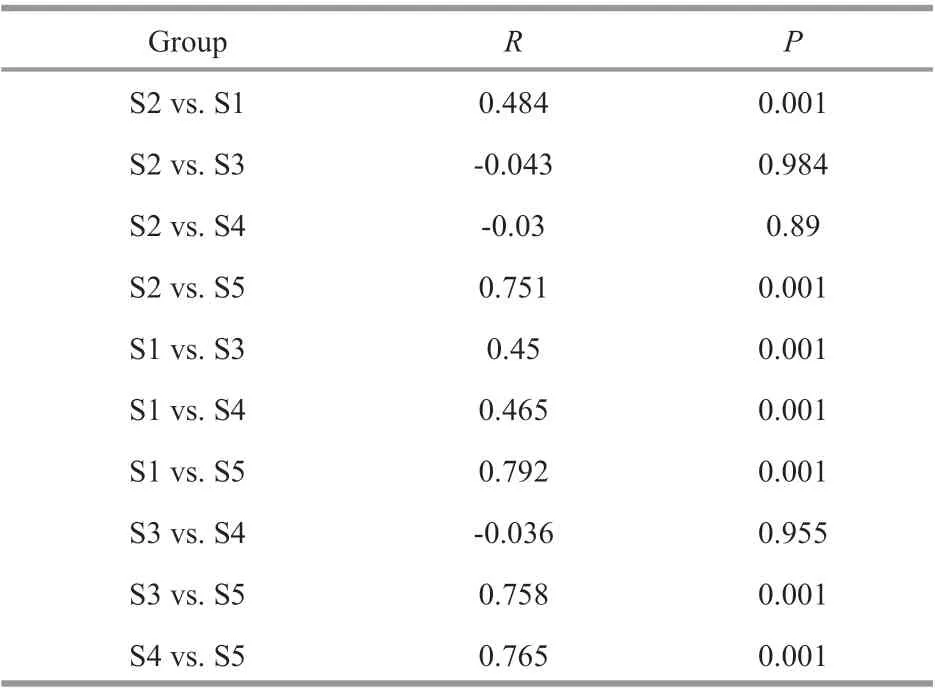
Table 3 Statistics of the analysis of similarity (ANOSIM)testing diff erences of phytoplankton community composition in diff erent stations
3.5 Relationship between phytoplankton communities in the closed and semi-closed lakes situated in the Jiangxinyu Islet
Phytoplankton communities were closely clustered, and the community composition at S1 and S5 sites were signif icantly separated from the other sites (Fig.5; Table 3). Although the two lakes are very close with a straight-line distance of about 50 m, the phytoplankton communities in the closed lake were signif icantly diff erent from those found in the semi-closed lake. Furthermore, the phytoplankton community at S2 was more similar to that at S1 than those at other sampling sites in the semi-closed lake.However, the phytoplankton communities among sampling sites in the semi-closed lake did not show a signif icant diff erence.
3.6 Physicochemical parameter
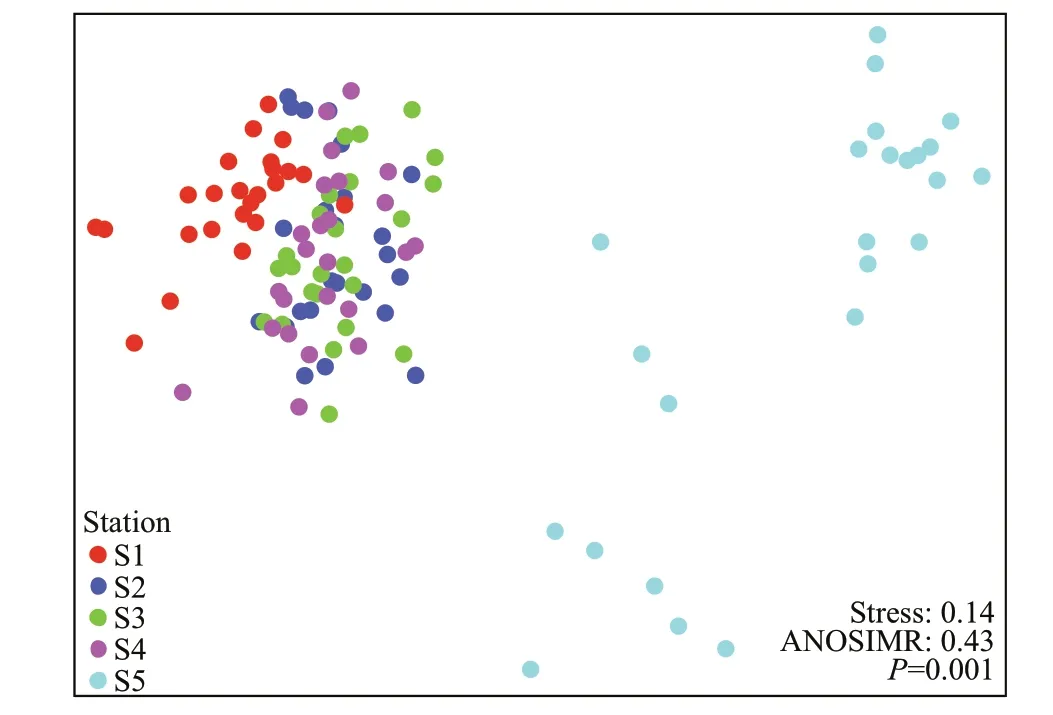
Fig.5 Non-metric multidimensional scaling (NMDS) of phytoplankton community composition in the f ive sampling sites in the closed lake (S1), semi-closed lake(S2, S3, S4), and the Oujiang River near the exit (S5)
The concentrations of chlorophyllain the water at the surface of both lakes and the Oujiang River exhibited a similar trend with a peak in summer.In addition, during a same sampling time, the chlorophyll-aconcentration in the closed lake was higher than that in the semi-closed one, and both were above that of the Oujiang River (Fig.6a).The concentration of chlorophyllain the closed lake, the semi-closed lake, and the Oujiang River were 12.7-87.8, 8.4-52.4, and 2.2-11.3 μg/L,respectively.
Surface water temperature presented an obvious seasonal pattern and showed no signif icant diff erences among diff erent sites (Fig.6b). Salinity in the Oujiang River was higher than that in the semi-enclosed lake,and both were higher than that in the enclosed lake except in summer due to high f looding tides (Fig.6c).The degree of water transparency in the closed lake was higher than that in the semi-closed lake, and both were much higher than that in the Oujiang River(Fig.6d). A similar concentration of TN was found in the water of both lakes, and this TN concentration was much lower than that detected in Oujiang River during the entire study period (Fig.6e). However, there was no signif icant diff erence in TP concentration in the water between the two lakes, and the water of both lakes showed a similar TP concentration to that found in the Oujiang River except for autumn (Fig.6f).
4 DISCUSSION
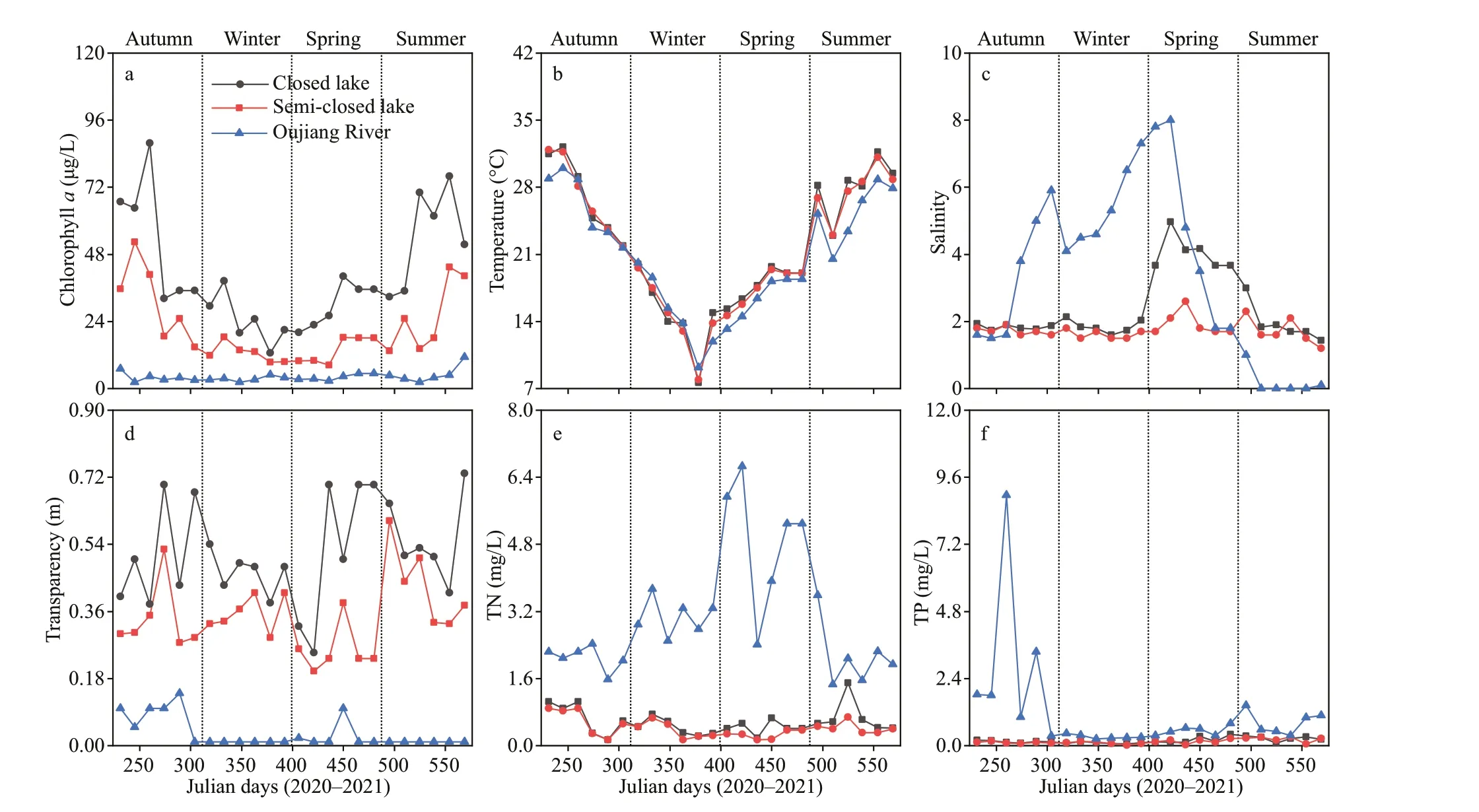
Fig.6 Water environmental parameters in the closed lake, the semi-closed lake, and the Oujiang River
Phytoplankton communities in freshwater ecosystems usually exhibit a stronger response to environmental changes and are considered good indicators of environmental change and ecosystem status because of their fast responses to environmental disturbances (Liu et al., 2015; Xue et al., 2018). The mixing of water bodies is also considered an eff ective method for controlling cyanobacterial blooms(Visser et al., 1996, 2016). In the present study, we found that the abundance, diversity, and dominant phytoplankton species in the semi-closed lake were profoundly aff ected by the water exchange caused by tides compared with those in the closed lake. In addition, the overwhelmingly dominant position of cyanobacteria throughout the year was weakened and tended to be replaced by diatoms and green algae in some of the months, thereby reducing the risk of cyanobacterial blooms.
The tide-dependent water exchange had little eff ect on the species composition of phytoplankton community between the closed and semi-closed lakes, but it exerted signif icant eff ects on the abundance, dominance, and diversity of the dominant species. It is well known that gas vesicles contained in the cyanobacterial cells can provide the cells with buoyancy in an aqueous environment, and give them an advantage when competing for light and CO2(Walsby, 1975; Pfeifer, 2012). Therefore, dense cyanobacterial blooms usually occur in lakes and reservoirs with stagnant waters, little wind mixing,and small f luctuations in the water level (Huisman,2018). In addition, if the vertical mixing rates exceed the f lotation velocities of the cyanobacteria, then it can no longer benef it from the gas vesicles that provide buoyancy, and they tend to be replaced by diatoms and green algae (Huisman et al., 2004; Visser et al., 2016).
In the present study, the numbers of species and taxonomical groups of phytoplankton found in the closed and semi-closed lakes are similar. However, the abundance of the total phytoplankton and dominance of cyanobacteria profoundly decreased and those of diatoms and green algae appreciably increased in the semi-closed lake compared with the closed lake,and the trends were more obvious in Site S4 near the exit where brackish water of the Oujiang River f lowed into the semi-closed lake during the period of astronomical tides. The diversity indexHand richness indexRof the phytoplankton species in the semi-closed lake were higher than those in the closed one, while the evenness indexEshowed the opposite trend. This suggested that the exchange of water in the semi-closed lake signif icantly increased the diversity of the phytoplankton community and reduced the cyanobacterial dominance, and this could decrease the risk of harmful cyanobacterial blooms (Gomaa et al., 2018). In addition, there was a distinct diff erence between the compositions of the phytoplankton communities in the two lakes, with the highest degree of similarity between S1 and S2 when compared with the other sampling sites in the semi-closed lake. These diff erences showed an obvious eff ect of the tidal water exchange on the phytoplankton community of the semi-closed lake. These changes could be attributed to the turbulence caused by the water exchange. In addition, it is reported that cyanobacteria are more easily replaced by diatoms and green algae under turbulence conditions (Yang et al., 2020). Furthermore,the development of cyanobacterial blooms takes time,shortening the residence time by increasing the water f low could weaken their competitive advantage,thereby, preventing the outbreak of cyanobacterial blooms (Verspagen et al., 2006; Mitrovic et al., 2011).
Chlorophyllais generally regarded as an eff ective indicator to algal blooms (Guo et al., 2018; Ding et al., 2020), and a concentration of 10 μg/L is usually regarded as the threshold for triggering algal bloom although some studies have selected higher thresholds,such as 20 μg/L and 30 μg/L (Wu and Xu, 2011; Liao et al., 2021). In the present study, the chlorophyllaconcentration in the water near the surface in the closed lake was much higher than 10 μg/L for most of the study period, and reached a peak of 87.8 μg/L in summer. This indicated that cyanobacterial blooms might occur all year round because the abundance of cyanobacteria detected during a one-year period was more than 96% that of the total abundance.Furthermore, the chlorophyll-aconcentration was higher than the threshold almost throughout the year in the semi-closed lake. In contrast, the chlorophyllaconcentration in the Oujiang River was below the threshold during the study period. Therefore,the decrease in chlorophyll-aconcentration and phytoplankton abundance in the semi-closed lake could be attributed to the exchange of water between the semi-closed lake and the Oujiang River.
Water temperature is always the main environmental factor aff ecting the growth of cyanobacterial species;and the global warming had favored the outbreak of cyanobacterial blooms (Huisman et al., 2018; Yan et al., 2020). However, in this study, water temperature varied in the same pattern and no signif icant diff erence was detected among the closed lake, the semi-closed lake, and the Oujiang River. Therefore,the signif icant decreases in phytoplankton abundance,chlorophyll-aconcentration, and diversity of dominant phytoplankton species in the semi-closed lake was unlikely to be caused by variation of water temperature.
On the other hand, the outbreak of algal bloom is mainly triggered by eutrophication. In general, TN and TP concentrations in stagnant water that exceed the eutrophication threshold of 0.2 mg/L and 0.02 mg/L,respectively, would indicate a high risk of algal bloom(Carey and Migliaccio, 2009; Guo et al., 2017; Rice and Westerhoff , 2017; Lv et al., 2020). All the TN and TP concentrations in the two lakes and Oujiang River were above the eutrophication thresholds, and were much higher in the Oujiang River than in the two lakes, indicating a much higher level of nutrients in the Oujiang River. Therefore, it was unlikely that the very low phytoplankton abundance and chlorophyllaconcentration in semi-closed lakes were caused by nutrient changes.
However, in comparison with that in the closed lake, the salinity in the semi-closed lake was signif icantly higher but the transparency signif icantly lower. In addition, it is reported that cyanobacteria are more sensitive to salinity increase than diatoms and green algae, and many cyanobacterial species are able to grow in freshwater only (Babu et al.,2020; Wiśniewska et al., 2021), thus the decrease in cyanobacterial abundance observed in the semiclosed lake was the result of higher salinity due to the mixing with seawater. The lower transparency in the semi-closed lake caused by water exchange with the Oujiang River suppressed the growth of phytoplankton and thus decreased the phytoplankton abundance and chlorophyll-aconcentration. Therefore, it is reasonable to speculate that the changes in salinity and transparency in the semi-closed lake shaped the phytoplankton community structure and reduced the risk of cyanobacterial blooms to a certain extent.
5 CONCLUSION
The water exchange caused by tidal movement signif icantly decreased the dominance of cyanobacterial species, and thus rose the dominance of diatoms and green algae in the semi-closed lake. In addition, the diversity index and richness index of the phytoplankton species in the semi-closed lake were higher than those in the closed lake, while the evenness index showed the opposite trend, indicating that water exchange was benef icial to the growth of diatoms and green algae, thereby increasing the diversity of phytoplankton species. The NMDS and ANOSIM analysis also illustrated a signif icant diff erence in phytoplankton community between the two lakes.The high salinity and low transparency of water in the semi-closed lake were duo to the variation in phytoplankton community structure, which reduced the risk of cyanobacterial bloom in the lake. These f indings provided a good example and insightful clue that the outbreak of cyanobacterial blooms could be prevented and controlled by applying hydrodynamic methods.
6 DATA AVAILABILITY STATEMENT
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
7 ACKNOWLEDGMENT
The authors highly appreciate Dr. Alan K CHANG(Wenzhou University) for his eff ort in the linguistic revision of the manuscript, and the anonymous reviewers for their very useful and insightful comments.
 Journal of Oceanology and Limnology2022年5期
Journal of Oceanology and Limnology2022年5期
- Journal of Oceanology and Limnology的其它文章
- Comparison of three f locculants for heavy cyanobacterial bloom mitigation and subsequent environmental impact*
- Eff ect of light intensity on bound EPS characteristics of two Microcystis morphospecies: the role of bEPS in the proliferation of Microcystis*
- Community structure of aerobic anoxygenic phototrophic bacteria in algae- and macrophyte-dominated areas in Taihu Lake, China*
- Eff ect of random phase error and baseline roll angle error on eddy identif ication by interferometric imaging altimeter*
- Estimating the evolution of sea state non-Gaussianity based on a phase-resolving model*
- Sodium acetate can promote the growth and astaxanthin accumulation in the unicellular green alga Haematococcus pluvialis as revealed by a proteomics approach*
