Seasonal shifts in assembly dynamics of phytoplankton communities in a humans-aff ected river in NE China*
Zhenxiang LI , Xinxin LU ,2,**, Yawen FAN ,2,**
1 College of Life Science and Technology, Harbin Normal University, Harbin 150025, China
2 Key Laboratory of Biodiversity of Aquatic Organisms, Harbin Normal University, Harbin 150025, China
Abstract Identifying seasonal shift in phytoplankton community is essential for understanding the signif icance of eutrophication and f inding biological indicators of ecological health of a lotic system.Phytoplankton communities, as well as the seasonal changes in the Ashi River Basin (ASRB) of Heilongjiang Province were investigated from April 2018 to January 2019. A survey in April (spring), July (summer),October (autumn), and January (winter) at 16 sampling sites was conducted. The composition, abundance,and biodiversity indices of phytoplankton were studied and 127 taxa of phytoplankton were identif ied.Among them, Bacillariophyta dominated the phytoplankton communities in the whole year. There were signif icant spatio-temporal changes in the structures of the phytoplankton communities during the study period. Trophic state index (TSI) show that the nutritional status of the ASRB was at mesotrophic-middle eutrophic levels. Redundancy analysis (RDA) revealed that total nitrogen (TN), water temperature (WT),oxidation reduction potential (ORP), pH, and dissolved oxygen (DO) were the critical factors in the dynamic phytoplankton community structure. The multivariate regression tree (MRT) analysis showed that Chlamydomonas microsphaerella Pascher et Jahoda, Melosira granulata (Ehrenberg) Ralfs, Merismopedia tenuissima Lemmermann, and Asterionella formosa Hassall were valuable indicators in the determination of water quality in ASRB. Our f indings provide a scientif ic basis for water quality protection and management at basin scale.
Keyword: Ashi River Basin; eutrophication; community structure; succession; indicator
1 INTRODUCTION
The spatial and temporal distribution pattern of phytoplankton is a comprehensive indicator of ecological health (Cao et al., 2018). Community succession pattern is an eff ective tool for the control and management of the health of river ecosystems and an essential indicator of the restoration and reconstruction of degraded ecosystems (Huang et al., 2012). Phytoplankton is essential for primary productivity in river ecosystems, and their diversity and community structures can usually be used to determine the responses to the changes in nutrient levels in environment (Litchman et al., 2010). For example, local environmental conditions, temperature,light, grazing pressure, and nutrient supply strongly regulate phytoplankton reproduction and primary production through bottom-up and top-down controls(Kerimoglu et al., 2013). Phytoplankton occupies a unique niche in the ecosystem and plays an essential role in the biogeochemical cycle (Aufdenkampe et al., 2011; Ye et al., 2013). The phytoplankton community distribution pattern is widely used for the assessment of water quality in aquatic ecosystems(Zhao et al., 2020). Sommer et al. (2012) used the well-known Plankton Ecology Group (PEG) model to describe the seasonal changes in the richness and density of phytoplankton in a temperate mesotrophic lake. Since then, this model has been applied in many comparative studies of diff erent types of lakes and rivers (Varol, 2019). However, there are few studies on the species composition and community structure changes of phytoplankton in rivers inf luenced by anthropogenic activities in NE China.
Eutrophication is an abnormal change in structure and function of an aquatic ecosystem and the apparent deterioration of water environment when a lake contains excessive nutrients, especially nitrogen and phosphorus (Wu et al., 2019). Globally, eutrophication is the most pervasive water quality challenge as seem in severe algae blooms (Foden et al., 2011). Recently,more studies focused on using phytoplankton as a tool for the assessment of eutrophication (Foden et al., 2011). Compared with the physical and chemical indices, phytoplankton is widely distributed and abundant, and responds quickly to environmental changes in the ecosystem; and the phytoplankton community structure is an excellent biological indicator to trophic state (Abell et al., 2010).Phytoplankton community structures provide unique information on the condition of aquatic ecosystem,and their succession traits can be used to indicate changes in water quality (Holopainen et al., 2008).Moreover, the appearance of some indicator species can eff ectively ref lect the state of water quality in the area (Stenger-Kovács et al., 2007). Accordingly,phytoplankton was used to assess water quality in the Water Framework Directive (WFD, 2000/60/EC). Rivers are lotic ecosystems, and can not only irrigate the land, but also undertake transportation roles. Anthropogenic activities along rivers lead to hydrological alterations and further the changes in local phytoplankton communities. Discharge of many pollutants decrease diversity and stability of phytoplankton community structure, thus aff ecting the biological integrity of the ecological service functions of river ecosystem (Sabater-Liesa et al., 2018).Understanding ecological changes in the regularity of rivers under human pressure requires countermeasure for sustainable watershed management. However,such studies in the ASRB were few.
With fast industrialization and urbanization,rivers in Heilongjiang Province have been polluted to varying degrees in recent years. China’s Eco-Environmental Status Report (2018) shows that water of the Songhua River Basin was slightly polluted. The Ashi River is a primary tributary to the Songhua River.The Xiquanyan Reservoir located in the upper reaches of the river is a backup water source for Harbin City,the capital of Heilongjiang Province in NE China.Recently, water pollution in the ASRB has gradually attracted more and more attention. The water quality of ASRB had been documented in several studies (Guo et al., 2005; Ma et al., 2015). However, systematic studies on phytoplankton community succession and the use of phytoplankton communities as indicators of water quality are scarce. This study examines the seasonal succession pattern of the phytoplankton community and the environmental factors driving the seasonal succession of the phytoplankton community.We hypothesized that: (1) the PEG model is a relevant approach for studying the traits of the phytoplankton community succession in ASRB; (2) indicator species can respond to water pollution in the ASRB. This study was to develop the succession matrices of phytoplankton community, to explore the eff ect of environmental factors on phytoplankton community assembly, and to determine relevant phytoplankton indicators to water quality.
2 MATERIAL AND METHOD
2.1 Study area and sampling sites
The Ashi River is located in the south of the Songhua River Basin, some 80 km from Harbin.The study areas are located between longitude 126°43′E-127°36′E and latitude 45°08′N-45°50′N,in total length of 213 km (Fig.1), along which the Xiquanyan Reservoir is connected in the upper part of the main section of the ASRB. The area features a temperate continental monsoon, with below-freezing temperatures from November to April in ASRB, and the annual average temperature is 3.6 °C. Sixteen sites along the river were selected for sampling.S13-S16 were located upstream of the river mostly forest covered; S15-S16 were in the water inlet of the reservoir, and S14 was in the outlet of the Xiquanyan Reservoir (Fig.1; Supplementary Table S1). Sites S1-S9 were inf luenced by domestic sewage discharge and industrial pollution, while sites S10 to S12 were aff ected by agricultural activity. Many small- and medium-sized enterprises were located in this area,thus industrial wastewater was the main source of pollution. Samples were collected every three months from April 2018 to January 2019, representing the water conditions in spring, summer, autumn, and winter. However, due to meteorological reasons in the winter, samples were not collected at some sites.
2.2 Environmental data
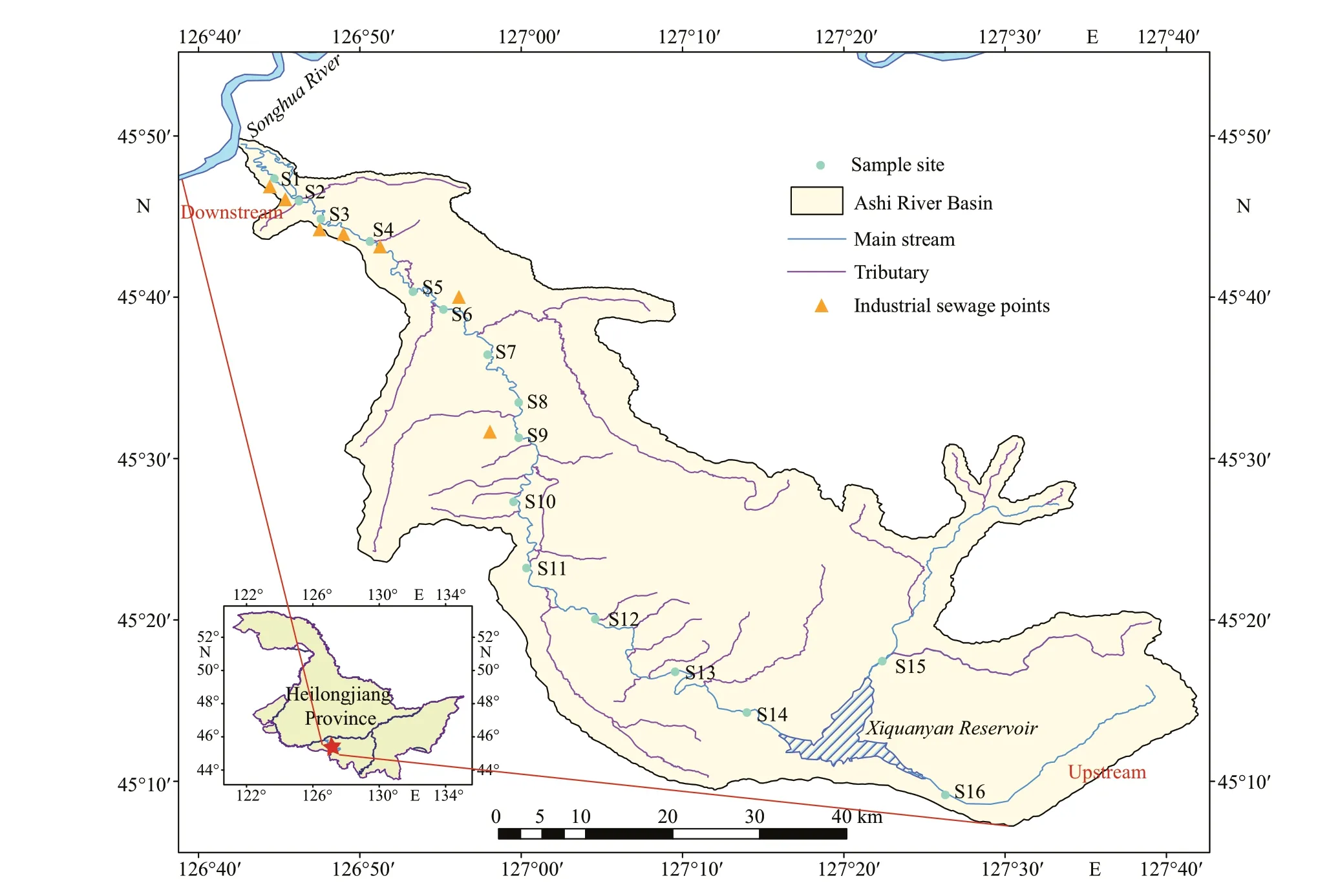
Fig.1 Map of ASRB indicating sample sites
Surface water samples (0-0.5 m) were collected from each sample site using brown glass bottles in triplicate for analysis. Phytoplankton samples (1 L)were immediately f ixed with 1.5% acidic Lugol’s solution and stored in the dark at 20 °C until further processing. In this study, a multi-parameter water quality analyzer (Hydrolab DS5, Hach Company,Loveland, CO, USA) was used to record physical and chemical properties in all sample sites, such as water temperature (WT), dissolved oxygen (DO),electrical conductivity (Cond.), pH, and oxidationreduction potential (ORP). The chemical indicators of the water environment, including total phosphorus(TP) (MEP, 1989a), total nitrogen (TN) (MEP, 2012),potassium permanganate index (CODMn) (MEP,1989b), and biochemical oxygen demand after 5 days(BOD5) (MEP, 2009), were measured following the corresponding standard methods.
2.3 Plankton analysis
Before counting species, the f inal volume was concentrated from 1 L to 30 mL by siphoning and thorough shaking, after which a 100-μL plankton counting box chamber was used for counting.Phytoplankton identif ication was conducted at 400×magnif ication using a light microscope (Optec B302,Chongqing, China) (Yuan et al., 2018).
2.4 Calculation of diversity indices
The dominant species of phytoplankton were determined based on the dominance value,Y, for each species (Guo et al., 2005; Ma et al., 2015) as follows:
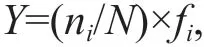
whereniandNare the numbers of individuals of speciesiand the total number of individuals of all species within site,fiis the occurrence frequency of the speciesi.
Shannon-Wiener index (Shannon et al., 1949),Margalef richness index (Margalef, 1967), the Pielou evenness index (Pielou, 1966), and the Simpson diversity index (Simpson, 1949) were also calculated.
2.5 Data analysis
Statistical analyses were performed to quantitatively determine the impacts of environmental factors on the phytoplankton. All data were logarithmically transformed to obtain factors of equal weight. A detrended correspondence analysis (DCA) was f irst performed to test the character of variability in the phytoplankton assemblage. The length of the f irst DCA gradient was 2.0 standard deviations for our dataset, indicating that the species responded linearly to the environmental factors. It justif ied the further use of the redundancy analysis (RDA). DCA and RDA were performed using CANOCO software,Canonical Community Ordination version 4.5 for Windows. The signif icance analysis was performed using SPSS 20.0 for Windows (SPSS Inc., Chicago,Illinois). Independent-samplest-test was conducted to evaluate the diff erences in the environmental data and species abundance. Statistical signif icance was set at *P<0.05, **P<0.01.
The impacts of environmental variables on microbial diversity were conducted by multivariate regression tree (MRT) analysis. A 1 000 cross-validation process was used to decrease the structural complexity of MRT and predict the critical relationship between multispecies data and environmental variables; it was carried out with the packages ‘mvpart’ and‘MVPARTwrap’ within the ‘R’ program.
A comprehensive trophic state index (TSI) was used to describe the trophic status in ASRB (Wang et al., 2002). The equations for TSI were as follows:
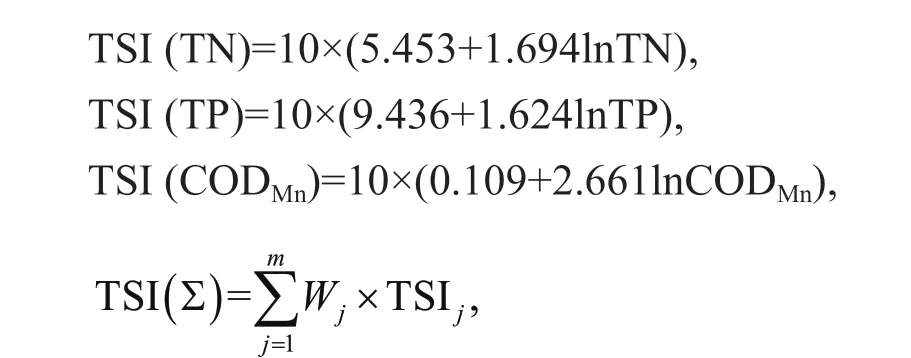
where TSI(∑) is trophic state index,mis evaluate the number of parameters,Wjis correlation weights of the nutrient state index for thejthparameter, TSIjis nutritional state index for thejthparameter.
Evaluation standard: 0<TSI<30 oligotrophic,30≤TSI≤50 mesotrophic, TSI>50 eutrophic,50<TSI≤60 light eutrophic, 60<TSI≤70 middle eutrophic, TSI>70 high eutrophic. The interpolation map was constructed by ArcGIS software using the inverse distance weighting method.
3 RESULT
3.1 Physical and chemical properties and the trophic status of ASRB
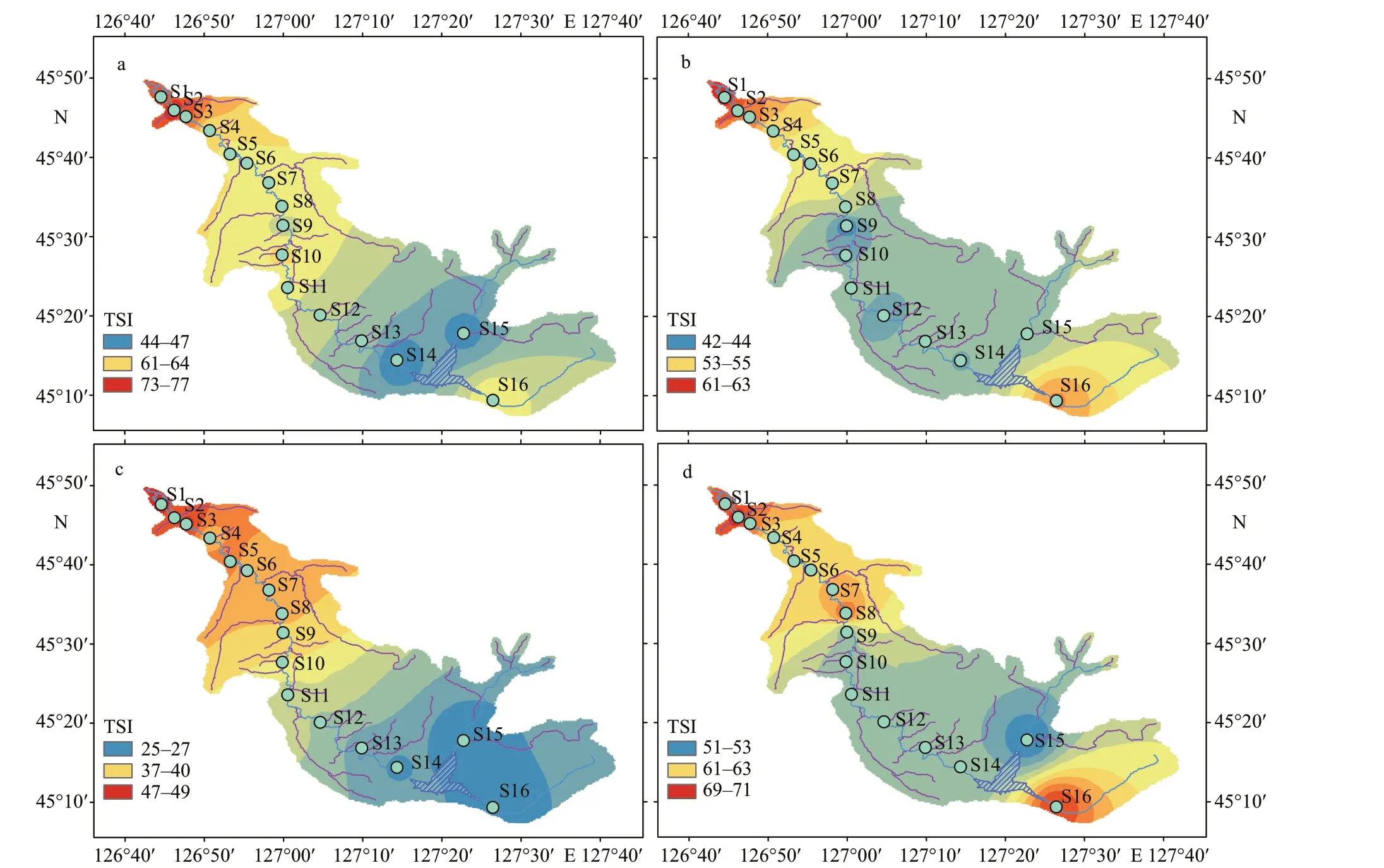
Fig.3 Spatial distribution of trophic state index in ASRB during diff erent seasons
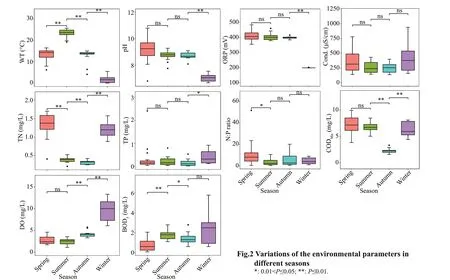
Most of the physical and chemical factors had signif icant diff erence in diff erent seasons (Fig.2;Supplementary Table S2). Generally, in spring,the values of pH and TN were higher than those in other seasons, while the values of DO and BOD5were lower than those in other seasons. There was no signif icant diff erence in pH, ORP, Cond., and TP between summer and autumn, but TN, CODMn, and BOD5were signif icantly higher in summer than in autumn. Although WT, pH, and ORP in winter were the lowest throughout the year, TP, DO, Cond., and BOD5were higher than those in other seasons. The WT ranged from 25.62 °C to -0.02 °C, reaching its highest value in summer. The water was alkaline,the pH values ranged from 10.78 to 6.68, and there was no signif icant diff erence in the values between summer and autumn. The values for Cond. varied from 136 μS/cm (S14 in autumn) to 939 μS/cm (S2 in winter). ORP varied from 199 mV to 434 mV, the highest at S11. TN was higher in the spring and winter months than in summer and autumn. The highest TN concentrations were found at S1 (1.7 mg/L). TP did not change signif icantly in spring, summer, and autumn. The highest TP concentrations were mainly recorded in winter: ranging from 0.02 to 2.40 mg/L.The N∶P ratio varied from 0.52 (S1 in summer) to 51.64 (S15 in spring), and the average value in spring was the highest. CODMnconcentrations ranged from 1.57 mg/L to 10.31 mg/L, the highest value was found at S16 in winter, followed by S1 (9.89 mg/L) and S3(9.87 mg/L) in spring, and the lowest value was found at S16 in autumn. DO values ranged from 1.05 mg/L to 12.31 mg/L, and the values in autumn and winter were signif icantly higher than those in spring and summer. The BOD5was signif icantly lower in spring,and the highest value was found at S16 (6.40 mg/L) in winter, followed by S1 (5.80 mg/L) in spring, and the lowest value was found at S12 (0.07 mg/L) in spring.
The seasonal and spatial variations of TSI are shown in Fig.3. The trophic state of the water in the ASRB was best in autumn, followed by summer,spring, and winter. The upstream water quality of the ASRB was better than that of the downstream. The TSI values in winter and spring were 61.21±7.08 and 59.26±8.52, respectively, being signif icantly higher than those in summer and autumn (P<0.05). It was at the middle eutrophic level in winter, light eutrophic level in spring and summer. The nutritional status in autumn was better than that in other seasons. In relation to the spatial distribution, the area near the reservoir (S14-S16) has a low nutritional status and was at oligotrophic to light eutrophic levels; the nutritional status of the lower reaches of the river(S1-S7) was signif icantly higher than that of other areas (P<0.05), which is at the mesotrophic to high eutrophic levels.
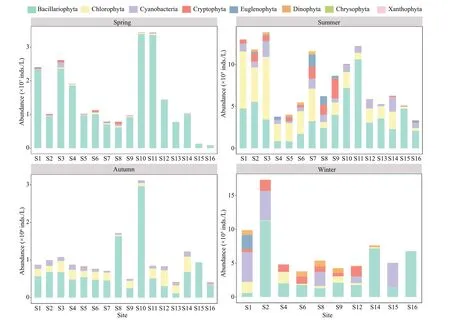
Fig.4 Variation in abundances of phytoplankton during the study period
3.2 Phytoplankton assemblage and seasonal succession
In this study, 127 taxa of phytoplankton belonging to 73 genera were identif ied including 66 species of Chlorophyta, 33 species of Bacillariophyta, 16 species of Cyanobacteria, 4 species of Cryptophyta, 4 species of Euglenophyta, 2 species of Dinophyta, 1 species of Chrysophyta, and 1 species of Xanthophyta.According to the dominance index (Y≥0.02),37 species of dominant algae, with dominance indexes between 0.02 and 0.81 (Supplementary Table S3) were identif ied. Five algae, namelyCyclotella meneghinianaKützing,Navicula radiosaKützing,Nitzschia palea(Kützing) W. Smith,Limnothrix redekeiVan Goor, andPseudanabaena limnetica(Lemmermann) Komárek were dominant throughout the year.The phytoplankton abundance in spring reached the highest compared to other seasons.The highest abundance appeared at S10 in spring(3.45×107inds./L) and the lowest at S16 in summer(3.35×105inds./L; Fig.4).
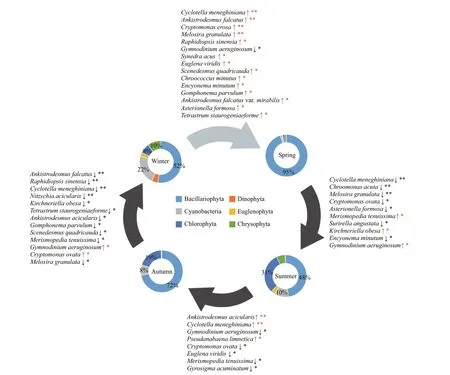
Fig.5 Seasonal shifts in the assembly dynamics of phytoplankton communities in ASRB
Succession at the species level was compared among the four seasons (Fig.5). There were signif icant diff erences (P<0.05) between adjacent seasons among the 24 dominant species. The diatoms predominated the assemblages in spring, accounting for 95% of the total abundance. From spring to summer, f ive of the diatoms(C.meneghiniana,Asterionellaformosa,Encyonema minutum(Hilse) Mann,Surirella angustataKützing,andM.granulata) had signif icantly decreased.Then, there was a sharp increase in Chlorophyta and Cyanobacteria in summer, as they dominated by 31% and 10% respectively, includingMerismopedia tenuissima, andKirchneriella obesa(W. West)Schmidle. In autumn,M.tenuissimadecreased, whileAnkistrodesmus acicularis(A. Braun) KorschikoffandP.limneticaincreased signif icantly. Although the status of diatom had gradually increased (from 48%to 72%), the abundance ofGyrosigma acuminatum(Kützing) Rabenhorst had decreased signif icantly.In the following autumn and winter, the dominance of the diatoms declined again. Dominant algae, such asC.meneghinianaKützing,Nitzschia acicularis(Kützing) W. Smith,Gomphonema parvulum(Kützing) Kützing, andM.granulata, decreased signif icantly. Chlorophyta and Cyanobacteria(Ankistrodesmus falcatus(A. Braun) Korschikoff ,Raphidiopsis sinensiaJao,K.obesa,Tetrastrum staurogeniaeforme(Schroeder) Lemmermann,A.acicularis,Scenedesmus quadricauda(Turpin)Brébisson, andM.tenuissima) also decreased signif icantly. Meanwhile,Gymnodinium aeruginosumSteinandCryptomonas ovataEhrenberg increased signif icantly. In the transition from winter to spring,the abundance of diatoms, includingC.meneghiniana,M.granulata,E.minutum,G.parvulum,Synedra acusKützing, andA.formosaincreased signif icantly.A.falcatus,R.sinensia,S.quadricauda,Chroococcus minutus(Kützing) Nägeli,Ankistrodesmus falcatusvar.mirabilis(West & West) G. S. West, andT.staurogeniaeformealso increased. At the same time,G.aeruginosumsignif icantly decreased from winter to spring.
3.3 Phytoplankton diversity indices
In this study, we calculated the multiple diversity indexes of the ASRB (Table 1). The Shannon Wiener(H′) indices varied from 0.6 to 4.29. The highest value appeared at the S1 (4.29) in summer, and the lowest values at S11 (0.60) and S10 (0.64) in spring.The Margalef index (Dm) ranged from 0.85 to 4.47,with the maximum occurring at S1 in summer, and the minimum occurring at S15 in winter. Species evenness index (J) varied from 0.12 (S11 in spring)to 0.84 (S13 in autumn), and average values were the lowest in spring. For the Simpson index (D), the highest value was obtained in S1 and S7 (0.92) in summer, and the lowest value appeared at S11 (0.13)in spring.
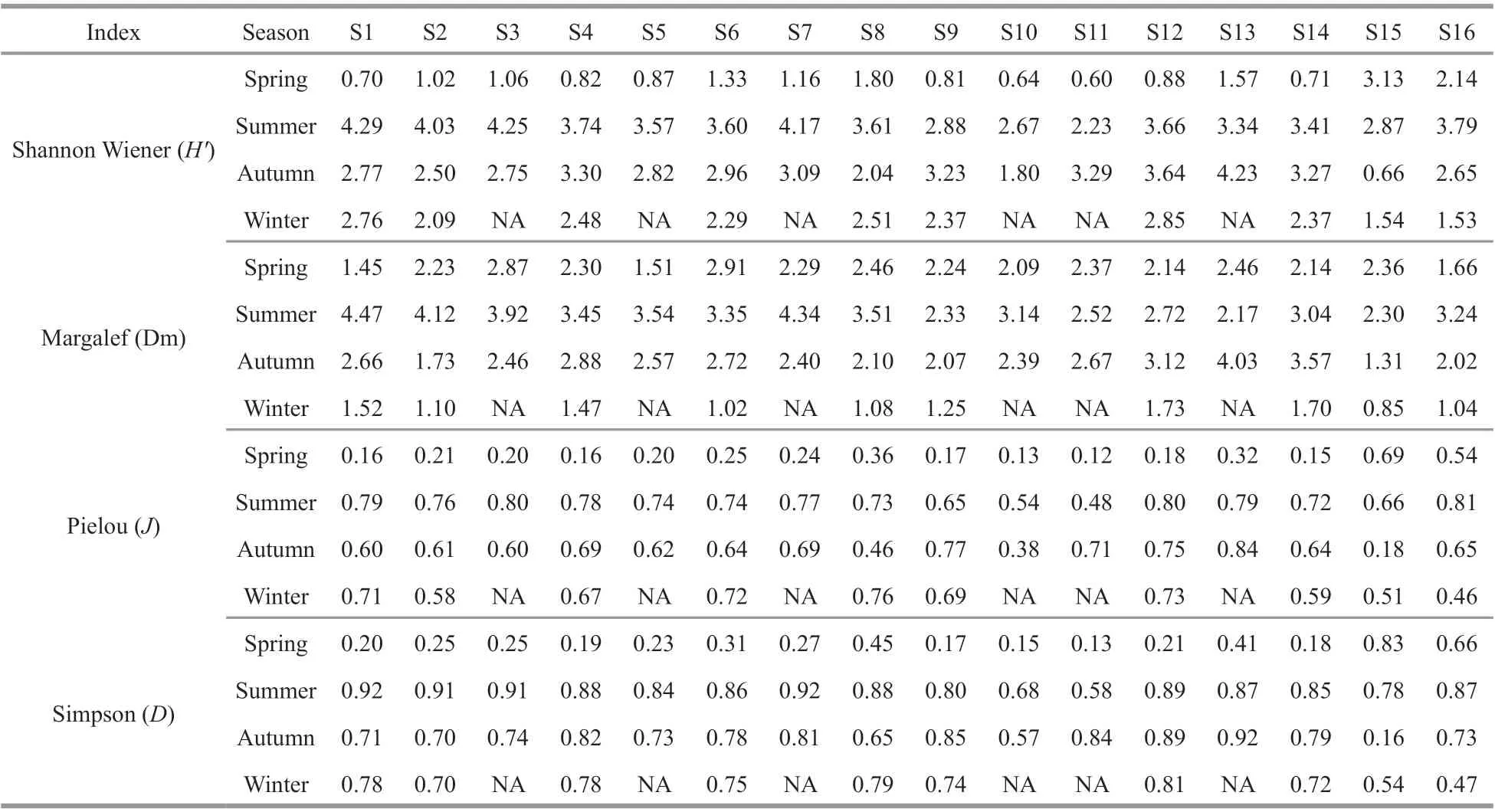
Table 1 α-diversity indexes of phytoplankton in ASRB during the study period
3.4 Relationship between phytoplankton and environmental factors
The redundancy analysis (RDA) shows the relationship between phytoplankton communities and the environmental factors. The eigenvalues of the f irst two axes were 0.128 and 0.096, accounting for 34.6%and 60.6%, respectively, of the cumulative variance of the relationship between species-environmental variables (Fig.6). Based on the results, the TN(R2=0.937 8) contents were strongly related to axis 1, while DO (R2=0.881 4), ORP (R2=-0.827 2), pH(R2=-0.792 1), and WT (R2=-0.702 3) were strongly related to axis 2.
Figure 6a shows an apparent seasonal variation during the study period. The ordination graph clearly shows that the succession of the phytoplankton community are mainly driven by physical and chemical factors. It shows that the succession of phytoplankton was under the inf luence of CODMnand TN in spring, and is restricted by water temperature in summer. In winter, the phytoplankton communities are closely associated with the concentration of DO.
As shown in Fig.6b, two predominant species,S.angustataandEuglena viridisEhrenberg, are highly positively correlated with CODMn.Chroomonas acutaUtermhöl is highly correlated with TN.A.falcatusandG.parvulumare inf luenced by temperature. Eight dominant species (Actinasturm hantzschiiLagerheim,A.acicularis,Ankistrodesmus angustusBernard,Spirulina sprincepsW. et G. S. West,N.radiosa,Diatoma vulgareBorger,N.palea, andL.redekei)are associated with BOD5.C.microsphaerellaandG.aeruginosumexhibit particular linkage to high DO.
3.5 MRT analysis and indicators
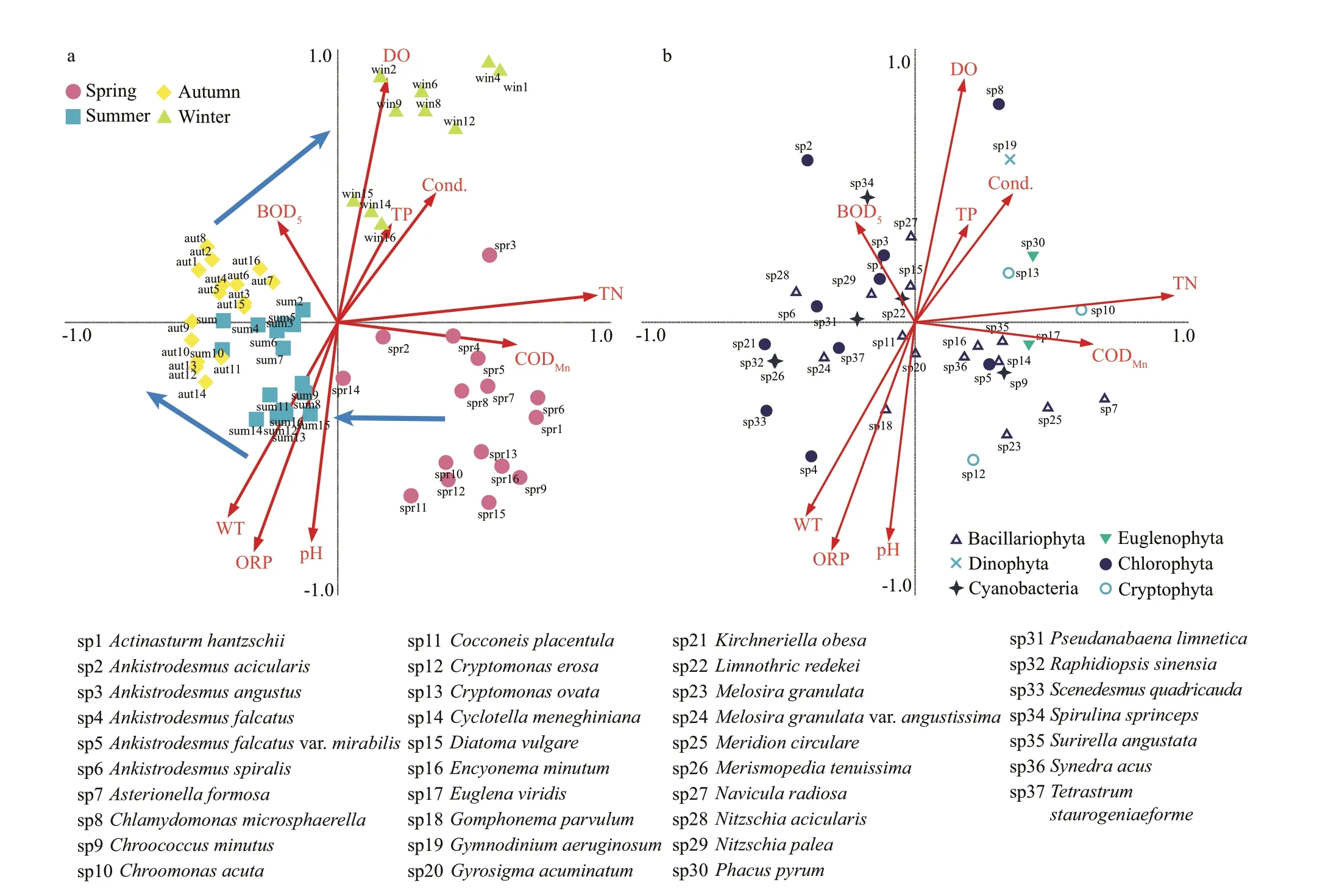
Fig.6 RDA of all sample sites during the study period
MRT analysis revealed the relationship between environmental variables and the composition of the phytoplankton community (Fig.7; Supplementary Table S4). The three groups were split based on the DO and TN. In total, the tree explains 25.4% of the variance of the phytoplankton community, suggesting that the phytoplankton community was split by the DO f irst, accounting for 13.4% of the variation, and then the phytoplankton communities in Group 1 were separated from those in Groups 2 and 3. The phytoplankton communities in Groups 2 and 3 were split by TN concentrations, accounting for 12% of the variation. Further analysis revealed that 24 of the 37 dominant species were signif icant indicators (P<0.05),among which Group 1 contained three indicators,C.microsphaerella,G.aeruginosum,A.acicularis;Group 2 contained nine indicators, includingM.granulata,M.tenuissima,S.quadricauda, andK.obesa. A total of 12 species were included in Group 3, includingA.formosa,C.ovata,C.acuta,andS.angustata.
4 DISCUSSION
4.1 Temporal-spatial variations of phytoplankton community in ASRB
Phytoplankton are located at the bottom of the food chain. They play critical roles in global carbon cycle(Zguna et al., 2019). Phytoplankton plays important roles in aquatic ecosystems, as they release oxygen during photosynthesis and aid in the energy exchange process (Lansac-Tôha et al., 2019). The temporalspatial distribution patterns of phytoplankton are
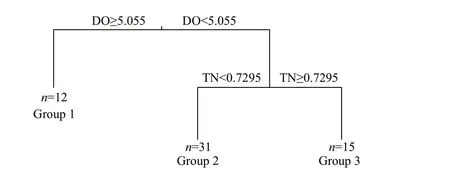
Fig.7 Multivariate regression tree (MRT) analysis of the correlation between environmental variables and phytoplankton community composition
nessential in the evaluation of the functions of aquatic ecosystems; it aff ects ecological processes, functions,and stability and ref lects changes in ecological environment (Guo et al., 2014).
Following the f irst exploration of the biodiversity of the phytoplankton community in the ASRB, 127 taxa of phytoplankton were identif ied. The richness was found higher in the ASRB than those of other aquatic ecosystems in Heilongjiang Province, such as Genhe River (61 taxa) (Li et al., 2019) and Xingkai Lake Basin (108 taxa) (Yuan et al., 2018). The highest phytoplankton abundance was recorded as 3.45×107inds./L in this study. These results might be attributed to the extreme anthropogenic activity-based ecological disturbance and a large amount of nutrient input in ASRB. Bacillariophyta and Chlorophyta were more abundant, whereas Cyanobacteria and Cryptophyta were less abundant. Bacillariophyta is considered the most critical taxonomic group in temperate rivers in terms of abundance and diversity(Rojo et al., 1994). In the rivers, Bacillariophyta and Chlorophyta usually dominate, followed by Cryptophyta and Cyanobacteria, whereas other phylum usually have less abundance (Rojo et al.,1994).
Total phytoplankton abundance in this study indicated an apparent increasing trend in spring and probably ref lects the change in nutrient concentrations.TN reaches its highest value and the N∶P ratio also reaches its highest value in spring. N∶P ratio also plays a vital role in phytoplankton reproduction(Masithah et al., 2019). The higher N∶P ratio in the ASRB promotes the reproduction of phytoplankton in spring. Storm rainfall events are usually recorded in summer in temperate rivers. Previous studies have shown that rainfall is the main natural disturbance driving hydrological changes (Bookhagen and Burbank, 2010). The amount of precipitation in summer (rainfall period) added to the river through surface runoff , leading to increase in turbidity. In addition, low light availability due to high-river discharge and fast f low velocity are the main factors contributing to the reduction of phytoplankton abundance (Bradford et al., 2013). The nutrient levels in autumn were similar to those in summer, the rainfall decreased, and water transparency increased.The average phytoplankton abundance in summer was (7.91±3.63)×105inds./L, and the abundance in autumn was (1.01±0.65)×106inds./L. During the study period, the lowest phytoplankton abundance was recorded in winter ((6.95±4.09)×105inds./L). These results can be attributed to the thicker ice layer in winter and the low light utilization for phytoplankton reproduction. Only a few diatoms and Cyanobacteria dominated in the winter. The phytoplankton Shannon Weaner, Margalef, and Simpson indexes of the ASRB were the highest in summer, followed by autumn, suggesting that the phytoplankton community structure was relatively stable in spring and autumn. These results were also conf irmed by the Pielou index in the ASRB. Although the number of phytoplankton species in spring (86) was higher than that in autumn, the dominance ofC.meneghinianain spring was signif icantly higher than that of other species, resulting in lower diversity indexes in spring than in the other seasons. Average Pielou index was 0.26±0.16 in spring, indicating that the phytoplankton community structures were unstable.
In this study, we observed that diatoms bloom in spring, then dominate throughout the year,Chlorophyta dominates in summer and autumn, while Cyanobacteria has a higher dominance in winter. The PEG model suggests that the seasonal succession of the phytoplankton community changes from Cryptophyta and Bacillariophyta in winter and spring, followed by Chlorophyta in summer and then Cyanobacteria in late summer and early autumn. These results show that although the PEG model can be used to describe the seasonal succession of phytoplankton communities in deep-water lakes in the temperate zone, there are specif ic diff erences in the ASRB. Diatoms like to live in low- and medium-temperature f lowing water and are the leading ecological group in river ecosystems(Cibic et al., 2018). Under clear ice, light conditions may promote diatoms and provide conditions for the quick start of the spring bloom (Sommer et al., 2012).In this study, the order of diatom abundance changes is spring > autumn > winter > summer.
Compared with winter, the water temperature in spring gradually rises and the ice layer melts, which promotes the reproduction of diatoms. However,higher water temperature will also limit the growth of diatoms. A previous study noted that the diatoms could be dormant in warm water temperature; thus,the reproduction of diatoms stops in summer (Saros and Anderson, 2015). Combined with the ecological characteristics and changes in environmental factors,we found that the water temperature in summer was higher and induced dormancy in diatoms, while the temperature in other seasons was suitable for the reproduction of diatoms. The higher water temperature in summer is more suitable for the mass reproduction of Chlorophyta and Cyanobacteria, giving them a higher dominant position in summer and autumn. In winter, the purif ication ability of the water is poor and decomposers have a slower reproduction rate,and a large amount of pollutants is discharged into the ASRB from upstream. Pollutants can stimulate the rapid division of Cyanobacteria, which eventually causes the density of Cyanobacteria in winter to be higher than that in other seasons.
This study reveals a gradual increase in the abundance and diversity of phytoplankton, from upstream to downstream, throughout the year. These results can be explained by the extra time taken for the phytoplankton to grow and the inf low of more nutrients from upstream to downstream, which is a typical characteristic of rivers (Ha et al., 1998). Among them, sites (S1-S9) located downstream of the river in urban areas were subject to human interference much more than those located in the upstream in the ASRB.The intermediate disturbance hypothesis (IDH)suggests that moderate interference can increase species diversity (Liu et al., 2019), consistent with the IDH. Many pollutants were collected downstream of the river, promoting the increase in the abundance and diversity of phytoplankton located downstream. Low discharge coincided with high nutrient concentrations in the S10 and S11 sites, resulting in phytoplankton abundance reaching its maximum at these sites. These sites had the lowest evenness indexes, suggesting the domination by only a few species and the precarious community structures. Reservoirs might alter the physical and chemical conditions of the rivers and cause upstream and downstream variations in the composition and abundance of the phytoplankton communities (Tornés et al., 2014). Sabater-Liesa et al. (2018) reported that the responses of phytoplankton and environmental variables were not uniform in the Ebro River, and they revealed spatial variability discontinuities upstream and downstream of the reservoirs. Tornés et al. (2014) suggested that reservoirs could change the spatial pattern of phytoplankton density. However, in this study, the abundance of phytoplankton in S14, which is located downstream of the reservoir, was higher than those of S15 and S16, which were located upstream of the reservoir. However, phytoplankton species increased downstream. These results indicate that the reservoirs do not cause signif icant f luctuations in phytoplankton abundance and diversity of the ASRB.
4.2 Driving factor of phytoplankton community
The relationship between phytoplankton and environmental factors is highly dynamic and have been the focus of many studies (Li et al., 2019).Various human activities increasingly inf luence the nutrient composition of the rivers (Liu et al., 2011).As with many other river systems, the richness,diversity, and succession patterns of phytoplankton were closely associated with anthropogenic pollution(Nassar and Fahmy, 2016; Tian et al., 2017).Phytoplankton succession is mainly determined by the interactions of environmental factors (Nassar and Fahmy, 2016; Tian et al., 2017). RDA reveals that the TN, WT, ORP, pH, and DO were critically linked to the phytoplankton community succession.More precisely, the main factors driving the change in phytoplankton community structures in spring was the TN; in summer and autumn they were WT, ORP,and pH; and it was DO in winter.
Nitrogen is one of the many elements required for reproduction and metabolism in phytoplankton (Tang et al., 2018). TN concentration is the key signal for assessing the pollution of aquatic systems. In this study, the RDA showed thatA.formosawas positively correlated with TN. Notably, the average concentration of TN was as high as 1.26 mg/L during spring and winter in ASRB. The dominance ofA.formosawas 0.06 and 0.11, in spring and winter, respectively; the density ofA.formosais usually the highest at the sites where the TN was higher, such as S1, S8, S9, and S10.A previous report showed thatA.formosais usually an indicator of nitrogen enrichment (Slemmons et al.,2017), which is consistent with the results obtained in this study. Additionally, we found thatC.ovataandC.acutawere located in the right of the f irst RDA axis and were closely related to the increase in TN.Early studies have shown thatC.ovataandC.acutaare highly adaptable to temperature and light, and that their growth is favoured by higher levels of nutrients(Greisberger and Teubner, 2007). These results are consistent with the closeness of these species to TN.
Warm temperatures provide favorable conditions for phytoplankton reproduction (Tucker and van der Ploeg, 1993). In previous studies, water temperature was the predominant environmental factor inf luencing the structure of the phytoplankton community (Ma et al., 2014). In this study, RDA shows that most of the Cyanobacteria and Chlorophytawere located on the left of the second RDA axis; the correlation between WT and Chlorophyta was higher than that between WT and Cyanobacteria. It is clear that the adaptability of Cyanobacteria to water temperature diff ers from that of Chlorophyta; Chlorophyta has the broadest range of adaptation to water temperature, followed by Cyanobacteria (McQueen and Lean, 1987).
The existence, migration, and transformation of various organic and inorganic substances in water are connected to the redox reactions (Kedziorek et al.,2008). The ORP ref lects the strength of the oxidizing and reducing properties of the water; the higher the ORP, the better the water quality (Ioka et al., 2016). In the ASRB, the ORP in winter was signif icantly lower than that in other seasons, while the ORP in spring varied widely (367-483 mV). The RDA showed thatN.radiosa,S.sprinceps, andPhacus pyrum(Ehrenberg) Stein were distributed above the second RDA axis, and they were related to the decline in ORP and increase in TP. The lower ORP in the areas subject to severe human disturbance located downstream of the ASRB was consistent with high concentrations of TP. A previous study indicated that the source of TP might be the lower ORP, which promoted the release of phosphorus in the water by the sediment, and led to the deepening of the eutrophication of the water(Shenker et al., 2005).
The reproduction and colonization of phytoplankton are closely associated with pH (Shi et al., 2009).Alkaline water with high pH is more conducive for the formation of organic matter, which is necessary for increase in phytoplankton reproduction (Liu et al., 2010). In the ASRB, phytoplankton community structures were signif icantly aff ected by pH in spring and summer, and this period has higher pH and phytoplankton abundance, which were consistent with previous research (Liu et al., 2010). The pH range of 7.5 to 9.0 was more conducive for the reproduction of Bacillariophyta (Unrein et al., 2010). RDA shows that most of the Bacillariophyta and Chlorophyta are located below the f irst RDA axis; they are positively linked to pH, and negatively correlated with TP. Previous studies have shown that the form of phosphorus in water is closely related to the pH; when pH>8, phosphorus mainly exists as orthophosphate,the main form absorbed by algae (Kunoh and Niwa,1997). Phytoplankton reproduction is closely related to pH; increased phytoplankton abundance leads to high pH (Zhang et al., 2019). Contrarily, CO2is the primary raw material for photosynthesis by phytoplankton,and its decline can reduce photosynthesis, thereby inhibiting phytoplankton reproduction (Keys et al.,2018).
DO is a key factor for phytoplankton reproduction and metabolism as shown in earlier studies (Xu et al., 2010). Increased DO concentration has negative eff ects, not only on growth, but also on the biomass of individual phytoplankton species. In this study,the DO concentration changed seasonally, and the DO in spring and summer was signif icantly lower than that in autumn and winter. These results might be attributed to the phytoplankton abundance which increased signif icantly in spring and summer.Although photosynthesis increases O2concentrations during daytime, higher phytoplankton abundance also accelerates the reduction of dissolved oxygen in the water column. In addition, the lower water temperature in winter is also one of the reasons for the increase in DO (Cardo et al., 2012). RDA shows that four dominant algae species (G.aeruginosum,S.sprinceps,C.microsphaerella, andN.radiosa)were positively correlated with DO, indicating that they make specif ic contribution to the DO in water.G.aeruginosumcan adapt to low light intensity and temperature (Erturk et al., 2014). Thicker ice layers in winter prevent the penetration of sunlight and the light available in the water becomes weaker, resulting in a signif icantly higher density of this species in winter than in other seasons. We speculated that other factors unmeasured in our study might drive the dynamics of phytoplankton communities, such as rainfall, hydrology, suspended solids, and the relationship between phytoplankton community and other communities, and interspecif ic relationships.Thus, it is necessary to gain further insight into other factors that aff ect the ASRB phytoplankton community structure change.
4.3 Phytoplankton as indicators for water quality assessment
Phytoplankton growth and distribution are deeply dependent on environmental factors, such as changes in nutrient concentrations (Guo et al., 2019). Currently,phytoplankton is usually used as a biological indicator in water quality evaluation (Guo et al., 2019). In this study, the sites were divided into three groups for MRT analysis, and Group 1 mainly included winter sample sites. Our results showed that winter presents higher trophic state indexes throughout the year. Analysis of the indicators showed thatC.microsphaerellawas the species with the highest indicator value (0.67)in Group 1. At the same time,C.microsphaerellaappeared in large numbers in winter sites. Kivrak(2006) reported that a river with high eutrophication,low water levels, and longer residence time resulted in high abundance ofC.microsphaerella. Thus,C.microsphaerellais generally considered a sign of eutrophication. It is similar to the hydrological status of the ASRB in winter in this study. We speculated thatC.microsphaerellain the ASRB could also be used as an indicator for eutrophication.M.granulatahas the highest indicator value (0.68) in Group 2,mainly in summer and autumn sites. TSI indicates that its water environment is at a mesotrophic state.Studies have reported thatM.granulatausually indicates the mesotrophic level (Li et al., 2011; Varol,2019). In the ASRB in summer and autumn, most sites were at the mesotrophic level; only seven sites in summer were at light eutrophic level, indicating thatM.granulatacan also be a good indicator of ASRB waters during these seasons. The indicator value ofM.tenuissimain Group 2 was 0.57, whileM.tenuissimausually indicates the mesotrophic level(Barinova et al., 2008), which is also applicable in the ASRB.A.formosahad a higher indicator value(0.81) in Group 3, which were spring sites. We found thatA.formosahad a higher abundance in spring;meanwhile, it has been proven in multiple studies to indicate eutrophication levels (Slemmons et al.,2017; Szabó et al., 2020). This f inding is consistent with the results of TSI. The TSI in spring was 59.26 ±8.52, which was close to the middle eutrophic level,indicating thatA.formosawas an indicator of water quality in the ASRB. In summary, we believe thatC.microsphaerella,M.granulata,M.tenuissima, andA.formosacan eff ectively indicate changes in water quality and be used as relevant tools for indicating water quality for ASRB.
5 CONCLUSION
This study demonstrated the relationship between phytoplankton community and environmental factors in the ASRB. High phytoplankton richness with 127 species from 7 phyla and 73 genera was recorded in the ASRB. TSI showed that the water quality of ASRB is at mesotrophic to middle eutrophic levels. The signif icant seasonal variation of phytoplankton community and environmental factors revealed that phytoplankton community shift is a relevant indicator for water quality assessment.TN, WT, ORP, pH, and DO are crucial predictors of phytoplankton community structures. In addition,C.microsphaerella,M.granulata,M.tenuissima, andA.formosaare potential valuable indicators for the determination of water quality in ASRB. Our f indings provide important information on water quality maintenance and management at the basin scale.
6 DATA AVAILABILITY STATEMENT
The authors declare that the data supporting the f indings of this study are available within the article.
 Journal of Oceanology and Limnology2022年5期
Journal of Oceanology and Limnology2022年5期
- Journal of Oceanology and Limnology的其它文章
- Comparison of three f locculants for heavy cyanobacterial bloom mitigation and subsequent environmental impact*
- Eff ect of light intensity on bound EPS characteristics of two Microcystis morphospecies: the role of bEPS in the proliferation of Microcystis*
- Community structure of aerobic anoxygenic phototrophic bacteria in algae- and macrophyte-dominated areas in Taihu Lake, China*
- Tidal water exchanges can shape the phytoplankton community structure and reduce the risk of harmful cyanobacterial blooms in a semi-closed lake*
- Eff ect of random phase error and baseline roll angle error on eddy identif ication by interferometric imaging altimeter*
- Estimating the evolution of sea state non-Gaussianity based on a phase-resolving model*
